Connectivity Alterations in Vascular Parkinsonism: A Structural Covariance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
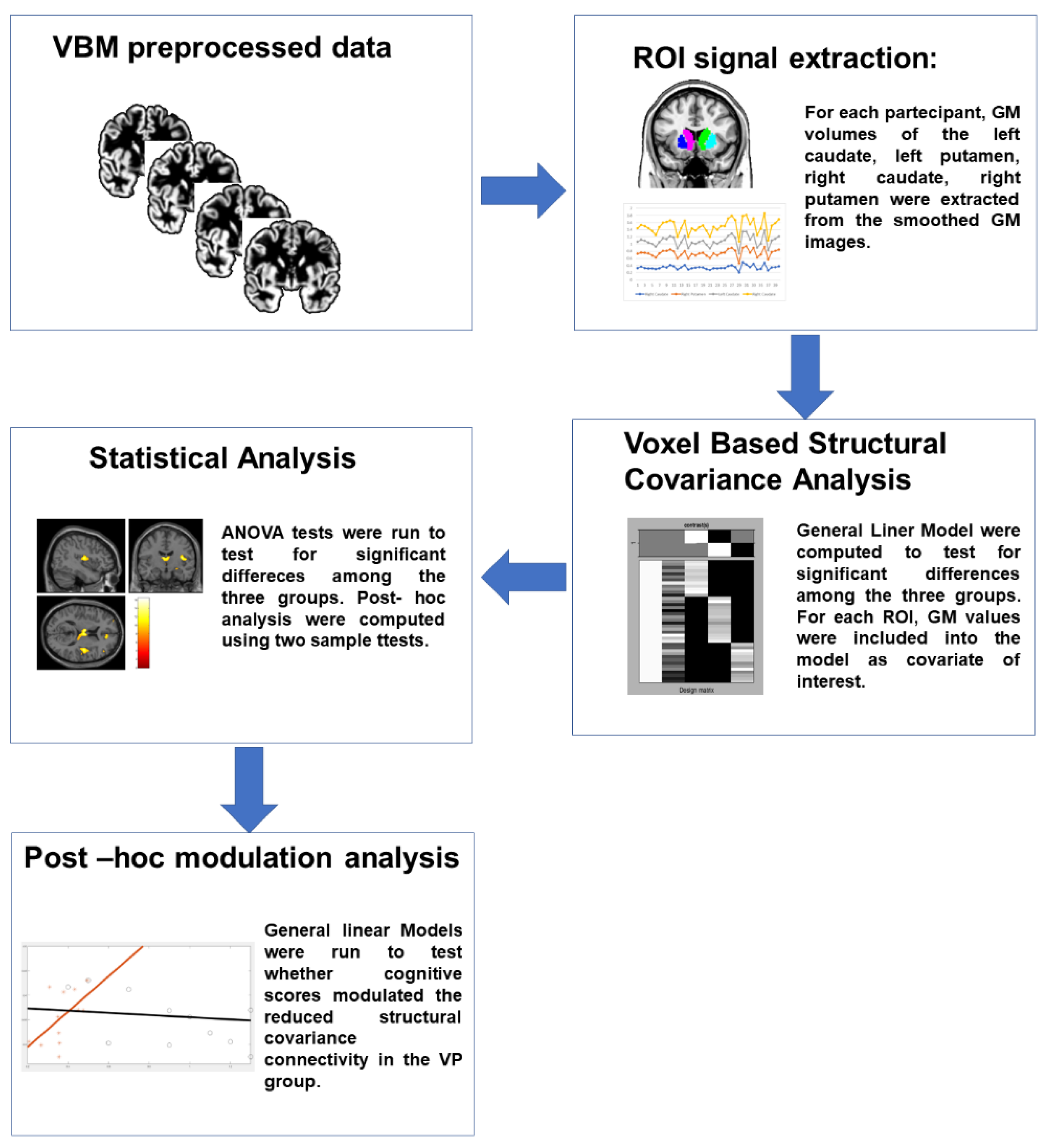
2.2. MRI Protocol, Data Processing and Analysis
2.3. Modulation Analysis of Structural Covariance
2.4. DAT-SPECT Imaging
2.5. Statistical Analysis
3. Results
3.1. Structural Covariance Analysis
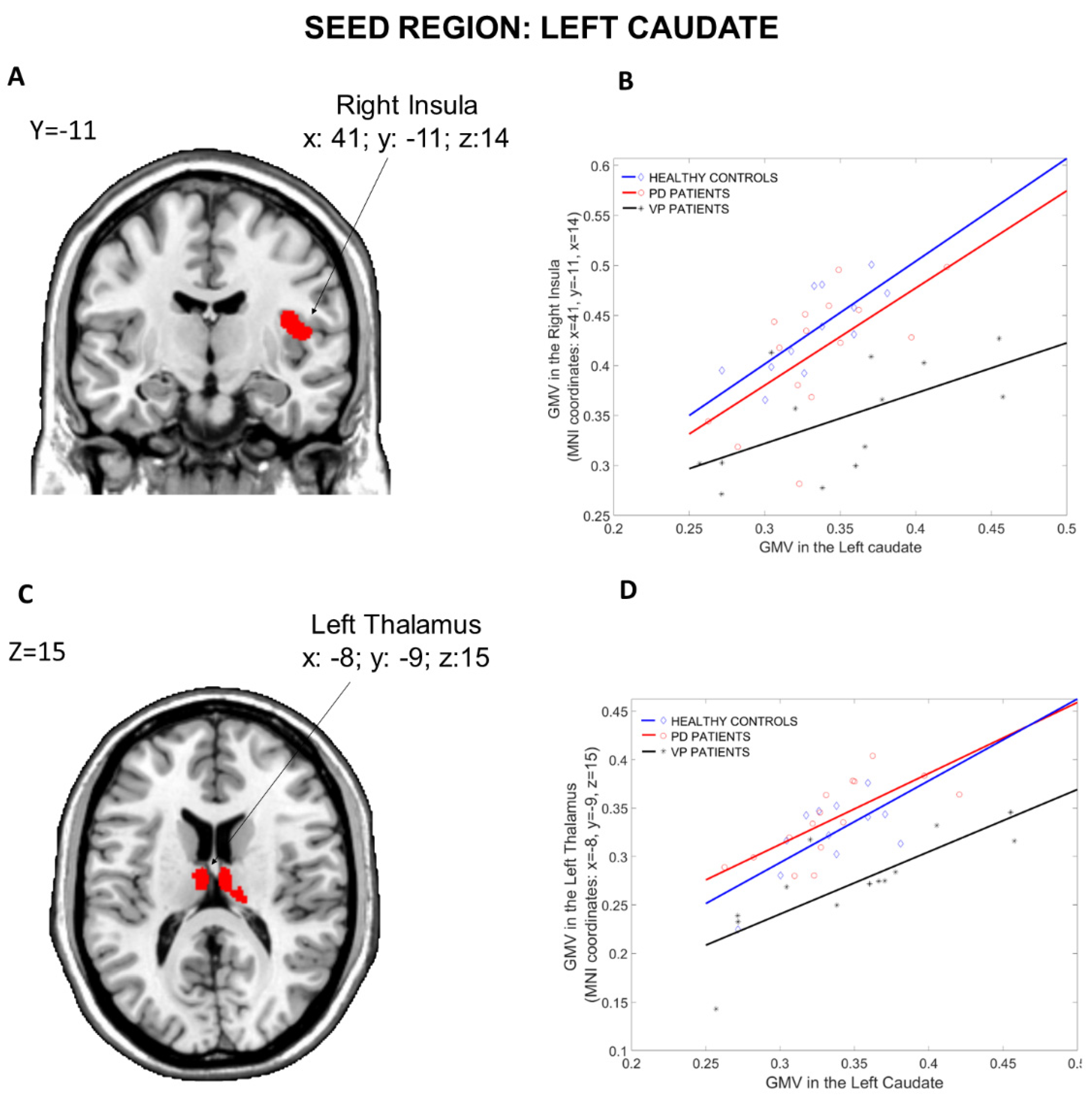
3.1.1. Seed Region: Left Caudate
3.1.2. Seed Region: Left Putamen
3.1.3. Seed Region: Right Caudate
3.1.4. Seed Region: Right Putamen
3.2. Modulation Analysis of Structural Covariance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.C.; Hsu, J.L.; Leemans, A. Diffusion tensor imaging of vascular parkinsonism: Structural changes in cerebral white matter and the association with clinical severity. Arch. Neurol. 2012, 69, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winikates, J.; Jankovic, J. Clinical correlates of vascular parkinsonism. Arch. Neurol. 1999, 56, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Salsone, M.; Caligiuri, M.E.; Vescio, V.; Arabia, G.; Cherubini, A.; Nicoletti, G.; Morelli, M.; Quattrone, A.; Vescio, B.; Nisticò, R.; et al. Microstructural changes of normal-appearing white matter in Vascular Parkinsonism. Parkinsonism Relat. Disord. 2019, 63, 60–65. [Google Scholar] [CrossRef]
- Antonini, A.; Vitale, C.; Barone, P.; Cilia, R.; Righini, A.; Bonuccelli, U.; Abbruzzese, G.; Ramat, S.; Petrone, A.; Quatrale, R.; et al. The relationship between cerebral vascular disease and parkinsonism: The VADO study. Parkinsonism Relat. Disord. 2012, 18, 775–780. [Google Scholar] [CrossRef]
- Alexander-Bloch, A.; Giedd, J.N.; Bullmore, E. Imaging structural covariance between human brain regions. Nat. Rev. Neurosci. 2013, 14, 322–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Liu, Z.; Kang, Q.; Lu, L.; Zhu, Y.; Xu, R. Cortical Structural Connectivity Alterations and Potential Pathogenesis in Mid-Stage Sporadic Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 650371. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, H.L.; Lu, C.H.; Lee, C.Y.; Chou, K.H.; Chen, M.H.; Yu, C.C.; Lai, Y.R.; Chiang, P.L.; Lin, W.C. The corticolimbic structural covariance network as an early predictive biosignature for cognitive impairment in Parkinson’s disease. Sci. Rep. 2021, 11, 862. [Google Scholar] [CrossRef]
- Li, R.; Zou, T.; Wang, X.; Wang, H.; Hu, X.; Xie, F.; Meng, L.; Chen, H. Basal ganglia atrophy-associated causal structural network degeneration in Parkinson’s disease. Hum. Brain Mapp. 2022, 43, 1145–1156. [Google Scholar] [CrossRef]
- Zijlmans, J.C.; Daniel, S.E.; Hughes, A.J.; Révész, T.; Lees, A.J. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov. Disord. 2004, 19, 630–640. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson Disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R.L. Unified Parkinson’s disease rating scale. In Recent Developments in Parkinson’s Disease II; MacMillan: New York, NY, USA, 1987; pp. 153–163. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini Mental State. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Appollonio, I.; Leone, M.; Isella, V.; Piamarta, F.; Consoli, T.; Villa, M.L.; Forapani, E.; Russo, A.; Nichelli, P. The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol. Sci. 2005, 26, 108–116. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F. Modified Card Sorting Test: Normative data. J. Clin. Exp. Neuropsychol. 2004, 26, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Laiacona, M.; lnzaghi, M.G.; De Tanti, A.; Capitani, E. Wisconsin card sorting test: A new global score, with ltalian norms, and its relationoship with the Weigl sorting test. Neurol. Sci. 2000, 21, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Manual for the Wechsler Adult Intelligence Scale Revised; Psychological Corporation: New York, NY, USA, 1981. [Google Scholar]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G.; Fadda, L.; Gallassi, R.; Lorusso, S.; Marfia, G.; Marra, C.; Nocentini, U.; Parnetti, L. The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.L.; Varney, N.R.; Hamsher, K.D. Visuospatial judgment. A clinical test. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef]
- Benton, A.L.; Hamsher, K.D.; Rey, G.J. Multilingual Aphasia Examination; AJA Associates: Iowa City, IA, USA, 1994. [Google Scholar]
- The Italian Group on the Neuropsychological Study of Aging. Token test in Italian standardization and classification of Neuropsychological tests. Ital. J. Neurol. Sci. 1987, 8, 120–123. [Google Scholar]
- Hamilton, M. Hamilton anxiety rating scale (HAM-A). J. Med. (Cincinnati) 1959, 61, 81–82. [Google Scholar]
- Beck, A.T.; Ward, C.; Mendelson, M. Beck Depression Inventory (BDI). Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Gaser, C.; Dahnke, R.; Kurth, F.; Luders, E. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. bioRxiv, 2022; preprint. [Google Scholar]
- Xu, Q.; Zhang, Q.; Liu, G.; Dai, X.; Xie, X.; Hao, J.; Yu, Q.; Liu, R.; Zhang, Z.; Ye, Y.; et al. BCCT: A GUI toolkit for brain structural covariance connectivity analysis on MATLAB. Front. Hum. Neurosci. 2021, 15, 641961. [Google Scholar] [CrossRef]
- Bernhardt, B.C.; Rozen, D.A.; Worsley, K.J.; Evans, A.C.; Bernasconi, N.; Bernasconi, A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: Insights from MRI-based morphometric correlation analysis. Neuroimage 2009, 46, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sharda, M.; Khundrakpam, B.S.; Evans, A.C.; Singh, N.C. Disruption of structural covariance networks for language in autism is modulated by verbal ability. Brain Struct. Funct. 2016, 221, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Salsone, M.; Bagnato, A.; Novellino, F.; Cascini, G.L.; Paglionico, S.; Cipullo, S.; Morelli, M.; Pugliese, P.; Nicoletti, G.; Messina, D.; et al. Cardiac MIBG scintigraphy in Primary Progressive Freezing Gait. Parkinsonism Relat. Disord. 2009, 15, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Yeo, B.T.T.; Spreng, R.N. Towards a Universal Taxonomy of Macro-Scale Functional Human Brain Networks. Brain Topogr. 2019, 32, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downar, J.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci. 2000, 3, 277–283. [Google Scholar] [CrossRef]
- Calhoon, G.G.; O’Donnell, P. Closing the gate in the limbic striatum: Prefrontal suppression of hippocampal and thalamic inputs. Neuron 2013, 78, 181–190. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, R.A.; Nour, M.M.; Dahoun, T.; Jauhar, S.; Pepper, F.; Expert, P.; Veronese, M.; Adams, R.A.; Turkheimer, F.; Mehta, M.A.; et al. Mesolimbic Dopamine Function Is Related to Salience Network Connectivity: An Integrative Positron Emission Tomography and Magnetic Resonance Study. Biol. Psychiatry 2019, 85, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; Nour, M.M.; Veronese, M.; Rogdaki, M.; Bonoldi, I.; Azis, M.; Turkheimer, F.; McGuire, P.; Young, A.H.; Howes, O.D. A Test of the Transdiagnostic Dopamine Hypothesis of Psychosis Using Positron Emission Tomographic Imaging in Bipolar Affective Disorder and Schizophrenia. JAMA Psychiatry 2017, 74, 1206–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Jellinger, K.A. Vascular Parkinsonism. Therapy 2008, 5, 237–255. [Google Scholar] [CrossRef]
- Vale, T.C.; Barbosa, M.T.; Caramelli, P.; Cardoso, F. Vascular Parkinsonism and cognitive impairment: Literature review, Brazilian studies and case vignettes. Dement. Neuropsychol. 2012, 6, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Alster, P.; Migda, B.; Madetko, N.; Duszyńska-Wąs, K.; Drzewińska, A.; Charzyńska, I.; Starczyński, M.; Szepelska, A.; Królicki, L.; Friedman, A. The Role of Frontal Assessment Battery and Frontal Lobe Single-Photon Emission Computed Tomography in the Differential Diagnosis of Progressive Supranuclear Palsy Variants and Corticobasal Syndrome—A Pilot Study. Front. Neurol. 2021, 12, 630153. [Google Scholar] [CrossRef]
- Lima, C.F.; Meireles, L.P.; Fonseca, R.; Castro, S.L.; Garrett, C. The Frontal Assessment Battery (FAB) in Parkinson’s disease and correlations with formal measures of executive functioning. J. Neurol. 2008, 255, 1756–1761. [Google Scholar] [CrossRef]
- Salsone, M.; Arabia, G.; Manfredini, L.; Quattrone, A.; Chiriaco, C.; Vescio, B.; Sturniolo, M.; Morelli, M.; Nistico, R.; Novellino, F.; et al. REM-Sleep Behavior Disorder in Patients with Essential Tremor: What Is Its Clinical Significance? Front. Neurol. 2019, 10, 315. [Google Scholar] [CrossRef] [Green Version]
- Blacker, D.; Lee, H.; Muzikansky, A.; Martin, E.C.; Tanzi, R.; McArdle, J.J.; Moss, M.; Albert, M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch. Neurol. 2007, 64, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.R.; Chen, J.; Yang, Z.J.; Zhang, H.; Fu, Y.; Shen, Y.; He, P.; Mao, C.; Liu, C. Rapid Eye Movement Sleep Behavior Disorder Symptoms Correlate with Domains of Cognitive Impairment in Parkinson’s Disease. Chin. Med. J. (Engl.) 2016, 129, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, F.; Onaka, H.; Kohara, S.; Ryo, M.; Takahashi, W.; Nogawa, S. Early detection of cognitive impairment in Parkinson’s disease with the use of the Wisconsin Card Sorting Test: Correlations with Montreal Cognitive Assessment and smell identification test. J. Neural Transm. 2019, 126, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kuruvilla, A. Vascular parkinsonism: What makes it different? Postgrad. Med. J. 2011, 87, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, C.; Morgante, L.; Antonini, A.; Barone, P.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, M.G.; Ceravolo, R.; Priamo Study Group; et al. Non-motor symptoms in atypical and secondary parkinsonism: The PRIAMO study. J. Neurol. 2010, 257, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Stenc Bradvica, I.; Janculjak, D.; Butkovic-Soldo, S.; Vladetic, M. Cognitive dysfunction in idiopathic and vascular parkinsonism. Med. Glas. 2011, 8, 209–215. [Google Scholar]
- Schoenberg, M.R.; Dawson, K.A.; Duff, K.; Patton, D.; Scott, J.G.; Adams, R.L. Test performance and classification statistics for the Rey auditory verbal learning test in selected clinical samples. Arch. Clin. Neuropsychol. 2006, 21, 693–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estévez-González, A.; Kulisevsky, J.; Boltes, A.; Otermín, P.; García-Sánchez, C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: Comparison with mild cognitive impairment and normal aging. Int. J. Geriatr. Psychiatry 2003, 18, 1021–1028. [Google Scholar] [CrossRef]
- Balthazar, M.L.; Yasuda, C.L.; Cendes, F.; Damasceno, B.P. Learning, retrieval, and recognition are compromised in aMCI and mild AD: Are distinct episodic memory processes mediated by the same anatomical structures? J. Int. Neuropsychol. Soc. 2010, 16, 205–209. [Google Scholar] [CrossRef]
- Eckert, M.A.; Menon, V.; Walczak, A.; Ahlstrom, J.; Denslow, S.; Horwitz, A.; Dubno, J.R. At the heart of the ventral attention system: The right anterior insula. Hum. Brain Mapp. 2009, 30, 2530–2541. [Google Scholar] [CrossRef] [Green Version]
- Dobbins, I.G.; Simons, J.S.; Schacter, D.L. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J. Cogn. Neurosci. 2004, 16, 908–920. [Google Scholar] [CrossRef]
- Cosentino, S.; Brickman, A.M.; Griffith, E.; Habeck, C.; Cines, S.; Farrell, M.; Shaked, D.; Huey, E.D.; Briner, T.; Stern, Y. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia 2015, 75, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shany-Ur, T.; Lin, N.; Rosen, H.J.; Sollberger, M.; Miller, B.L.; Rankin, K.P. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain 2014, 137, 2368–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombello, J.M.; Cooper, C.M.; Fatt, C.C.; Grannemann, B.D.; Carmody, T.J.; Jha, M.K.; Mayes, T.L.; Greer, T.L.; Yezhuvath, U.; Aslan, S.; et al. Neural substrates of emotional conflict with anxiety in major depressive disorder: Findings from the Establishing Moderators and biosignatures of Antidepressant Response in Clinical Care (EMBARC) randomized controlled trial. J. Psychiatr. Res. 2022, 149, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.C.; Davis, R.M.; Zarate, C.A., Jr.; Drevets, W.C. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. 2013, 213, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leh, S.E.; Petrides, M.; Strafella, A.P. The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology 2010, 35, 70–85. [Google Scholar] [CrossRef]



| Healthy Controls (N = 12) | PD Patients (N = 15) | VP Patients (N = 13) | Group Differences | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | F, T, p Values | |
| Gender distribution | 9 m, 3 f | 9 m, 6 f | 9 m, 4 f | F = 0.33, p = 0.72 |
| Age | 73.9 ± 5.7 | 70.2 ± 4.3 | 75.4 ± 7.3 | F = 2.93, p = 0.07 |
| MMSE | 28.33 ± 1.58 | 25.9 ± 2.6 | 25.2 ± 2.7 | F = 5.44, p = 0.009 |
| TOKEN | 31.05 ± 1.71 | 30.15 ± 2.33 | 21.94 ± 11.69 | F = 4.82, p = 0.01 |
| COWAT | 26.95 ± 9.17 | 24.53 ± 9.98 | 14.55 ± 6.36 | F = 5.46, p = 0.04 |
| RAVLT-I.R. | 41.78 ± 6.67 | 36.41 ± 9.80 | 28.40 ± 8.61 | F = 7.52, p = 0.002 |
| RAVLT-D.R. | 7.98 ± 2.90 | 7.48 ± 4.10 | 4.5 ± 3.36 | F = 1.48, p = 0.24 |
| Digit Span fw | 5.5 ± 1.07 | 5.29 ± 0.74 | 4.25 ± 1.00 | F = 6.08, p = 0.006 |
| Digit Span bw | 3.67 ± 0.49 | 4 ± 0.82 | 2.92 ± 0.79 | F = 7.35, p = 0.002 |
| JLO-V | 23 ± 4.57 | 21.77 ± 4.81 | 17.67 ± 4.42 | F = 4.42, p = 0.02 |
| MCST | 5.33 ± 1.23 | 4.31 ± 1.31 | 2.33 ± 1.15 | F = 18.24, p < 0.001 |
| WEIGL | 13.58 ± 2.27 | 10.99 ± 2.13 | 5.74 ± 3.55 | F = 26.03, p < 0.001 |
| FAB | 14.98 ± 2.16 | 13.63 ± 2.24 | 11.08 ± 2.68 | F = 8.42, p = 0.001 |
| BDI | 8.41 ± 5.73 | 8.77 ± 4.13 | 9.67 ± 4.48 | F = 0.12, p = 0.88 |
| HAMA | 8.83 ± 4.19 | 7.77 ± 3.56 | 9.92 ± 4.38 | F = 1.96, p = 0.16 |
| Disease Duration | - | 5.5 ± 3.6 | 4.7 ± 3.6 | T = 0.57, p = 0.57 |
| UPDRS (Total Score) | - | 34.7 ± 9.3 | 33.3 ± 9.11 | T = 0.42, p = 0.68 |
| UPDRS-ME | - | 21.9 ± 8.7 | 24.4 ± 5.6 | T = −0.88, p = 0.39 |
| DAT-SPECT (Putamen/Caudate—Right) | - | 1.01 ± 0.39 | 1.43 ± 0.88 | T = −1.40, p = 0.17 |
| DAT-SPECT (Putamen/Caudate—Left) | - | 1.06 ± 0.52 | 1.35 ± 0.58 | T = −1.22, p = 0.24 |
| ANOVA Comparisons among the Three Groups | |||||||
| Brain Region | MNI Coordinates | F value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Left Thalamus | −8 | −9 | 15 | 16.40 | 4.28 | 0.001 ** | 1460 |
| Right Insula | 41 | −11 | 14 | 13.82 | 3.96 | 0.027 ** | 711 |
| Post hoc comparisons | |||||||
| PD > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Left Thalamus | −8 | −9 | 15 | 5.48 | 4.62 | 0.034 * | 2993 |
| Left Hippocampus | −15 | −36 | 3 | 5.07 | 4.36 | 0.02 ** | 1049 |
| HC > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Insula | 41 | −11 | 14 | 5.13 | 4.40 | <0.001 ** | 2918 |
| Right Thalamus | 23 | −30 | −3 | 4.73 | 4.13 | 0.026 ** | 975 |
| ANOVA Comparisons among the Three Groups | |||||||
| Brain Region | MNI Coordinates | F value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Hippocampus | 21 | −26 | −9 | 16.98 | 4.34 | 0.001 ** | 1426 |
| Left Hippocampus | −12 | −36 | 3 | 15.81 | 4.21 | 0.049 ** | 590 |
| Post hoc comparisons | |||||||
| PD > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Left Hippocampus | −12 | −36 | 3 | 5.55 | 4.67 | 0.028 * | 4318 |
| Right Hippocampus | 21 | −26 | −9 | 5.50 | 4.64 | 0.05 * | |
| HC > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Hippocampus | 27 | −11 | −11 | 5.66 | 4.57 | 0.02 * | 2132 |
| Right Insula | 41 | −11 | 14 | 4.95 | 4.28 | 0.001 ** | 1895 |
| Left Rectus | −9 | 23 | −14 | 4.64 | 4.07 | 0.016 ** | 1102 |
| Right Cerebellum Crus 2 | 17 | −78 | −36 | 3.97 | 3.58 | 0.019 ** | 1053 |
| ANOVA Comparisons among the Three Groups | |||||||
| Brain Region | MNI Coordinates | F value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Left Thalamus | −6 | −11 | 15 | 16.13 | 4.25 | 0.004 ** | 1110 |
| Right Insula | 41 | −12 | 14 | 13.34 | 3.89 | 0.032 ** | 681 |
| Post hoc comparisons | |||||||
| PD > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Left Thalamus | −6 | −11 | 15 | 5.43 | 4.59 | 0.038 * | 2801 |
| Left Para Hippocampus | −18 | −33 | −11 | 4.62 | 4.05 | 0.032 ** | 919 |
| Right Cerebellum Crus 2 | 14 | −81 | −36 | 4.14 | 3.71 | 0.037 ** | 883 |
| HC > VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Insula | 41 | −12 | 14 | 5.07 | 4.36 | <0.001 ** | 2702 |
| Left Frontal- med-orb (BA 10) | −5 | 47 | −8 | 4.74 | 4.14 | 0.004 | 1560 |
| Right Cerebellum Crus 2 | 12 | −78 | −36 | 4.11 | 3.69 | 0.02 ** | 1054 |
| ANOVA Comparisons among the Three Groups | |||||||
| Brain Region | MNI Coordinates | F value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Thalamus | 23 | −30 | −3 | 16.55 | 4.29 | 0.001** | 1566 |
| Left Thalamus | −14 | −35 | 5 | 14.44 | 4.04 | 0.004** | 631 |
| Post hoc comparisons | |||||||
| PD> VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Hippocampus Left Thalamus | 23 −14 | −27 −35 | −9 5 | 5.36 | 4.55 | 0.04 * | 4370 |
| HC> VP | |||||||
| Brain Region | MNI Coordinates | T value | Z-value | p value | Cluster extent | ||
| x | y | z | |||||
| Right Hippocampus | 29 | −12 | −11 | 5.40 | 4.57 | 0.04 * | 253 |
| Right Para Hippocampal region | 23 | −29 | −17 | 5.24 | 4.47 | 0.004 ** | 1523 |
| Left Frontal -Med- Orb | −9 | 35 | −12 | 4.86 | 4.22 | 0.009 ** | 1299 |
| Right Insula | 41 | −11 | 14 | 4.84 | 4.21 | 0.001 ** | 1959 |
| A. Seed region: Right Caudate; Target Region: Right Insula | |
| Cognitive variable | Significance of the interaction term |
| COWAT | T = −2.26; p = 0.029 |
| RAVLT- DR | T = −2.33; p = 0.045 |
| MCST | T = −2.59; p = 0.029 |
| BDI | T = −3.09; p = 0.013 |
| HAMA | T = −2.64; p = 0.027 |
| B. Seed region: Right Caudate; Target Region: Left Thalamus | |
| BDI | T = −2.64; p = 0.027 |
| C. Seed region: Right Putamen; Target Region: Left Thalamus | |
| MCST | T = −2.28; p = 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novellino, F.; Salsone, M.; Riccelli, R.; Chiriaco, C.; Argirò, G.; Quattrone, A.; Madrigal, J.L.M.; Ferini Strambi, L.; Quattrone, A. Connectivity Alterations in Vascular Parkinsonism: A Structural Covariance Study. Appl. Sci. 2022, 12, 7240. https://doi.org/10.3390/app12147240
Novellino F, Salsone M, Riccelli R, Chiriaco C, Argirò G, Quattrone A, Madrigal JLM, Ferini Strambi L, Quattrone A. Connectivity Alterations in Vascular Parkinsonism: A Structural Covariance Study. Applied Sciences. 2022; 12(14):7240. https://doi.org/10.3390/app12147240
Chicago/Turabian StyleNovellino, Fabiana, Maria Salsone, Roberta Riccelli, Carmelina Chiriaco, Giuseppe Argirò, Andrea Quattrone, José L. M. Madrigal, Luigi Ferini Strambi, and Aldo Quattrone. 2022. "Connectivity Alterations in Vascular Parkinsonism: A Structural Covariance Study" Applied Sciences 12, no. 14: 7240. https://doi.org/10.3390/app12147240






