Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. CNTs Synthesis
2.2. Synthesis of Composites
2.3. Characterization of Samples
2.4. Gas Sensing
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhu, A. Study on the synthesis of PANI/CNT nanocomposite and its anticorrosion mechanism in waterborne coatings. Prog. Org. Coat. 2021, 159, 106447. [Google Scholar] [CrossRef]
- Liao, Y.Z.; Zhang, C.; Wang, X.; Li, X.G.; Ippolito, S.J.; Kalantar-Zadeh, K.; Kaner, R.B. Carrier Mobility of Single-Walled Carbon Nanotube-Reinforced Polyaniline Nanofibers. J. Phys. Chem. C 2011, 115, 16187–16192. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energy Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, B. Carbon Nanotube-Based Organic Thermoelectric Materials for Energy Harvesting. Polymers 2018, 10, 1196. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818. [Google Scholar] [CrossRef]
- Oluigbo, C.J.; Xu, Y.; Louis, H.; Yusuf, A.B.; Yaseen, W.; Ullah, N.; Xie, J. Controllable fabrication of abundant nickel-nitrogen doped CNT electrocatalyst for robust hydrogen evolution reaction. Appl. Surf. Sci. 2021, 562, 150161. [Google Scholar] [CrossRef]
- Byeon, H.; Gu, B.; Kim, H.-J.; Lee, J.H.; Seo, I.; Kim, J.; Kim, J.-K. Redox chemistry of nitrogen-doped CNT-encapsulated nitroxide radical polymers for high energy density and rate-capability organic batteries. Chem. Eng. J. 2021, 413, 127402. [Google Scholar] [CrossRef]
- Muangrat, W.; Wongwiriyapan, W.; Yordsri, V.; Chobsilp, T.; Inpaeng, S.; Issro, C.; Domanov, O.; Ayala, P.; Pichler, T.; Shi, L. Unravel the Active Site in Nitrogen-Doped Double-Walled Carbon Nanotubes for Nitrogen Dioxide Gas Sensor. Phys. Status Solidi A 2018, 215, 1800004. [Google Scholar] [CrossRef]
- Li, B.; Sun, X.; Su, D. Calibration of the basic strength of the nitrogen groups on the nanostructured carbon materials. Phys. Chem. Chem. Phys. 2015, 17, 6691–6694. [Google Scholar] [CrossRef]
- Pang, Z.; Yildirim, E.; Pasquinelli, M.A.; Wei, Q. Ammonia Sensing Performance of Polyaniline-Coated Polyamide 6 Nanofibers. ACS Omega 2021, 6, 8950–8957. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Lu, G. The room temperature gas sensor based on Polyaniline@flower-like WO 3 nanocomposites and flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 259, 505–513. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Le, X.V.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Synergistic enhancement of ammonia gas-sensing properties at low temperature by compositing carbon nanotubes with tungsten oxide nanobricks. Vacuum 2019, 168, 108861. [Google Scholar] [CrossRef]
- Toušek, J.; Rutsch, R.; Toušková, J. Explanation of the high conductivity of HCl protonated polyaniline films. Mater. Chem. Phys. 2021, 260, 124153. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.; Bourdo, S.E.; Saini, V.; Watanabe, F.; Barnes, B.; Ghosh, A.; Biris, A.S. Tuning the work function of polyaniline via camphorsulfonic acid: An X-ray photoelectron spectroscopy investigation. RSC Adv. 2015, 5, 33–40. [Google Scholar] [CrossRef]
- Gong, B.; Ikematsu, A.; Waki, K. Impacts of structure defects and carboxyl and carbonyl functional groups on the work function of multiwalled carbon nanotubes. Carbon 2017, 114, 526–532. [Google Scholar] [CrossRef]
- Popov, E.O.; Kolosko, A.G.; Filippov, S.V. Electrical field admissible values for the classical field emitter regime in the study of large area emitters. AIP Adv. 2019, 9, 015129. [Google Scholar] [CrossRef] [Green Version]
- Benko, A.; Duch, J.; Gajewska, M.; Marzec, M.; Bernasik, A.; Nocuń, M.; Piskorz, W.; Kotarba, A. Covalently bonded surface functional groups on carbon nano-tubes: From molecular modeling to practical applications. Nanoscale 2021, 13, 10152–10166. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, J.M.; Hwang, S.K.; Lee, S.H.; Lee, H.-J.; Lee, B.R.; Park, H.I.; Kim, J.-S.; Yoo, S.; Song, M.H.; et al. A ZnO/N-doped carbon nanotube nanocomposite charge transport layer for high performance optoelectronics. J. Mater. Chem. 2012, 22, 12695–12700. [Google Scholar] [CrossRef]
- Davletkildeev, N.A.; Stetsko, D.V.; Bolotov, V.V.; Stenkin, Y.A.; Korusenko, P.M.; Nesov, S.N. Determination of work function in the individual carbon nanotubes using electrostatic force microscopy. Mater. Lett. 2015, 161, 534–537. [Google Scholar] [CrossRef]
- Bolotov, V.V.; Kan, V.E.; Biryukov, M.Y.; Knyazev, E.V.; Shelyagin, R.V.; Korusenko, P.M.; Nesov, S.N.; Sten’kin, Y.A. Origin of the LowFrequency Band in Raman Spectra of MultiWalled Carbon Nanotubes Synthesized by the CVD Method. Phys. Solid State 2013, 55, 1459–1462. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Fedoseenko, S.I.; Iossifov, I.E.; Gorovikov, S.A.; Schmidt, J.-S.; Follath, R.; Molodtsov, S.L.; Adamchuk, V.K.; Kaindl, G. Development and present status of the Russian–German soft X-ray beamline at BESSY II. Nucl. Instrum. Methods Phys. Res. Sect. A 2001, 470, 84–88. [Google Scholar]
- Korusenko, P.M.; Nesov, S.N.; Iurchenkova, A.A.; Fedorovskaya, E.O.; Bolotov, V.V.; Povoroznyuk, S.N.; Smirnov, D.A.; Vinogradov, A.S. Comparative Study of the Structural Features and Electrochemical Properties of Nitrogen-Containing Multi-Walled Carbon Nanotubes after Ion-Beam Irradiation and Hydrochloric Acid Treatment. Nanomaterials 2021, 11, 2163. [Google Scholar] [CrossRef]
- Wiggins-Camacho, J.D.; Stevenson, K.J. Effect of Nitrogen Concentration on Capacitance, Density of States, Electronic Conductivity, and Morphology of N-Doped Carbon Nanotube Electrodes. J. Phys. Chem. C 2009, 113, 19082–19090. [Google Scholar] [CrossRef]
- Tomczyk, M.M.; Boncel, S.; Herman, A.; Krawczyk, T.; Jakóbik-Kolon, A.; Pawlyta, M.; Kuźnik, N. Oxygen Functional Groups on MWCNT Surface as Critical Factor Boosting T2 Relaxation Rate of Water Protons: Towards Improved CNT-Based Contrast Agents. Int. J. Nanomed. 2020, 15, 7433–7450. [Google Scholar] [CrossRef]
- Nxumalo, E.N.; Coville, N.J. Nitrogen Doped Carbon Nanotubes from Organometallic Compounds: A Review. Materials 2010, 3, 2141–2171. [Google Scholar] [CrossRef] [Green Version]
- Bobenko, N.G.; Bolotov, V.V.; Egorushkin, V.E.; Korusenko, P.M.; Melnikova, N.V.; Nesov, S.N.; Povoroznyuk, S.N. Experimental and theoretical study of electronic structure of disordered MWCNTs. Carbon 2019, 153, 40–51. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedorovskaya, E.O.; Okotrub, A.V.; Maximovskiy, E.A.; Vyalikh, D.V.; Chen, X.; Song, H. Electronic state of polyaniline deposited on carbon nanotube or ordered mesoporous carbon templates. Phys. Status Solidi B Basic Res. 2011, 248, 2484–2487. [Google Scholar] [CrossRef]
- Nesov, S.N.; Korusenko, P.M.; Sachkov, V.A.; Bolotov, V.V.; Povoroznyuk, S.N. Effects of preliminary ion beam treatment of carbon nanotubes on structures of interfaces in MOx/multi-walled carbon nanotube (M =Ti,Sn) composites: Experimental and theoretical study. J. Phys. Chem. Solids 2022, 169, 110831. [Google Scholar] [CrossRef]
- Eklund, K.; Karttunen, A.J. Effect of the Dopant Configuration on the Electronic Transport Properties of Nitrogen-Doped Carbon Nanotubes. Nanomaterials 2022, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.A.; Davletkildeev, N.A.; Sokolov, D.V. Work function tuning of the individual polyaniline/carbon nanotube nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 443, 012021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Chen, G. Carbon and carbon composites for thermoelectric applications. Carbon Energy 2020, 2, 408–436. [Google Scholar] [CrossRef]
- Davletkildeev, N.A.; Sokolov, D.V.; Mosur, E.Y.; Bolotov, V.V.; Lobov, I.A. Determination of the Conductivity of Individual Carbon Nanotubes Based on Image Profile Analysis of Electrostatic Force Microscopy. Instrum. Exp. Tech. 2019, 62, 578–581. [Google Scholar] [CrossRef]
- Chen, D.-J.; Lei, S.; Wang, R.-H.; Pan, M.; Chen, Y.-Q. Dielectrophoresis Carbon Nanotube and Conductive Poly-aniline Nanofiber NH3 Gas Sensor. Chin. J. Anal. Chem. 2012, 40, 145–149. [Google Scholar] [CrossRef]
- Eising, M.; O’Callaghan, C.; Eduardo Cava, C.; Schmidt, A.; Gorgatti Zarbin, A.J.; Ferreira, M.S.; Roman, L.S. The role of carbon nanotubes on the sensitivity of composites with polyaniline for ammonium sensors. Carbon Trends 2021, 3, 100026. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonium detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Silveira, F.; Duarte, G.; Tachinski, C.; Piletti, R.; Fiori, J.; Peterson, M.; Riella, H.; Fiori, M. Polyaniline synthesis using hydrofluoric acid as doping agent:comparative evaluation with polyaniline doped with sulfuric acid. J. Appl.Polym. Sci. 2013, 128, 430–435. [Google Scholar] [CrossRef]
- Yang, Z.; Dou, X. Emerging and Future Possible Strategies for Enhancing 1D Inorganic Nanomaterials-Based Electrical Sensors towards Explosives Vapors Detection. Adv. Funct. Mater. 2016, 26, 2406–2425. [Google Scholar] [CrossRef]
- Dong, Y.; Han, W.; Choi, H. Polyaniline Coated Core-Shell Typed Stimuli-Responsive Microspheres and Their Electrorheology. Polymers 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, U.; Malik, S. Graphene oxide/polyaniline nanostructures: Transformation of 2D sheet to 1D nanotube and in situ reduction. Chem. Commun. 2012, 48, 10862. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, M.; Wang, G.; Wang, Q.; Yan, M.; Bi, H. Morphology-dependent electrochemical performance of nitrogen-doped carbon dots@polyaniline hybrids for supercapacitors. Int. J. Energy Res. 2019, 43, 7529–7540. [Google Scholar] [CrossRef]

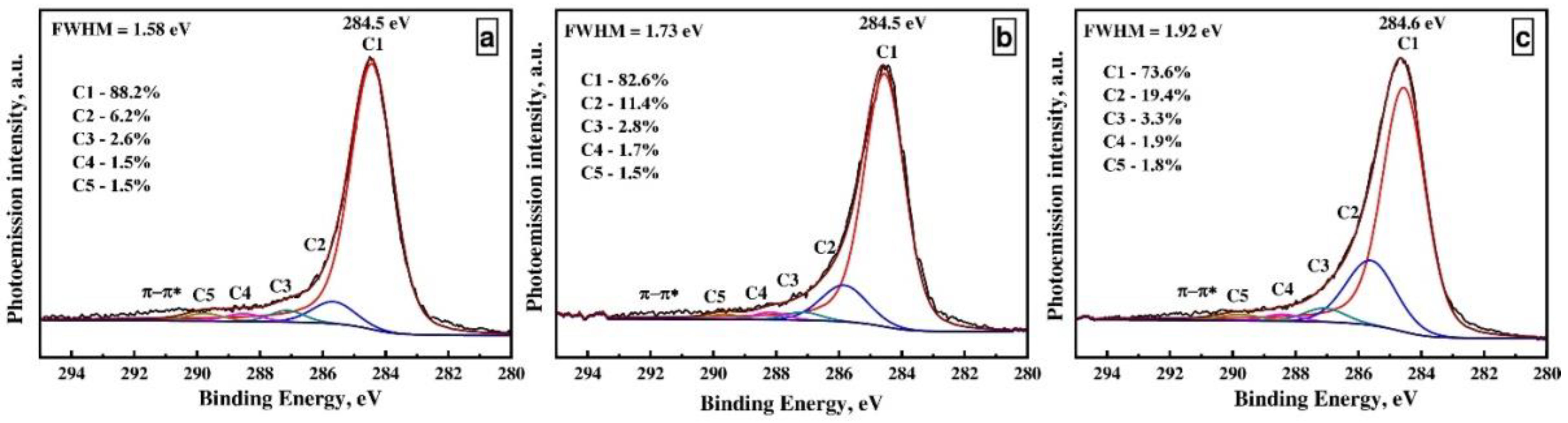
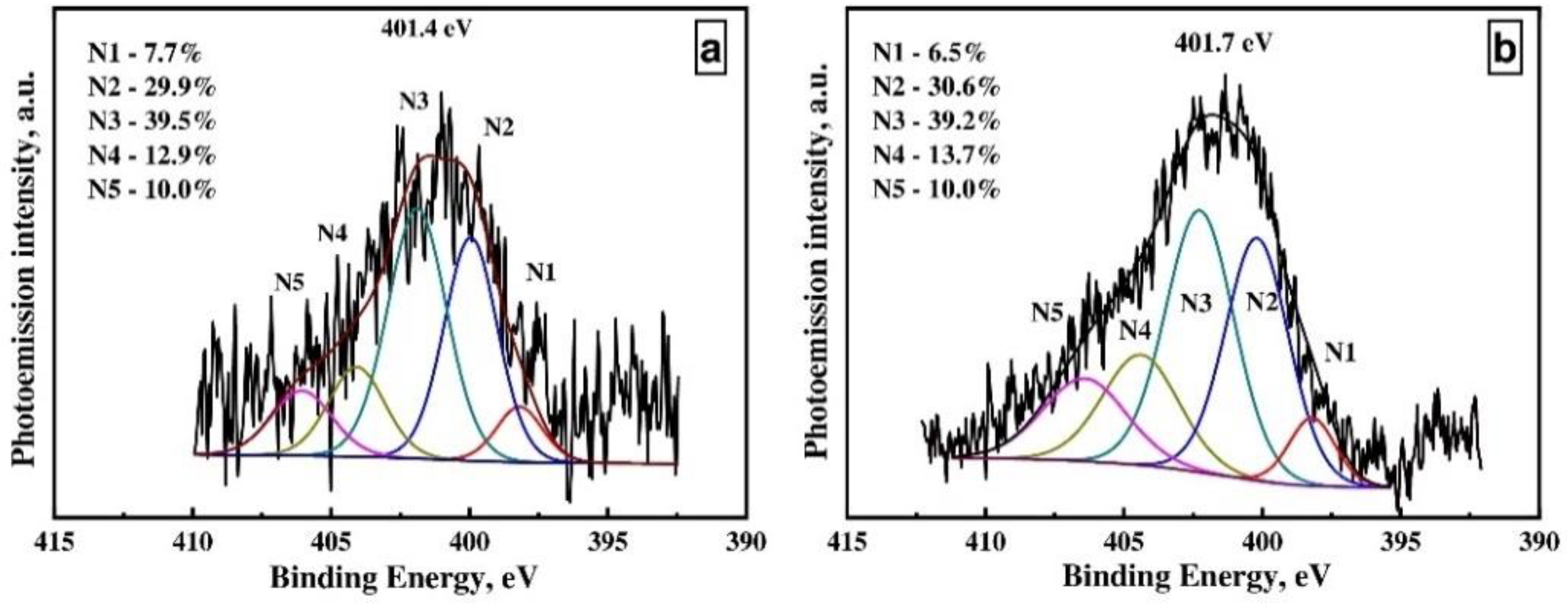

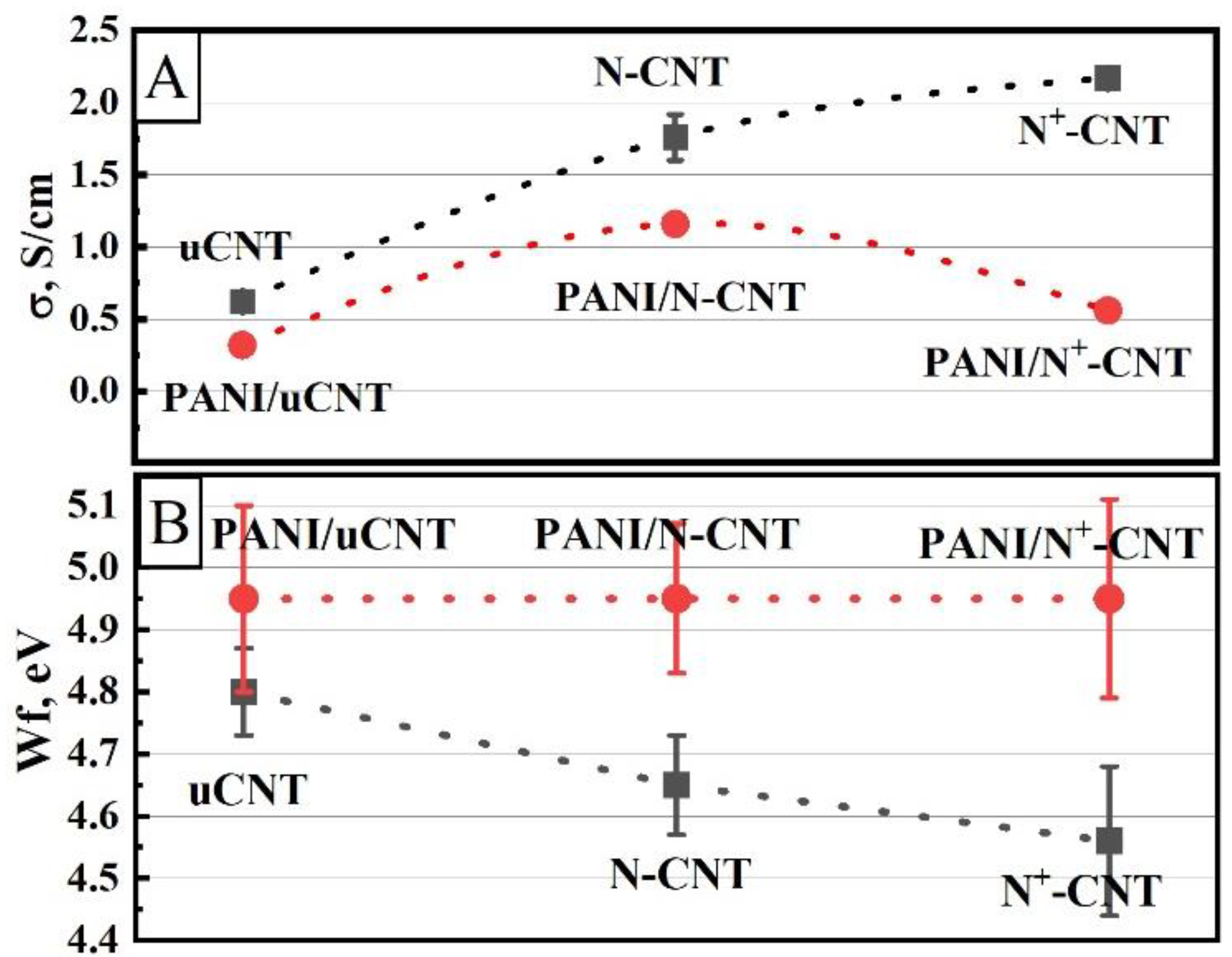
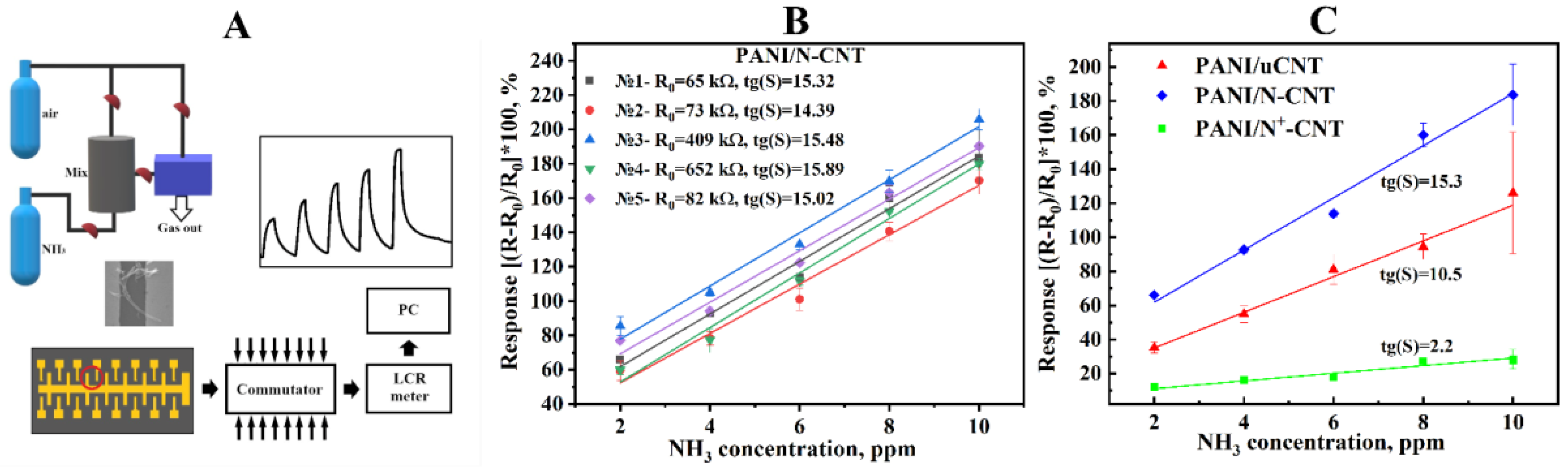

| CNTs Type | Concentration, at. % | ||
|---|---|---|---|
| [C] | [N] | [O] | |
| uCNT | 96.0 | - | 4.0 |
| N-CNT | 93.4 | 2.1 | 4.5 |
| N+-CNT | 92.6 | 4.0 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobov, I.A.; Davletkildeev, N.A.; Nesov, S.N.; Sokolov, D.V.; Korusenko, P.M. Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite. Appl. Sci. 2022, 12, 7169. https://doi.org/10.3390/app12147169
Lobov IA, Davletkildeev NA, Nesov SN, Sokolov DV, Korusenko PM. Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite. Applied Sciences. 2022; 12(14):7169. https://doi.org/10.3390/app12147169
Chicago/Turabian StyleLobov, Ivan A., Nadim A. Davletkildeev, Sergey N. Nesov, Denis V. Sokolov, and Petr M. Korusenko. 2022. "Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite" Applied Sciences 12, no. 14: 7169. https://doi.org/10.3390/app12147169
APA StyleLobov, I. A., Davletkildeev, N. A., Nesov, S. N., Sokolov, D. V., & Korusenko, P. M. (2022). Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite. Applied Sciences, 12(14), 7169. https://doi.org/10.3390/app12147169








