Abstract
In this study, we investigated the effects of additional ultraviolet radiation (UV) on the main growth fluorescent lamp light on pigment content and essential oil accumulation in sweet basil (Ocimum basilicum L.). Three different UV light sources from light-emitting diodes and discharge lamps, which emit UV in the UV-A (315–400 nm), UV-B (280–315 nm) and UV-C (100–280 nm) ranges, were tested for basil plant growing. The plants, growing under additional UV-A and UV-B from mercury lamps, on the 60th growing day were higher than control plants by 90% and 53%, respectively. The fresh leaf mass of the UV-A irradiated basil plants was 2.4-fold higher than the control plant mass. The dry mass/fresh mass ratio of the UV-A and UV-B irradiated plants was higher by 45% and 35% in comparison to the control plants. Leaf area was increased by 40% and 20%, respectively. UV-C affected the anthocyanin content most strongly, they increased by 50%, whereas only by 27% and 0% under UV-A and UV-B. Any UV addition did not affect the essential oil total contents but altered the essential oil compositions. UV-A and UV-B increased the linalool proportion from 10% to 20%, and to 25%, respectively, in contrast to UV-C, which reduced it to 3%. UV-C induced the eugenol methyl ether accumulation (17%) and inhibited plant growth. Moreover, UV increased the proportion of α-guaiene, β-cubebene and α-bulnesene and decreased the proportion of sabinene and fenchone. Thus, we concluded that UV (except UV-C) used jointly with main light with PPFD 120 ± 10 μmol photons·m−2·s−1 for sweet basil cultivation may be justified to stimulate basil growth and optimize the essential oil accumulation.
1. Introduction
Sweet basil (Ocimum basilicum L.) is a popular herb whose flavor and aroma are caused by its high essential oil substances. Essential oils of plants are volatile secondary metabolites that can be divided onto three categories, i.e., monoterpenes, sesquiterpenes and oxygenated terpenes [1]. Interest in studying the essential oils is due to their biological activity: antioxidant, antifungal and antibacterial [2,3].
Such factors as plant age, species and variety, light intensity, temperature and level of nutrition and irrigation significantly affect the sweet basil growth and essential oil content in their leaves [4,5,6,7]. The induction of essential oils can increase plant protection against adverse environmental factors [8,9]. It is known that the quality of light affects the content of secondary metabolites [10] and plant pigments [11] and the quantitative and qualitative composition of essential oil in plants [12,13]. The detailed information on the effects of light quality on the growth and phytonutrient accumulation of herbs is presented in the review [14].
Generally, an increase in photosynthetic photon flux density (PPFD) led to a significant increase in the total essential oil content. This is typical for such important components as 1,8-cineole, linalool and eugenol [15]; vice versa, in experiments with shading plants in the open field, a recession in illumination from 50–60 klx to 3–5 klx led to a decrease in the basil growth rate and the gross yield of essential oil [16]. However, changes in growth and development of plants caused by changes in illumination do not always correlate with the alteration of essential oil content. When plants were being shaded, the number and mass of leaves and inflorescences decreased but the total essential oil content, in particular eugenol, increased [17].
In addition to light intensity, spectral light quality strongly affects the essential oil production and accumulation. Red, blue and UV light enhanced the essential oil concentration in various herbs compared with white light or sunlight [18,19,20]. However, for sweet basil, blue light increased the total essential oils, while red light in contrast decreased the oil content [4]. Supplemental UV, generally inhibiting plant growth, increased the amount of essential oil, resulting in enhanced plant protection against UV [21]. UV-B (280–315 nm), or a combination of UV-A (315–400 nm) and UV-B, can increase essential oil concentration in O. basilicum, M. arvensis and G. uralensis [22,23,24]. Moreover, these light treatments could effectively alter the compositions of essential oil in plants [4,25].
An ultraviolet irradiation impact on plants is of great interest due to both its potentially negative effect and the possibility of biochemical and physiological process regulation to obtain high quality plants. It is interesting not only to observe UV-A and UV-B as part of the spectrum but also UV-C radiation (100–280 nm), which is the most damaging type of UV. With the help of photosensors, plants are able to perceive solar radiation in a wide spectral range from ultraviolet 260 nm to far red 780 nm [26]. Cryptochromes are activated by UV-A, blue and green wavelengths. UV-B regulates plant growth by photoreceptors responding to UV-B irradiation [27]. Ultraviolet irradiation induces a stress reaction that cause the synthesis of protective pigments and phenolic compounds, oxidative damage, partial inhibition of photosynthesis and decreased growth [28].
Sweet basil is usually grown in greenhouses under glass or film cover, which does not transmit ultraviolet radiation. In urban farms, which are also gaining popularity, plants are grown on racks in totally controlled environments using light-emitting diode (LED) irradiators (without UV range). The absence of UV radiation in the spectrum can lead to deterioration in the grown product quality.
The UV-B radiation affects the development and morphology of the plant [29]. In the absence of the UV-B wavelengths in irradiation spectrum, the peltate and capitate basil glandular trichomes do not develop in mature and young leaves, and essential oil sacs become wrinkled and only partially fill [30]. With UV-B treatment introduction in the first four days, the “normal” development of the glandular trichomes occurs. However, neither the number of glands nor the qualitative or quantitative composition of the volatiles depend on UV irradiation; the UV spectrum of light only affects a filling of the glandular trichomes in basil [31].
A high dose of continuous UV-B radiation (68 kJ m−2 day−1) leads to irreversible photodamage of photosystem II and inactivation of the oxygen-evolving complex of photosystem II, which ultimately leads to plant death [32]. However, higher doses (102 kJ m−2 day−1) with intermittent irradiation improve the basil nutritional qualities without affecting the photosynthetic apparatus efficiency [33]. It was also found that the UV-B radiation effect on the phenolic compound synthesis is different at various PPFD [34].
In contrast to UV-B studies, there is limited information on the specific effects of UV-A and UV-C radiation on secondary metabolites, aromatics and the growth of sweet basil plants. In this research, we tested three different UV light sources in the form of LEDs and discharge lamps, emitting in the UV-A, UV-B and UV-C ranges, in addition to the main visible light. The aim of our study is to determine the impact of UV irradiation of different ranges on productivity and the essential oil content of sweet basil plants.
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
Red leaf basil of “Red Ruby” variety (“Prestige”, Moscow, Russia) was chosen for the research. The plants were grown in a climate chamber in plastic pots (1-L capacity) using drip irrigation. Neutralized high-moor peat Agrobalt-C (Rostorfinvest, Moscow, Russia) was used as a substrate. Seeds were sown in pots. After germination, five plants were left in each pot and were cultured for eight weeks under assimilation light, as described below. Each variant had 4 replicates. The nutrient solution was prepared using deionized water.
The chemical composition of the nutrient solution was: N-NO3 9.64 mM; N-NH4 1.07 mM; P-PO4 1.00 mM; K 5.77 mM; Ca 2.00 mM; Mg 1.65 mM; S-SO4 1.75 mM; Fe 15.00 µM; B 20.00 µM; Cu 1.00 µM; Zn 5.00 µM; Mn 10.00 µM; Mo 1.00 µM. A chamber temperature was maintained by the microclimate system described earlier [35] in the day/night range of 23/16 ± 1.0 °C. The relative air humidity was 65 ± 5%.
2.2. Light Treatments
In the phytochamber, general illumination was provided by combined irradiators based on fluorescent lamps (FL) Osram Fluora 36W and Osram Lumilux 36W T8 L15 W/827 (OSRAM Licht AG, Munich, Germany). The spectral composition and the PPFD level were chosen optimally for basil plants using a 16-h photoperiod [36,37]. Light intensity was measured for each variant at a height of about 20 cm from the pot surface using a TKA-VD spectrocolorimeter (NTP TKA, St. Petersburg, Russia). The average PPFD was 120 ± 10 μmol photons·m−2·s−1. The phytochamber was divided into four equal compartments, separated by an opaque material. In three compartments of the chamber, UV irradiators of various ranges were additionally installed: mercury lamps UV-A 365 nm FERON T8 (FERON, Moscow, Russia), mercury lamps UV-A/UV-B Arcadia T8 (Arcadia, Croydon, England) and UV-C LED 275 nm (Seoulviosys Co., Seoul, Korea) (Table 1). The UV irradiators were turned on simultaneously with the basic light. An intensity of the used UV emitters is comparable to the UV component of sunlight for the Moscow summer season for 2019 and 2020 [38] (with the exception of UV-C, which is absent under normal conditions). UV irradiance was monitored using a portable TKA-PKM UV radiometer (NTP TKA, St. Petersburg, Russia). The fourth chamber compartment with only fluorescent lamps (FL) was used as the control variant.

Table 1.
Spectral properties of radiation sources. The values are mean ± standard error (SE, n = 5). FL—fluorescence lamp spectrum.
2.3. Morphological Parameters
An assessment of biometric parameters of morphological organs of sweet basil plants was carried out on the 30th and 60th day after germination. Five plants from four pots were randomly selected from each variant in four replications for measuring fresh plant mass, leaf mass, dry mass, plant height and leaf surface area.
The mass of the aboveground part of the plants was determined by weighing using a Sartorius LA230S Laboratory Scale (Goettingen, Germany). The height of the plants was measured using a ruler. The leaf area was determined using the LI-COR LI-3100 AREA METER photoplanimeter (Lincoln, OR, USA).
2.4. Determination of the Pigment Composition
Chlorophyll and carotenoid quantitative analysis was carried out by extracting them from plant tissues with solvent (100% acetone). The optical density of the pigment extract was determined at wavelengths of 662, 644 and 440.5 nm using 10 mm cuvettes [39]. The concentration of chlorophylls a and b, as well as carotenoids, were calculated according to Holm–Wettstein for 100% acetone in mg / g of fresh mass [40,41].
To extract anthocyanins, initial weighed fresh leaf portion (0.3 g) was ground with 1% HCl. A flask with extract was kept in a water bath at 40–45 °C for 15 min and then the extract was filtered. Total anthocyanin content was calculated using the absorption index of cyanidin-3,5-diglycoside in 1% solution of hydrochloric acid at 510 nm wavelength [37].
2.5. The Total Content and Essential Oil Composition
Gas chromatography–mass spectrometry (GC-MS) analysis and gas chromatography with flame-ionization detection (GC-FID) were applied to determine the essential oil profile, quantity and concentration in freshly picked leaves. Fresh leaves of all variants were extracted with hexane. The extraction was carried out in 100 mL flasks with a plant material fresh mass to solvent volume ratio of 1 g per 3 mL, respectively. The flask with the extract was placed in an ultrasonic stirrer Elmasonic S60H (Singen, Germany) (exposure time was 15 min). The samples were shaken gently with added sodium sulfate anhydrous (Na2SO4) for dehydration [42]. After the extraction process was completed, the solution was centrifuged and filtered through a syringe filter.
For a qualitative definition a 1 μL volume of the extract was injected into GC-MS Agilent 8890 GC (Santa Clara, CA, USA), equipped with a 30 m 0.25 mm fused silica capillary column Agilent Carbowax (Santa Clara, CA, USA) (i.d. 0.25 μm). The measurements were carried out according to the established technique [42]. Identification of sample components was performed by comparing their comparison of mass spectra of the electron ionization compounds of the samples with the GC-MS library NIST MS Library Bundle 2020.
For the quantitative oil determination, fresh leaves of one variant were similarly extracted with hexane, and hexadecane was added as an internal standard for quantitative calculations. The relative percentage of the oil constituents was calculated from the GC peak areas. The identification of compounds was performed by comparison of the NIST Chemistry WebBook. A volume of 1 μL of the extract was injected into gas chromatograph with a flame ionization detector (FID) Shimadzu (Kyoto, Japan) (model LC-20, software GCSolution v. 2.3 (Kyoto, Japan)). Nitrogen was used as a carrier gas at a constant rate of 1 mL/min. Samples were injected in splitless mode. The injector temperature was 250 °C and the temperature of the transmission line and the detector was 270 °C. The temperature gradient increased from 50 °C to 260 °C at a rate of 5 °C/min.
2.6. Data Analysis
All experiments were carried out fourfold. The statistical processing of measurement results and plotting were performed in Pyton 3.9. To estimate the statistical significance by the considered parameters, the independent two-sample t-test at p < 0.05 significant levels was applied. To evaluate the correction for multiple comparisons, the library “statsmodels” (Holm method) was used.
3. Results
3.1. Morphological Parameters
The results of the statistical analysis showed that additional UV irradiation had a significant effect on the morphological characteristics of basil plants (Table 2, Figure 1). Significant differences were observed in plant height, leaf surface area and fresh and dry mass. The additional irradiation of plants with UV-A light range led to a drastic increase in plant growth rate. UV-A added jointly with UV-B had a moderate positive effect on the 60th day. On the 30th day, “UV-A” and “UV-A+UV-B” treatments had a comparable effect. UV-C treatment, in contrast, had a negative effect on plant growth.

Table 2.
Morphological parameters of sweet basil “Red Ruby” plants on the 30th and 60th days of cultivation. Values represent mean SEM (n = 20). Letters indicate significant differences among treatment and control samples (p < 0.05).

Figure 1.
Typical basil plants on the 60th day from germination.
3.2. Photosynthetic Pigment Content
Exposure to additional UV-A and UV-B irradiation significantly influenced the content of chlorophyll a and b and carotenoids (Figure 2), which, however, was not consistent with other studies [43,44]. The significant increase in the total amount of anthocyanins was observed under additional UV-A and UV-C irradiation. The increase in the content of photosynthetic pigments in plants can be due to the activation of protecting mechanisms against UV irradiation [45,46].
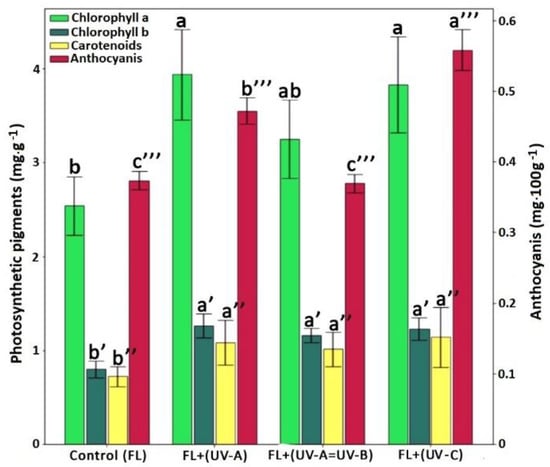
Figure 2.
Chlorophyll a, chlorophyll b, carotenoid and anthocyanin concentration in sweet basil of “Red Ruby” variety on the 60th day of cultivation. Values represent mean SEM (n = 20). Letters indicate significant differences among treatment and control samples (p < 0.05).
3.3. Essential Oil Components
A chromatographic analysis of essential oil is represented in Table 3. The study identified a total of 21 different essential oil components and unidentified oil (about 3–4% in total). The major components were eugenol (up to 54%), linalool (from 10% to 20%), eucalyptol (10%) and sabinene (up to 5%). It was demonstrated that the additional irradiation of plants with UV did not affect the essential oil total content. At the same time, the essential oil composition changed. Generally, UV-treatments decreased the proportion of sabinene and fenchone and increased the linalool, α-guaiene, β-cubebene and α-bulnesene fraction. The concentration of other oil components remained practically unchanged. However, in some cases, the effect of UV-C differed from that of other types of ultraviolet. For example, UV-A and UV-B increased the linalool proportion from 10% to 20–25%, whereas UV-C reduced it to 3%. UV-C induced the eugenol methyl ether accumulation (17%) content, which remained unchanged under UV-A and UV-B. Moreover, under UV-C the proportion of fenchone practically did not change. Thus, UV, jointly with main growth light, may be applied to change the essential oil profile.

Table 3.
Essential oil composition in “Red Ruby” sweet basil plants on the sixtieth day of cultivation. Averages with standard error of the mean of four measurements are shown. Letters indicate significant differences among treatment and control samples (p < 0.05).
4. Discussion
4.1. Effect of UV Radiation on Plant Morphology and Pigment Content
It is known that basil varieties with purple leaves are more sensitive to UV-A radiation, which is reflected in the decrease of leaf area and plant height [43]. However, in our experiments, UV-A irradiation had a stimulating effect: on the 60th day, the height, the leaf surface area and the fresh and dry mass of the “Red Ruby” variety basil plants significantly increased. This can be explained by the fact that at low PPFD, UV-A can be used as an additional source of light energy to intensify the photosynthesis [47,48]. There are differences in the UV-A susceptibility of different plant species. UV-A had a positive effect on the fresh mass and the number of lettuce shoots and had practically no effect on the dry mass [49]. The increase in height and plant mass may be associated with the higher photosynthetic activity of UV-A radiation compared with UV-B and UV-C. It has been found that soybean plant height increases when UV-A irradiation is screened in a glass greenhouse [50], while UV-A does not affect the leaf area and plant height of peppers and eggplants and decreased the length of internodes [51]. The combined illumination with UV-A and UV-B also positively influenced the height, leaf area and fresh and dry sweet basil plant mass at the first stage of cultivation (30 days). It is known that small doses of UV-B irradiation (2–4 kJ m−2 day−1) lead to an increase in leaf surface area, fresh and dry mass and the total content of phenolic compounds of sweet basil [52]. It was previously established that a seven-day illumination of basil with UV-B increases plant height, photosynthetic leaf area and fresh and dry biomass [43]. In general, our data are consistent with previous studies. Unlike UV-A and UV-B radiation, the UV-C strongly suppresses plant growth [49], which is also confirmed by our experiment (Table 2).
4.2. UV Radiation Stimulates the Biosynthesis of Anthocyanins
According to our data, UV-A and UV-C irradiation had a positive effect on the anthocyanin content in basil leaves, while the combination of UV-A and UV-B did not change the anthocyanin content. It is known that repeated exposure to UV-C enhances the accumulation of phenolic compounds [50], and anthocyanin flavonoids have a function as a protective filter of the cell against the destructive photodynamic action of light and UV irradiation [45,46,53]. The absorption maximum of anthocyanin refers to 265–280 nm and 510–560 nm.
Flavonoids are of interest due to their antioxidant activity, anti-inflammatory and anticarcinogenic effects [54]. It is known that the biosynthesis of flavonoids is influenced by UV-B, UV-A and visible radiation (400–700 nm) [25]. UV-A radiation at a wavelength of 373 nm increased the accumulation of anthocyanins in young lettuce leaves [55]. At the same time, in green-leaved lettuce varieties, UV-A irradiation increased the content of nitrites [56]. UV-A improves antioxidant properties (affects the total content of phenol, anthocyanins and ascorbic acid) in basil plants [44]. In our case, UV-C radiation had the greatest effect on the biosynthesis of flavonoids. The anthocyanin content in this variant was higher than others. In our previously conducted research [57], the “Red Ruby” basil variety also showed a strong dependence on the average anthocyanin concentration in leaves on the type of UV radiation. The maximum influence was caused by UV-C on the 60th day of cultivation, which indicates a greater response to UV stress during the budding and flowering stages.
4.3. UV Radiation Changes the Composition of Essential Oil
Depending on the UV irradiation range, the essential oil concentration and its component composition changed. The content of linalool was higher in plants grown under UV-A and UV-B irradiation, while the eugenol content was higher in the control variant. Additionally, under UV-C radiation, eugenol methyl ester (ME) is additionally synthesized.
ME is found in a number of plants (over 450 species from 80 families, including the angiosperms and gymnosperms) and plays a role in attracting pollinators. Essential oils containing eugenol are used in food, perfumery and pharmaceutical industries [58]. ME is a naturally occurring genotoxic carcinogenic compound with DNA-binding ability and has a toxicological danger due to its structural similarity with known carcinogenic phenylpropanoids, for example, estragole and safrole [59]. From 2021, any product containing more than 0.01% methyl eugenol must be declared in accordance with the Regulation of the European Parliament and of the Council (16 December 2008) [60].
The “Red Ruby” variety belongs to the basil chemotype rich in ME [61]. Researchers have established a strong dependence of eugenol and ME content on seasonal changes (mainly solar radiation, but also on temperature and relative humidity). Other studies show that sweet basil which was grown in a greenhouse up to the first internodes under natural light, and subsequently moved under artificial light with a PAR 400–700 nm spectrum with additional UV-B irradiation for 18 days (up to six developed leaves and a height of 12–15 cm), had a lower content of methyleugenol [30,62]. During ontogenesis, the activity of eugenol O-methyltransferase (EOMT) is constantly decreasing, while the level of eugenol increases. UV-B radiation apparently suppresses the activity of EOMT already in young leaves, which indicates a UV-induced change in the enzymatic defense systems in sweet basil leaves. In our study the UV-A and UV-B irradiation depressed the activity of EOMT and prevented ME synthesis. However, in other research, UV-B irradiation had a positive effect on eugenol, phenylpropanoid and ME and decreased the content of linalool in young basil leaves and increased it in adult plants [24].
The scheme of changes in the component composition of essential oil depending on the impact of ultraviolet radiation of various ranges is shown in Figure 3. An amount of phenylpropanoids responsible for plant protective reaction to aggressive environmental influences, including ultraviolet irradiation, increased only in the variant with the use of additional UV-C irradiation. UV-A and UV-B irradiation in this experiment did not cause a stress increase in phenylpropanoids, rather was stimulating and necessary for optimal growth.
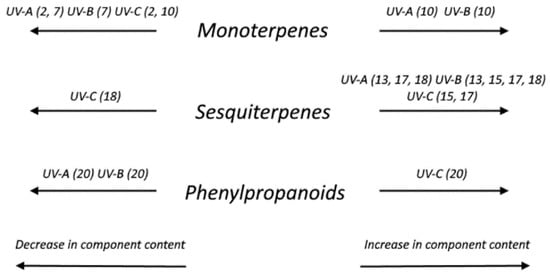
Figure 3.
Scheme of the changing of essential oil component composition of the “Red Ruby” variety sweet basil under ultraviolet irradiation influence of the different ranges on the 60th day of cultivation.
It is known that additional illumination with UV-B wavelengths increases the aromatic volatile compound level in sweet basil leaves [31,62]. The essential oil content is much higher in young leaves, and the main aromatic components are a-pinene, p-pinene, 1,8-cineole and linalool [63]. The essential oil content can be increased with UV-B irradiation and at later stages of growing [64]. In contrast, our research showed, that the basil leaf total oil content had no statistically significant differences between variants. It can be explained by different reactions of basil varieties to UV-B radiation impact [65].
In general, it can be said that exposure to UV-A and UV-B irradiation changes the composition of terpenes in “Red Ruby” variety sweet basil essential oil towards more complex sesquiterpenes that affect the plant aroma.
5. Conclusions
In this complex study, the complex effect of additional UV-A, UV-B and UV-C irradiation on the biometric and biochemical parameters of sweet basil of “Red Ruby” variety was investigated. The study highlights that basil, when grown with increased UV-A irradiation values, has strong increased characteristics of fresh mass, height and leaf surface area, probably due to a change in the intensity of photosynthesis. UV-A induces changes in chlorophyll and anthocyanin concentrations, as well as in the essential oil composition of sweet basil plants. In comparison with other studies, the specific basil variety reaction to the researched ranges of UV irradiation was established.
Thus, ultraviolet of UV-A and UV-B ranges is capable of changing both productivity and the taste and aromatic properties of basil, increasing predominantly sesquiterpene content.
Author Contributions
Conceptualization and methodology, N.A.S., A.A.S. and S.V.G.; validation, A.S.I.; formal analysis, N.A.S. and A.S.I.; investigation and resources, N.O.C.; writing—original draft preparation, N.A.S., A.A.S. and Y.A.P.; writing—review and editing, N.O.C., R.R.S. and D.V.Y.; visualization, Y.A.P. and N.O.C.; supervision and funding acquisition, A.S.D.; project administration, A.S.D. and A.Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant of the Ministry of Science and Higher Education of the Russian Federation for large scientific projects in priority areas of scientific and technological development (subsidy identifier 075-15-2020-774).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No additional data available.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
References
- Veloso, R.A.; Castro, H.G.; Barbosa, L.C.A.; Cardoso, D.P.; Chagas Júnior, A.F.; Scheidt, G.N. Essential oil composition and content of different accessions and cultivars of basil (Ocimum basilicum L.). Rev. Bras. Plantas Med. 2014, 16, 364–371. [Google Scholar] [CrossRef]
- Anwar, F.; Alkharfy, K.M.; Mehmood, T.; Bakht, M.A.; Najeeb-ur-Rehman. Variation in Chemical Composition and Effective Antibacterial Potential of Ocimum basilicum L. Essential Oil Harvested from Different Regions of Saudi Arabia. Pharm. Chem. J. 2021, 55, 187–193. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Çilesiz, Y.; Korkmaz, E.; Mustafa, Z.; Baloch, F.S.; Karaköy, T.; Aasim, M. An Overview of O. basilicum (L.) in Turkey. In Ocimum basilicum: Taxonomy, Cultivation and Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 51–71. [Google Scholar]
- Amaki, W.; Yamazaki, N.; Ichimura, M.; Watanabe, H. Effects of light quality on the growth and essential oil content in sweet basil. Acta Hortic. 2011, 907, 91–94. [Google Scholar] [CrossRef]
- Chang, X.M.; Alderson, P.G.; Wright, C.J. Effect of temperature integration on the growth and volatile. oil content of basil (Ocimum basilicum L.). J. Hortic. Sci. Biotechnol. 2005, 80, 593–598. [Google Scholar] [CrossRef]
- Lachowicz, K.J.; Jones, G.P.; Briggs, D.R.; Bienvenu, F.E.; Palmer, M.V.; Mishra, V.; Hunter, M.M. Characteristics of Plants and Plant Extracts from Five Varieties of Basil (Ocimum basilicum L.) Grown in Australia. J. Agric. Food Chem. 1997, 45, 2660–2665. [Google Scholar] [CrossRef]
- Morales, M.R.; Simon, J.E.; Charles, D.J. Comparison of essential oil content and composition between field and greenhouse grown genotypes of methyl cinnamate basil (Ocimum basilicum L.). J. Herbs Spices Med. Plants 1993, 1, 25–30. [Google Scholar] [CrossRef]
- Aurore, G.S.; Abaul, J.; Bourgeois, P. Antibacterial and antifungal activities of the essential oils of Pimenta racemosa var. racemosa P. Miller (Myrtaceae). J. Essent. Oil Res. 1998, 10, 161–164. [Google Scholar] [CrossRef]
- Kim, D.H.; Ahn, Y.J. Contact and fumigant activities of constituents of Foeniculum vulgare fruit against three coleopteran stored-product insects. Pest Manag. Sci. 2001, 57, 301–306. [Google Scholar] [CrossRef]
- Nishimura, T.; Zobayed, S.M.A.; Kozai, T.; Goto, E. Medicinally Important Secondary Metabolites and Growth of Hypericum perforatum L. Plants as Affected by Light Quality and Intensity. Environ. Control Biol. 2007, 45, 113–120. [Google Scholar] [CrossRef]
- Ebisawa, M.; Shoji, K.; Kato, M.; Shimomura, K.; Goto, F.; Yoshihara, T. Effects of supplementary lighting of UV-B, UV-A and blue light during the night on growth and coloring in red-leaf lettuce. J. Shita 2008, 20, 158–164. [Google Scholar] [CrossRef]
- Milenkovic, L.; Stanojevic, J.; Cvetkovic, D.; Stanojevic, L.; Lalevic, D.; Sunic, L.; Fallik, E.; Ilic, Z.S. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019, 140, 111718. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light Quality Dependent Changes in Morphology, Antioxidant Capacity, and Volatile Production in Sweet Basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Gerda, M.; Wilfried, H. Effect of PAR and UV-B Radiation on the Quality and Quantity of the Essential Oil in Sweet basil (Ocimum basilicum L.). Acta Hortic. 2004, 659, 375–382. [Google Scholar] [CrossRef]
- Il’chenko, G.N.; Ageeva, T.T.; Berezkin, N.G. Influence of different illumination levels on growth and development of basil eugenol (Ocimum gratissimum L.). Oilseed Crops 2014, 2, 159–160. [Google Scholar]
- Bliznikas, Z.; Zukauskas, A.; Samuoliene, G.; Virsile, A.; Brazaityte, A.; Jankauskiene, J.; Duchovskis, P.; Novickovas, A. Effect of Supplementary Pre-Harvest LED Lighting on the Antioxidant and Nutritional Properties of Green Vegetables. Acta Hortic. 2012, 939, 85–91. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- Nishioka, N.; Nishimura, T.; Ohyama, K.; Sumino, M.; Malayeri, S.; Goto, E.; Inagaki, N.; Morota, T. Light Quality Affected Growth and Contents of Essential Oil Components of Japanese Mint Plants. Acta Hortic. 2008, 797, 431–436. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.; Kozai, T. Spectral quality and UV-B stress stimulate glycyrrhizin concentration of Glycyrrhiza uralensis in hydroponic and pot system. Plant Physiol. Biochem. 2005, 43, 1074–1081. [Google Scholar] [CrossRef]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2021, 956, 37–49. [Google Scholar] [CrossRef]
- Hikosaka, S.; Ito, K.; Goto, E. Effects of ultraviolet light on growth, essential oil concentration, and total antioxidant capacity of japanese mint. Environ. Control Biol. 2010, 48, 185–190. [Google Scholar] [CrossRef]
- Sun, R.; Hikosaka, S.; Goto, E.; Sawada, H.; Saito, T.; Kudo, T.; Ohno, T.; Shibata, T.; Yoshimatsu, K. In Effects of UV irradiation on plant growth and concentrations of four medicinal ingredients in Chinese licorice (Glycyrrhiza uralensis). Acta Hortic. 2012, 956, 643–648. [Google Scholar] [CrossRef]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Noguchi, A.; Amaki, W. Effects of light quality on the growth and essential oil production in Mexican mint. Acta Hortic. 2016, 1134, 239–244. [Google Scholar] [CrossRef]
- Folta, K.; Carvalho, S. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Jenkins, G. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Klein, R.M. Plants and near-ultraviolet radiation. Bot. Rev. 1978, 44, 1–127. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Robberecht, R.; Billings, W.D. A Steep Latitudinal Gradient of Solar Ultraviolet-B Radiation in the Arctic-Alpine Life Zone. Ecology 1980, 61, 600–611. [Google Scholar] [CrossRef]
- Ioannidis, D.; Bonner, L.; Johnson, C.B. UV-B is required for normal development of oil glands in Ocimum basilicum L. (sweet basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Sodi, A.M. UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy 2018, 9, 434. [Google Scholar] [CrossRef]
- Grishin, A.A.; Smirnov, A.A.; Grishin, V.A.; Dorokhov, A.A.; Chilingaryan, N.O. Climatic chambers with a system of controlled phytoirradiation for growing plants. Bull. Cent. Bot. Gard. 2011, 1, 56–60. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Maslennikov, P.; Chupakhina, G.; Skrypnick, L.; Fedurayev, P.; Poltavskaya, R. Content of anthocyanin and carotenoid pigments in medicinal plants. Bull. Mosc. Reg. State Univ. 2013, 1, 6. [Google Scholar]
- Solar and Meteorological Data Sets from NASA Research for Support of Renewable Energy, Building Energy Efficiency and Agricultural Needs. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 1 September 2021).
- Musiienko, M.; Parshykova, T.; Slavnyj, P. Spectrographic Methods in Practical Physiology, Biochemistry and Ecology of Plants; Fitosociocentr: Kyiv, Ukraine, 2001; p. 200. (In Ukrainian) [Google Scholar]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef]
- Fischer, R.; Nitzan, N.; Chaimovitsh, D.; Rubin, B.; Dudai, N. Variation in Essential Oil Composition within Individual Leaves of Sweet Basil (Ocimum basilicum L.) Is More Affected by Leaf Position than by Leaf Age. J. Agric. Food Chem. 2011, 59, 4913–4922. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viskelis, P.; Duchovskis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A. Supplementary UV-B irradiation effects on basil (Ocimum basilicum L.) growth and phytochemical properties. J. Food Agric. Environ. 2012, 10, 342–346. [Google Scholar] [CrossRef]
- Vastakaite, V.; Virsile, A.; Brazaityte, A.; Samuoliene, G.; Jankauskiene, J.; Sirtautas, R.; Duchovskis, P. The effect of blue light dosage on growth and antioxidant properties of microgreens. Sodin. Daržininkyste 2015, 34, 25–35. [Google Scholar] [CrossRef]
- Ballas, J.P.; Matter, S.F. UV-induced anthocyanin in the host plant Sedum lanceolatum has little effect on feeding by larval Parnassius smintheus. Alpine Bot. 2020, 130, 25–30. [Google Scholar] [CrossRef]
- Landi, M.; Agati, G.; Fini, A.; Guidi, L.; Sebastiani, F.; Tattini, M. Unveiling the shade nature of cyanic leaves: A view from the “blue absorbing side” of anthocyanins. Plant Cell Environ. 2020, 49, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, M.-M.; Son, K.-H. Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Dader, B.; Gwynn-Jones, D.; Moreno, A.; Winters, A.; Fereres, A. Impact of UV-A radiation on the performance of aphids and whiteflies and on the leaf chemistry of their host plants. J. Photochem. Photobiol. B Biol. 2014, 138, 307–316. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viskelis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. Sci. Food Agric. 2013, 93, 1266–1271. [Google Scholar] [CrossRef]
- Raymond, B.K. Flavonoids: Biosynthesis, Biological Effects and Dietary Sources; Nova Science Publishers: Hauppauge, NY, USA, 2009; ISBN 1-61761-914-0. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Li, Q.; Kubotab, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Virsile, A.; Brazaityte, A.; Vastakaite-Kairiene, V.; Miliauskiene, J.; Jankauskiene, J.; Novickovas, A.; Lauzike, K.; Samuoliene, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef] [PubMed]
- Proshkin, Y.A.; Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Burynin, D.A.; Ivanitskikh, A.S.; Panchenko, V.A. Assessment of Ultraviolet Impact on Main Pigment Content in Purple Basil (Ocimum basilicum L.) by the Spectrometric Method and Hyperspectral Images Analysis. Appl. Sci. 2021, 11, 8804. [Google Scholar] [CrossRef]
- Il’chenko, G.N.; Kislun, Y.V.; Ageeva, T.T. Methods of assessment of major components of the essential oil component of the plant material of basil (Ocimum L.). New Technol. 2016, 1, 91–99. [Google Scholar]
- De Vincenzi, M.; Silano, M.; Stacchini, P.; Scazzocchio, B. Constituents of aromatic plants: I. Methyleugenol. Fitoterapia 2000, 71, 216–221. [Google Scholar] [CrossRef]
- Regulation of the European Parliament and of the Council of 16 December 2008 No. 1272/2008 on the Classification, Labeling and Packaging of Chemicals and Mixtures. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 1 September 2021).
- Murárikova, A.; Tazky, A.; Neugebauerova, J.; Plankova, A.; Jampílek, J.; Mucaji, P.; Mikus, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Ziv-Raz, I.; Dudai, N.; Tadmor, Y.; Lastochkin, E.; Larkov, O.; Chaimovitsh, D.; Ravid, U.; Putievsky, E.; Pichersky, E.; et al. Biosynthesis of estragole and methyl-eugenol in sweet basil (Ocimum basilicum L.). Developmental and chemotypic association of allylphenol O-methyltransferase activities. Plant Sci. 2000, 160, 27–35. [Google Scholar] [CrossRef]
- Ichimura, M.; Noguchi, A.; Kimura, M. Changes in flavor and essential oil components by location of leaf in sweet basil (Ocimum basilicum L.). J. Agric. 2008, 53, 91–95. [Google Scholar]
- Nithia, J.; Shanthi, N. Effect of enhanced solar UV-b (280–320) radiation on physiology and volatile oil synthesis in Ocimum basilicum L. World J. Pharm. Pharm. Sci. 2015, 4, 687–698. [Google Scholar]
- Singh, S.K.; Verma, S.K.; Mathur, A.; Siddiqui, M.A.; Gupta, D.K.; Sharma, B.M. Alterations in antioxidative potential of Ocimum cultivars as a method to characterize UV-B tolerance. Recent Res. Sci. Technol. 2011, 3, 140–148. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).