Diagnostic Strategies for Urologic Cancer Using Expression Analysis of Various Oncogenic Surveillance Molecules—From Non-Coding Small RNAs to Cancer-Specific Proteins
Abstract
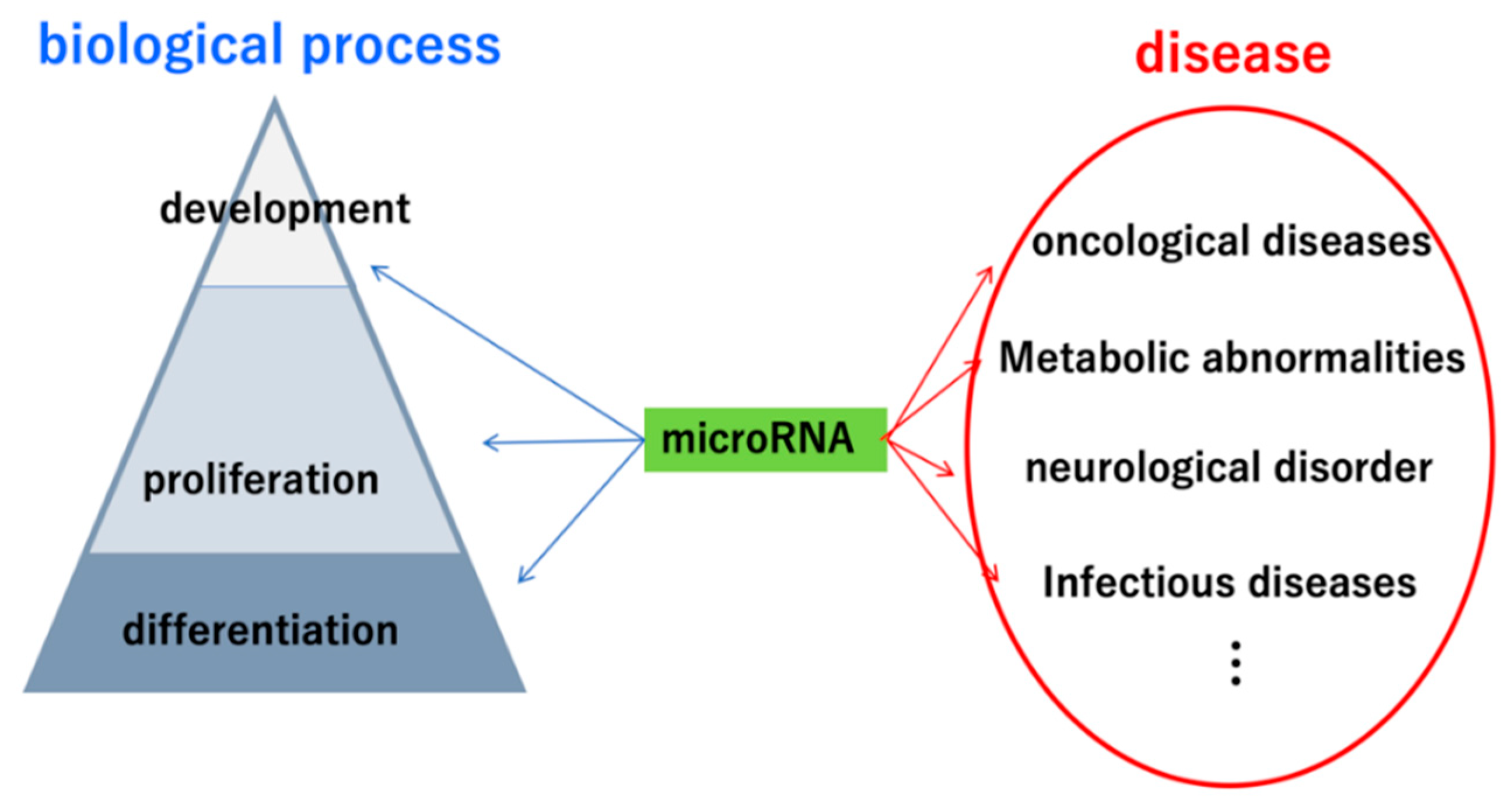
:1. Introduction
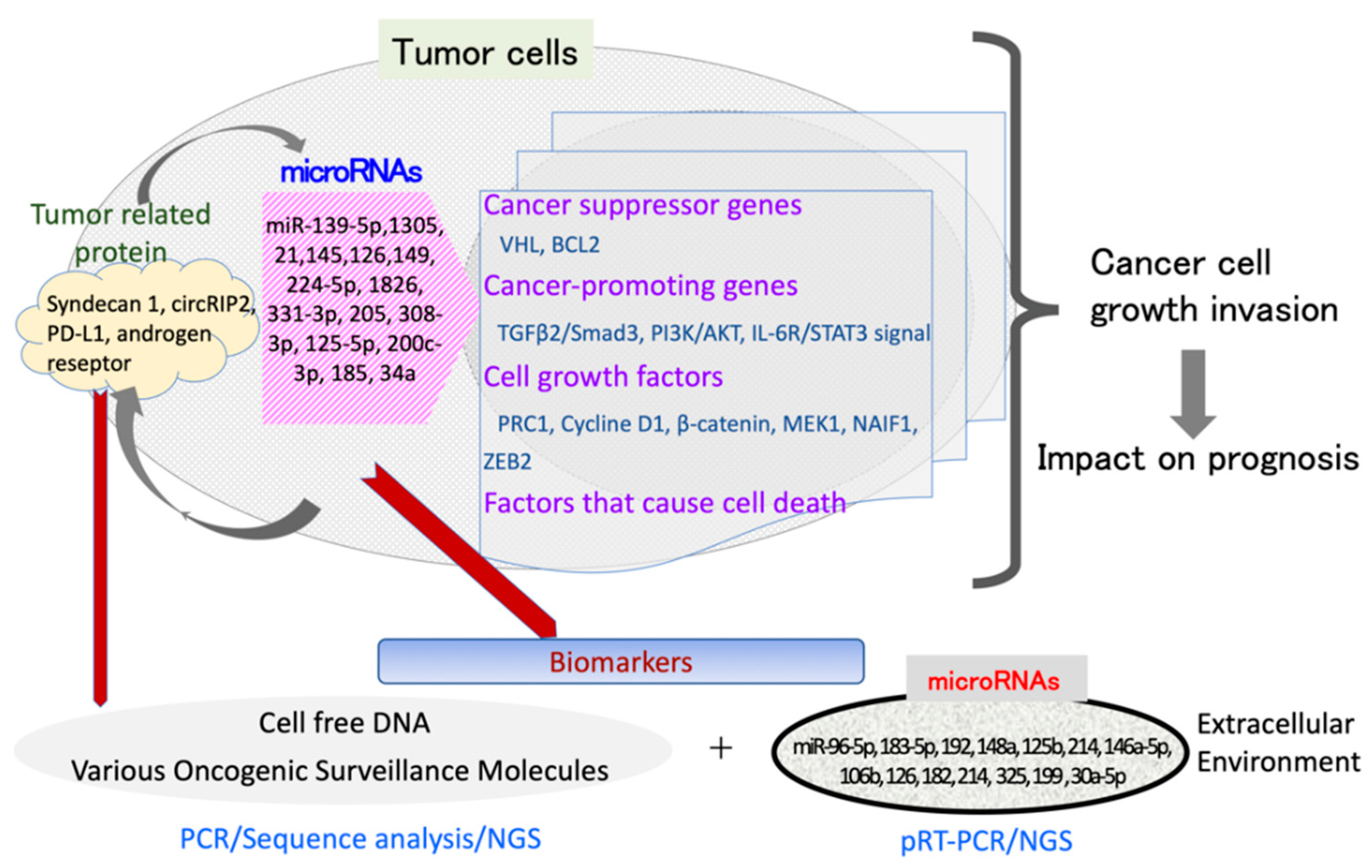
2. Importance of miRNAs in Urinary Tract Carcinoma
2.1. Renal Carcinoma
2.2. Bladder Carcinoma
2.3. Prostate Carcinoma
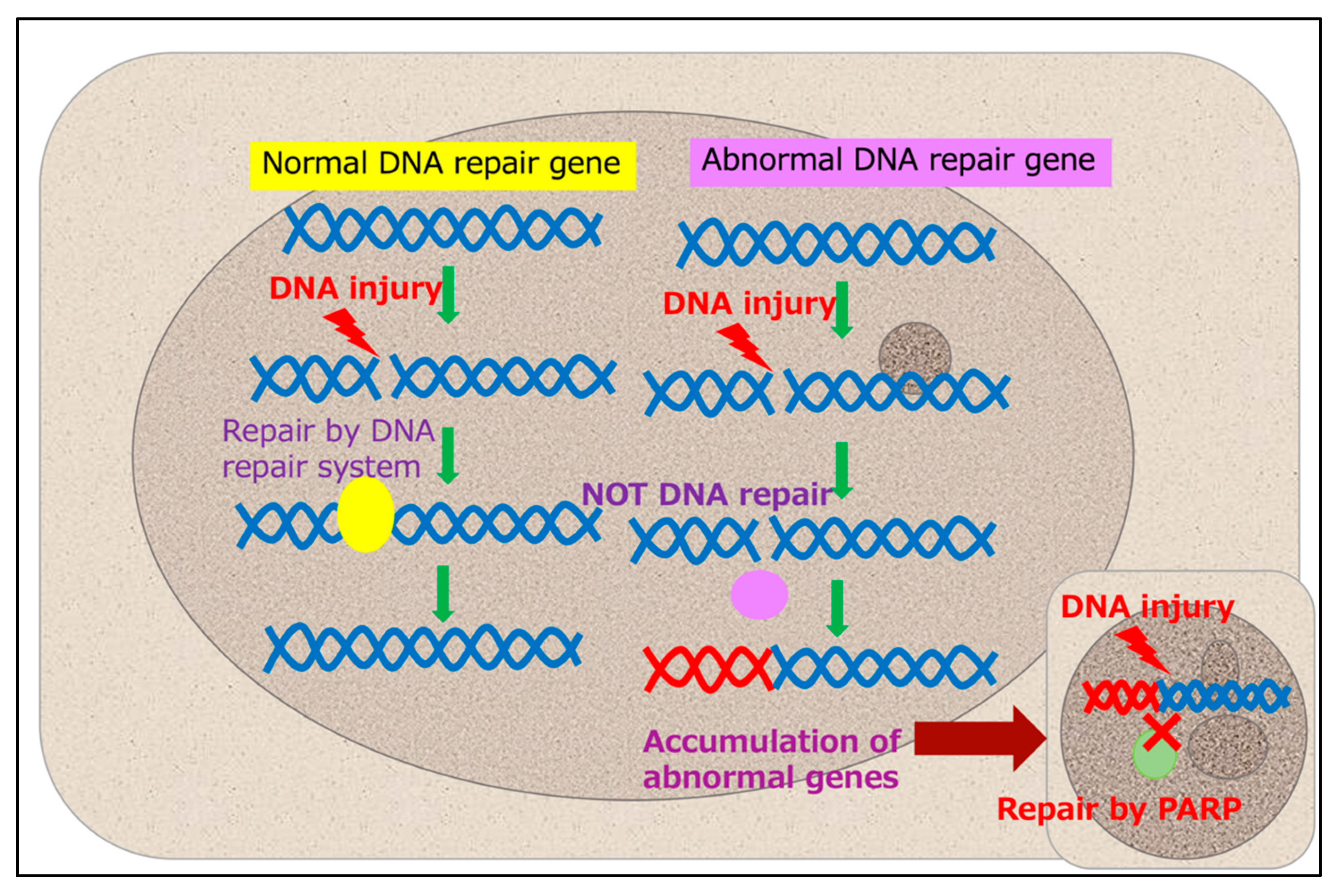
3. Problems and Prospects of Genetic Analysis via Liquid Biopsies
4. Development and Perspectives for Molecular Diagnostics Using Cell-Free DNA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR Mutations and Lung Cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. 2016, 27, iii4–iii15. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Cancer 2014, 14, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur. Urol. 2016, 71, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritterhouse, L.L.; Barletta, J.A. BRAF V600E mutation-specific antibody: A review. Semin. Diagn. Pathol. 2015, 32, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.S.; Gomes, I.; Casimiro, S.; Fernandes, I.; Costa, L. c-Met expression in renal cell carcinoma with bone metastases. J. Bone Oncol. 2020, 25, 100315. [Google Scholar] [CrossRef]
- Timar, J.; Kashofer, K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef]
- Vietri, M.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef]
- Fabris, L.; Ceder, Y.; Chinnaiyan, A.M.; Jenster, G.W.; Sørensen, K.D.; Tomlins, S.; Visakorpi, T.; Calin, G.A. The Potential of MicroRNAs as Prostate Cancer Biomarkers. Eur. Urol. 2016, 70, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Branicki, W.; Taheri, M. MicroRNA Signature in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 596359. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Inamoto, T.; Uehara, H.; Akao, Y.; Ibuki, N.; Komura, K.; Takahara, K.; Takai, T.; Uchimoto, T.; Saito, K.; Tanda, N.; et al. A Panel of MicroRNA Signature as a Tool for Predicting Survival of Patients with Urothelial Carcinoma of the Bladder. Dis. Mark. 2018, 2018, 5468672. [Google Scholar] [CrossRef] [Green Version]
- Pratap, P.; Raza, S.T.; Abbas, S.; Mahdi, F. MicroRNA-associated carcinogenesis in lung carcinoma. J. Cancer Res. Ther. 2018, 14, 249–254. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, P.; Fu, X.; Lin, W. Circular RNAs in renal cell carcinoma: Implications for tumorigenesis, diagnosis, and therapy. Mol. Cancer 2020, 19, 149. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Calin, G.A.; Croce, C.M. MicroRNA Signatures in Human Cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.-H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of Mammalian MicroRNA Targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Chkraborty, C.; Chin, K.-Y.; Das, S. miRNA-regulated cancer stem cells: Understanding the property and the role of miRNA in carcinogenesis. Tumor Biol. 2016, 37, 13039–13048. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Shirjang, S.; Baradaran, B. Micro-RNAs: The New Potential Biomarkers in Cancer Diagnosis, Prognosis and Cancer Therapy. Cell Mol. Biol. 2015, 61, 1–10. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26475381 (accessed on 19 June 2022).
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Fujimoto, K.; Konishi, N. Syndecan-1 responsive microRNA-126 and 149 regulate cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 2015, 456, 183–189. [Google Scholar] [CrossRef]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Hatakeyama, K.; Obayashi, C.; Fujimoto, K.; Konishi, N. microRNA-145 promotes differentiation in human urothelial carcinoma through down-regulation of syndecan-1. BMC Cancer 2015, 15, 818. [Google Scholar] [CrossRef] [Green Version]
- Petejova, N.; Martínek, A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. 2016, 160, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Li, L.; Kalantar-Zadeh, K. Obesity That Makes Kidney Cancer More Likely but Helps Fight It More Strongly. J. Natl. Cancer Inst. 2013, 105, 1848–1849. [Google Scholar] [CrossRef] [Green Version]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and Reproductive Toxicity—An Overview. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 493–502. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and screening for renal cancer. World J. Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Riese, W.; Goldenberg, K.; Allhoff, E.; Stief, C.; Schlick, R.; Liedke, S.; Jonas, U. Metastatic renal cell carcinoma (RCC): Spontaneous regression, long-term survival and late recurrence. Int. Urol. Nephrol. 1991, 23, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNichols, D.W.; Segura, J.W.; Deweerd, J.H. Renal Cell Carcinoma: Long-Term Survival and Late Recurrence. J. Urol. 1981, 126, 17–23. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Vedel, P.F.; Borgbjerg, J.; Andersen, G.; Borre, M. Renal cryoablation: Five- and 10-year survival outcomes in patients with biopsy-proven renal cell carcinoma. Scand. J. Urol. 2020, 54, 408–412. [Google Scholar] [CrossRef]
- Fedorko, M.; Pacik, D.; Wasserbauer, R.; Juracek, J.; Varga, G.; Ghazal, M.; Nussir, M.I. MicroRNAs in the Pathogenesis of Renal Cell Carcinoma and Their Diagnostic and Prognostic Utility as Cancer Biomarkers. Int. J. Biol. Markers 2016, 31, 26–37. [Google Scholar] [CrossRef]
- Go, H.; Kang, M.J.; Kim, P.-J.; Lee, J.-L.; Park, J.Y.; Park, J.-M.; Ro, J.Y.; Cho, Y.M. Development of Response Classifier for Vascular Endothelial Growth Factor Receptor (VEGFR)-Tyrosine Kinase Inhibitor (TKI) in Metastatic Renal Cell Carcinoma. Pathol. Oncol. Res. 2017, 25, 51–58. [Google Scholar] [CrossRef]
- Hirata, H.; Hinoda, Y.; Ueno, K.; Nakajima, K.; Ishii, N.; Dahiya, R. MicroRNA-1826 directly targets beta-catenin (CTNNB1) and MEK1 (MAP2K1) in VHL-inactivated renal cancer. Carcinogenesis 2011, 33, 501–508. [Google Scholar] [CrossRef]
- Lei, Z.; Klasson, T.D.; Brandt, M.M.; Van De Hoek, G.; Logister, I.; Cheng, C.; Doevendans, P.A.; Sluijter, J.P.G.; Giles, R.H. Control of Angiogenesis via a VHL/miR-212/132 Axis. Cells 2020, 9, 1017. [Google Scholar] [CrossRef]
- Ma, X.; Shen, D.; Li, H.; Zhang, Y.; Lv, X.; Huang, Q.; Gao, Y.; Li, X.; Gu, L.; Xiu, S.; et al. MicroRNA-185 inhibits cell proliferation and induces cell apoptosis by targeting VEGFA directly in von Hippel-Lindau–inactivated clear cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 169.e1–169.e11. [Google Scholar] [CrossRef]
- Mikhaylova, O.; Stratton, Y.; Hall, D.; Kellner, E.; Ehmer, B.; Drew, A.F.; Gallo, C.A.; Plas, D.R.; Biesiada, J.; Meller, J.; et al. VHL-Regulated MiR-204 Suppresses Tumor Growth through Inhibition of LC3B-Mediated Autophagy in Renal Clear Cell Carcinoma. Cancer Cell 2012, 21, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Chen, Z.; Chen, Y.; Wang, X.; Tang, N. MIR155HG is a prognostic biomarker and associated with immune infiltration and immune checkpoint molecules expression in multiple cancers. Cancer Med. 2019, 8, 7161–7173. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Hu, H.; Sun, W.; Chen, L.; Jin, S.; Xu, Q.; Liu, Y.; Yu, L.; Zeng, S. miR-224-5p Contained in Urinary Extracellular Vesicles Regulates PD-L1 Expression by Inhibiting Cyclin D1 in Renal Cell Carcinoma Cells. Cancers 2021, 13, 618. [Google Scholar] [CrossRef]
- Qu, F.; Ye, J.; Pan, X.; Wang, J.; Gan, S.; Chu, C.; Chu, J.; Zhang, X.; Liu, M.; He, H.; et al. MicroRNA-497-5p down-regulation increases PD-L1 expression in clear cell renal cell carcinoma. J. Drug Target. 2018, 27, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Ma, X.; Li, H.; Zhang, Y.; Wang, X.; Zhou, P.; Zhang, X. microRNA-107 functions as a candidate tumor suppressor gene in renal clear cell carcinoma involving multiple genes. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 205.e1–205.e11. [Google Scholar] [CrossRef]
- Unal, U.; Cecener, G.; Unlu, H.T.; Vuruskan, B.A.; Erdem, E.E.; Egeli, U.; Nazlioglu, H.O.; Kaygisiz, O.; Tunca, B.; Vuruskan, H. Investigation of VHL gene associated with miR-223 in clear cell renal cell carcinoma. Mol. Biol. Rep. 2021, 49, 2073–2083. [Google Scholar] [CrossRef]
- Valera, V.A.; Walter, B.A.; Linehan, W.M.; Merino, M.J. Regulatory Effects of microRNA-92 (miR-92) on VHL Gene Expression and the Hypoxic Activation of miR-210 in Clear Cell Renal Cell Carcinoma. J. Cancer 2011, 2, 515–526. [Google Scholar] [CrossRef]
- Wei, H.; Ke, H.-L.; Lin, J.; Shete, S.; Wood, C.G.; Hildebrandt, M.A. MicroRNA target site polymorphisms in the VHL-HIF1α pathway predict renal cell carcinoma risk. Mol. Carcinog. 2012, 53, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Niu, X.; Pan, H.; Zhang, Z.; Zhou, Y.; Qü, P.; Zhou, J. MicroRNA-497 targets hepatoma-derived growth factor and suppresses human prostate cancer cell motility. Mol. Med. Rep. 2016, 13, 2287–2292. [Google Scholar] [CrossRef]
- Wu, T.-K.; Wei, C.-W.; Pan, Y.-R.; Hsu, R.-J.; Wu, C.-Y.; Yu, Y.-L. The uremic toxin p-cresyl sulfate induces proliferation and migration of clear cell renal cell carcinoma via microRNA-21/ HIF-1α axis signals. Sci. Rep. 2019, 9, 3207. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Kaelin, W.G., Jr. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1519–1530. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Mytsyk, Y.; Dosenko, V.; Borys, Y.; Kucher, A.; Gazdikova, K.; Busselberg, D.; Caprnda, M.; Kruzliak, P.; Farooqi, A.A.; Lubov, M. MicroRNA-15a expression measured in urine samples as a potential biomarker of renal cell carcinoma. Int. Urol. Nephrol. 2018, 50, 851–859. [Google Scholar] [CrossRef]
- Oto, J.; Herranz, R.; Plana, E.; Sánchez-González, J.; Pérez-Ardavín, J.; Hervás, D.; Fernández-Pardo, Á.; Cana, F.; Vera-Donoso, C.; Martínez-Sarmiento, M.; et al. Identification of miR-20a-5p as Robust Normalizer for Urine microRNA Studies in Renal Cell Carcinoma and a Profile of Dysregulated microRNAs. Int. J. Mol. Sci. 2021, 22, 7913. [Google Scholar] [CrossRef]
- Outeiro-Pinho, G.; Barros-Silva, D.; Aznar, E.; Sousa, A.-I.; Vieira-Coimbra, M.; Oliveira, J.; Gonçalves, C.S.; Costa, B.M.; Junker, K.; Henrique, R.; et al. MicroRNA-30a-5pme: A novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J. Exp. Clin. Cancer Res. 2020, 39, 98. [Google Scholar] [CrossRef]
- Sun, I.O.; Lerman, L.O. Urinary microRNA in kidney disease: Utility and roles. Am. J. Physiol. Physiol. 2019, 316, F785–F793. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef]
- Fujii, T.; Shimada, K.; Nakai, T.; Ohbayashi, C. MicroRNAs in Smoking-Related Carcinogenesis: Biomarkers, Functions, and Therapy. J. Clin. Med. 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef]
- Adam, R.M.; DeGraff, D.J. Molecular mechanisms of squamous differentiation in urothelial cell carcinoma: A paradigm for molecular subtyping of urothelial cell carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 444–450. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, C.; Tang, Y.; Liu, X.; Liu, Z.; Li, G.; Mei, Y. Glandular differentiation in pT1 urothelial carcinoma of bladder predicts poor prognosis. Sci. Rep. 2019, 9, 5323. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Mitra, S.; Adhya, A.K.; Nayak, P. Urothelial carcinoma with villoglandular differentiation (UCVGD) with small cell neuroendocrine carcinoma of urinary bladder. BMJ Case Rep. 2019, 12, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Hualin, S.; Yu, J.; Song, H.; Zhu, S.; Sun, L.; Shang, Z.; Niu, Y. Squamous differentiation in patients with superficial bladder urothelial carcinoma is associated with high risk of recurrence and poor survival. BMC Cancer 2017, 17, 530. [Google Scholar] [CrossRef]
- Minato, A.; Noguchi, H.; Tomisaki, I.; Fukuda, A.; Kubo, T.; Nakayama, T.; Fujimoto, N. Clinical Significance of Squamous Differentiation in Urothelial Carcinoma of the Bladder. Cancer Control 2018, 25, 1073274818800269. [Google Scholar] [CrossRef]
- Davidson, P.J.; McGeoch, G.; Shand, B. Inclusion of a Molecular Marker of Bladder Cancer in a Clinical Pathway for Investigation of Haematuria May Reduce the Need for Cystoscopy. N. Z. Med. J. 2019, 132, 55–64. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31220066 (accessed on 19 June 2022).
- Karanović, S.; Ardin, M.; Tang, Z.; Tomić, K.; Villar, S.; Renard, C.; Venturini, E.; Lorch, A.H.; Lee, D.S.; Stipančić, Ž.; et al. Molecular profiles and urinary biomarkers of upper tract urothelial carcinomas associated with aristolochic acid exposure. Int. J. Cancer 2021, 150, 374–386. [Google Scholar] [CrossRef]
- Miremami, J.; Kyprianou, N. The Promise of Novel Molecular Markers in Bladder Cancer. Int. J. Mol. Sci. 2014, 15, 23897–23908. [Google Scholar] [CrossRef] [Green Version]
- Sapre, N.; Anderson, P.D.; Costello, A.J.; Hovens, C.M.; Corcoran, N.M. Gene-based urinary biomarkers for bladder cancer: An unfulfilled promise? Urol. Oncol. Semin. Orig. Investig. 2014, 32, 48.e9–48.e17. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Rizwani, W.; Ross, S.J.; Grossman, H.B.; Rosser, C.J. Urinary BTA: Indicator of bladder cancer or of hematuria. World J. Urol. 2012, 30, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Narayan, V.; Adejoro, O.; Schwartz, I.; Ziegelmann, M.; Elliott, S.; Konety, B.R. The Prevalence and Impact of Urinary Marker Testing in Patients with Bladder Cancer. J. Urol. 2018, 199, 74–80. [Google Scholar] [CrossRef]
- Pichler, R.; Tulchiner, G.; Fritz, J.; Schaefer, G.; Horninger, W.; Heidegger, I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med. Sci. 2017, 14, 811–819. [Google Scholar] [CrossRef] [Green Version]
- De Palma, G.; Di Lorenzo, V.F.; Krol, S.; Paradiso, A.V. Urinary exosomal shuttle RNA: Promising cancer diagnosis biomarkers of lower urinary tract. Int. J. Biol. Markers 2019, 34, 101–107. [Google Scholar] [CrossRef] [Green Version]
- El-Shal, A.S.; Shalaby, S.M.; Abouhashem, S.E.; Elbary, E.H.A.; Azazy, S.; Rashad, N.M.; Sarhan, W. Urinary exosomal microRNA-96-5p and microRNA-183-5p expression as potential biomarkers of bladder cancer. Mol. Biol. Rep. 2021, 48, 4361–4371. [Google Scholar] [CrossRef]
- Jiang, F.; Li, C.; Han, J.; Wang, L. Diagnostic Value of Combination of MicroRNA-192 in Urinary Sediment and B-Ultrasound for Bladder Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033819894573. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.-M.; Jeong, P.; Kim, Y.-H.; Byun, Y.J.; Xu, Y.; Kang, H.W.; Ha, Y.-S.; Kim, W.T.; Lee, J.-Y.; Woo, S.H.; et al. Urinary cell-free microRNA biomarker could discriminate bladder cancer from benign hematuria. Int. J. Cancer 2018, 144, 380–388. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Wang, L.; Dong, Z.; Du, L.; Yang, Y.; Guo, Y.; Wang, C. Downregulation of urinary cell-free microRNA-214 as a diagnostic and prognostic biomarker in bladder cancer. J. Surg. Oncol. 2015, 111, 992–999. [Google Scholar] [CrossRef]
- Zhang, D.-Z.; Lau, K.-M.; Chan, E.S.Y.; Wang, G.; Szeto, C.-C.; Wong, K.; Choy, R.K.W.; Ng, C.-F. Cell-Free Urinary MicroRNA-99a and MicroRNA-125b Are Diagnostic Markers for the Non-Invasive Screening of Bladder Cancer. PLoS ONE 2014, 9, e100793. [Google Scholar] [CrossRef]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2018, 110, 408–419. [Google Scholar] [CrossRef]
- Von Siebenthal, M.; Besic, M.; Gheinani, A.H.; Akshay, A.; Lizun-Platoni, S.; Kunz, N.; Burkhard, F.C.; Monastyrskaya, K. Urinary miRNA profiles discriminate between obstruction-induced bladder dysfunction and healthy controls. Sci. Rep. 2021, 11, 10204. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Yang, Y.; Li, Z.; Du, L.; Dong, Z.; Qu, A.; Jiang, X.; Li, P.; Wang, C. Urinary cell-free microRNA-106b as a novel biomarker for detection of bladder cancer. Med. Oncol. 2014, 31, 1–7. [Google Scholar] [CrossRef]
- Yoshino, H.; Seki, N.; Itesako, T.; Chiyomaru, T.; Nakagawa, M.; Enokida, H. Aberrant expression of microRNAs in bladder cancer. Nat. Rev. Urol. 2013, 10, 396–404. [Google Scholar] [CrossRef]
- Farrelly, C.; Lal, P.; Trerotola, S.O.; Nadolski, G.J.; Watts, M.M.; Gorrian, C.M.; Guzzo, T.J. Correlation of Peripheral Vein Tumour Marker Levels, Internal Iliac Vein Tumour Marker Levels and Radical Prostatectomy Specimens in Patients with Prostate Cancer and Borderline High Prostate-Specific Antigen: A Pilot Study. Cardiovasc. Interv. Radiol. 2016, 39, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Prorok, P.C.; Kramer, B.S. Prostate Cancer Screening—A Perspective on the Current State of the Evidence. N. Engl. J. Med. 2017, 376, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Anai, S.; Fujii, T.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J. Pathol. 2013, 231, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Li, B.; Li, L.; Li, Z.; Ji, H.; Yang, G.; Yin, F. MicroRNA-466 inhibits cancer cell migration and invasion in hepatocellular carcinoma by indirectly mediating the downregulation of ROCK2. Exp. Ther. Med. 2019, 18, 1493–1499. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Yu, B.; Liu, J. The Circular RNA circXPO1 Promotes Tumor Growth via Sponging MicroRNA-23a in Prostate Carcinoma. Front. Oncol. 2021, 11, 2925. [Google Scholar] [CrossRef]
- Fernandes, R.C.; Toubia, J.; Townley, S.; Hanson, A.R.; Dredge, B.K.; Pillman, K.A.; Bert, A.G.; Winter, J.M.; Iggo, R.; Das, R.; et al. Post-transcriptional Gene Regulation by MicroRNA-194 Promotes Neuroendocrine Transdifferentiation in Prostate Cancer. Cell Rep. 2021, 34, 108585. [Google Scholar] [CrossRef]
- Fu, Y.; Cao, F. MicroRNA-125a-5p regulates cancer cell proliferation and migration through NAIF1 in prostate carcinoma. OncoTargets Ther. 2015, 8, 3827–3835. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol. Carcinog. 2015, 55, 1378–1386. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Mao, J.; Hu, P.; Chen, Q.; Ding, W.; Pu, R. LncRNA TUC338 is overexpressed in prostate carcinoma and downregulates miR-466. Gene 2019, 707, 224–230. [Google Scholar] [CrossRef]
- Li, R.; Chen, Y.; Wu, J.; Cui, X.; Zheng, S.; Yan, H.; Wu, Y.; Wang, F. LncRNA FGF14-AS2 represses growth of prostate carcinoma cells via modulating miR-96-5p/AJAP1 axis. J. Clin. Lab. Anal. 2021, 35, e24012. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Chunyan, L.; Wang, L.; Li, C.; Xue, J.; Zhang, P.; Chen, W.; Jiang, A. MicroRNA-185 downregulates androgen receptor expression in the LNCaP prostate carcinoma cell line. Mol. Med. Rep. 2015, 11, 4625–4632. [Google Scholar] [CrossRef]
- Liu, H.; Hou, T.; Ju, W.; Xing, Y.; Zhang, X.; Yang, J. MicroRNA-122 downregulates Rho-associated protein kinase 2 expression and inhibits the proliferation of prostate carcinoma cells. Mol. Med. Rep. 2019, 19, 3882–3888. [Google Scholar] [CrossRef]
- Ma, J.; Wei, H.; Li, X.; Qu, X. Hsa-miR-149-5p Suppresses Prostate Carcinoma Malignancy by Suppressing RGS17. Cancer Manag. Res. 2021, 13, 2773–2783. [Google Scholar] [CrossRef]
- Rokavec, M.; Öner, M.G.; Li, H.; Jackstadt, R.; Jiang, L.; Lodygin, D.; Kaller, M.; Horst, D.; Ziegler, P.K.; Schwitalla, S.; et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1853–1867, Erratum in 2015, 125, 1362. [Google Scholar] [CrossRef] [Green Version]
- Stephan, C.; Jung, M.; Rabenhorst, S.; Kilic, E.; Jung, K. Urinary miR-183 and miR-205 do not surpass PCA3 in urine as predictive markers for prostate biopsy outcome despite their highly dysregulated expression in prostate cancer tissue. Clin. Chem. Lab. Med. 2015, 53, 1109–1118. [Google Scholar] [CrossRef]
- Stuopelyte, K.; Daniunaite, K.; Bakavicius, A.; Lazutka, J.R.; Jankevicius, F.; Jarmalaite, S. The utility of urine-circulating miRNAs for detection of prostate cancer. Br. J. Cancer 2016, 115, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-N.; Hu, S.; Shang, Y.-P.; Li, L.-Y.; Zhou, H.; Chen, J.-S.; Yang, J.-F.; Li, J.; Huang, Q.; Shen, C.-P.; et al. Relevance function of microRNA-708 in the pathogenesis of cancer. Cell. Signal. 2019, 63, 109390. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhu, Z.; Li, W.; Yu, G.; Jia, Z.; Wang, X. Prostate carcinoma cell-derived exosomal MicroRNA-26a modulates the metastasis and tumor growth of prostate carcinoma. Biomed. Pharmacother. 2019, 117, 109109. [Google Scholar] [CrossRef]
- Xie, X.; Dai, J.; Huang, X.; Fang, C.; He, W. MicroRNA-145 inhibits proliferation and induces apoptosis in human prostate carcinoma by upregulating long non-coding RNA GAS5. Oncol. Lett. 2019, 18, 1043–1048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, H.; Qin, Y.; Chen, C.; Yang, J.; Song, N.; Gu, M. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumor progression of prostate carcinoma. Ann. Transl. Med. 2019, 7, 141. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Fu, H.; Zhang, J. microRNA-205 and microRNA-338-3p Reduces Cell Apoptosis in Prostate Carcinoma Tissue and LNCaP Prostate Carcinoma Cells by Directly Targeting the B-Cell Lymphoma 2 (Bcl-2) Gene. Med. Sci. Monit. 2019, 25, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Xu, A.; Tian, Y.; Liang, C.; Wang, Z. MicroRNA-188 inhibits proliferation migration and invasion of prostate carcinoma by targeting at MARCKS. Am. J. Transl. Res. 2019, 11, 5019–5028. [Google Scholar] [PubMed]
- Fradet, Y.; Saad, F.; Aprikian, A.; Dessureault, J.; Elhilali, M.; Trudel, C.; Masse, B.; Piché, L.; Chypre, C. uPM3, a new molecular urine test for the detection of prostate cancer. Urology 2004, 64, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, X.; Guan, K.; Wang, D.; Bi, X.; Xiao, Z.; Xiao, Z.; Shan, X.; Hu, L.; Ma, J.; et al. Copy number variation of urine exfoliated cells by low-coverage whole genome sequencing for diagnosis of prostate adenocarcinoma: A prospective cohort study. BMC Med. Genom. 2022, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Cernei, N.; Gumulec, J.; Masarik, M.; Eckschlager, T.; Hrabec, R.; Zitka, O.; Adam, V.; Kizek, R. Determination of common urine substances as an assay for improving prostate carcinoma diagnostics. Oncol. Rep. 2014, 31, 1846–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Ku, J.Y.; Kang, B.J.; Kim, K.H.; Ha, H.K.; Kim, S. A Unique Urinary Metabolic Feature for the Determination of Bladder Cancer, Prostate Cancer, and Renal Cell Carcinoma. Metabolites 2021, 11, 591. [Google Scholar] [CrossRef]
- Neumann, E.; Hennenlotter, J.; Todenhöfer, T.; Scharpf, M.; Neumann, T.; Schilling, D.; Stenzl, A.; Bedke, J. The Value and Evaluability of the PCA3 Urine Assay in Prostate Carcinoma is Independent of the Tumor Localization. Adv. Ther. 2017, 34, 966–974. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Stopsack, K.H.; Whittaker, C.A.; Gerke, T.A.; Loda, M.; Kantoff, P.W.; Mucci, L.A.; Amon, A. Aneuploidy drives lethal progression in prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11390–11395. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Grasso, C.S.; Jordahl, K.M.; Kolb, S.; Nyame, Y.A.; Wright, J.L.; Ostrander, E.A.; Troyer, D.A.; Lance, R.; Feng, Z.; et al. Copy number alterations are associated with metastatic-lethal progression in prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 494–506. [Google Scholar] [CrossRef]
- Huang, X.; Liang, M.; Dittmar, R.; Wang, L. Extracellular MicroRNAs in Urologic Malignancies: Chances and Challenges. Int. J. Mol. Sci. 2013, 14, 14785–14799. [Google Scholar] [CrossRef] [Green Version]
- Koppers-Lalic, D.; Hackenberg, M.; de Menezes, R.; Misovic, B.; Wachalska, M.; Geldof, A.; Zini, N.; de Reijke, T.; Wurdinger, T.; Vis, A.; et al. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget 2016, 7, 22566–22578. [Google Scholar] [CrossRef] [Green Version]
- Mlcochova, H.; Hezova, R.; Stanik, M.; Slaby, O. Urine microRNAs as potential noninvasive biomarkers in urologic cancers. Urol. Oncol. Semin. Orig. Investig. 2013, 32, 41.e1–41.e9. [Google Scholar] [CrossRef]
- Puhka, M.; Thierens, L.; Nicorici, D.; Forsman, T.; Mirtti, T.; Hällström, T.A.; Serkkola, E.; Rannikko, A. Exploration of Extracellular Vesicle miRNAs, Targeted mRNAs and Pathways in Prostate Cancer: Relation to Disease Status and Progression. Cancers 2022, 14, 532. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Gupta, N.; Raja, K.D.; Singh, P.; Agarwal, S.; Sharma, A. Non-invasive diagnostic potential of microRNA-203 in liquid biopsy of urothelial carcinoma of bladder. Mol. Cell. Biochem. 2022, 1–10. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, S.; Fang, C.; Hu, X.; Zhang, Y.-S.; Zhang, L.-L.; Zeng, X.-T. Nanomaterials-Based Urinary Extracellular Vesicles Isolation and Detection for Non-invasive Auxiliary Diagnosis of Prostate Cancer. Front. Med. 2022, 8, 800889. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, S.; An, T.; Tang, Y.; Huang, Y.; Zheng, L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate 2017, 77, 1167–1175. [Google Scholar] [CrossRef]
- Zeuschner, P.; Linxweiler, J.; Junker, K. Non-coding RNAs as biomarkers in liquid biopsies with a special emphasis on extracellular vesicles in urological malignancies. Expert Rev. Mol. Diagn. 2019, 20, 151–167. [Google Scholar] [CrossRef]
- Fujii, T.; Uchiyama, T.; Matsuoka, M.; Myojin, T.; Sugimoto, S.; Nitta, Y.; Okabe, F.; Sugimoto, A.; Sekita-Hatakeyama, Y.; Morita, K.; et al. Evaluation of DNA and RNA quality from archival formalin-fixed paraffin-embedded tissue for next-generation sequencing—Retrospective study in Japanese single institution. Pathol. Int. 2020, 70, 602–611. [Google Scholar] [CrossRef]
- Nishikawa, T.; Fujii, T.; Tatsumi, S.; Sugimoto, A.; Sekita-Hatakeyama, Y.; Shimada, K.; Yamazaki, M.; Hatakeyama, K.; Ohbayashi, C. Molecular Analysis of Liquid-Based Cytological Specimen Using Virtually Positive Sputum with Adenocarcinoma Cells. Diagnostics 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Asano, A.; Shimada, K.; Tatsumi, Y.; Obayashi, C.; Konishi, N. Evaluation of RNA and DNA extraction from liquid-based cytology specimens. Diagn. Cytopathol. 2016, 44, 833–840. [Google Scholar] [CrossRef]
- Sekita-Hatakeyama, Y.; Nishikawa, T.; Takeuchi, M.; Morita, K.; Takeda, M.; Hatakeyama, K.; Nakai, T.; Uchiyama, T.; Itami, H.; Fujii, T.; et al. K-ras mutation analysis of residual liquid-based cytology specimens from endoscopic ultrasound-guided fine needle aspiration improves cell block diagnosis of pancreatic ductal adenocarcinoma. PLoS ONE 2018, 13, e0193692. [Google Scholar] [CrossRef]
- Laukhtina, E.; Pradere, B.; Mori, K.; Schuettfort, V.M.; Quhal, F.; Mostafaei, H.; Motlangh, R.S.; Katayama, S.; Grossmann, N.C.; Moschini, M.; et al. Catalog of prognostic tissue-based biomarkers in patients treated with neoadjuvant systemic therapy for urothelial carcinoma of the bladder: A systematic review. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 180–190. [Google Scholar] [CrossRef]
- Miron, B.; Hoffman-Censits, J.H.; Anari, F.; O’Neill, J.; Geynisman, D.M.; Zibelman, M.R.; Kutikov, A.; Viterbo, R.; Greenberg, R.E.; Chen, D.; et al. Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2020, 3, 544–547. [Google Scholar] [CrossRef]
- Mouliere, F.; Robert, B.; Peyrotte, E.A.; Del Rio, M.; Ychou, M.; Molina, F.; Gongora, C.; Thierry, A.R. High Fragmentation Characterizes Tumour-Derived Circulating DNA. PLoS ONE 2011, 6, e23418. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]

| miRNA | Target Molecules/Function | Target Cancer | Reference |
|---|---|---|---|
| miR-145 | Syndecan-1/suppressed cell proliferation | Bladder carcinoma | [25] |
| miR-1826 | β-cathenin, MEK1 | renal cell carcinoma | [37] |
| miR-185 | VEGFR/ Inhibits cell proliferation and induces cell apoptosis | renal cell carcinoma | [39] |
| miR-204 | Suppress tumor growth | renal cell carcinoma | [40] |
| miR-224-5p | Cyclin D1/Regulates PD-L1 expression | renal cell carcinoma | [42] |
| miR-497-5p | Regulates PD-L1 expression | renal cell carcinoma | [43] |
| miR-107 | Tumor suppressor | renal cell carcinoma | [44] |
| miR-92 | Regulates VHL gene expression | renal cell carcinoma | [46] |
| miR-15a | renal cell carcinoma | [52] | |
| miR-20a-5p | renal cell carcinoma | [53] | |
| miR-30a-5p | renal cell carcinoma | [54] | |
| miR-96-5p | Bladder carcinoma | [73] | |
| miR-192 | Bladder carcinoma | [74] | |
| miR-214 | Bladder carcinoma | [76] | |
| miR-99a, 125b | Bladder carcinoma | [77] | |
| miR-106b | Bladder carcinoma | [80] | |
| miR-23a | suppressed cell proliferation | Prostate carcinoma | [86] |
| miR-194 | Post-transcriptional regulation | Prostate carcinoma | [87] |
| miR-125-5p | NAIF1/regulates cell proliferation and migration | Prostate carcinoma | [88] |
| miR-185 | Androgen receptor | Prostate carcinoma | [92] |
| miR-122 | Rho associated protein kinase 2/inhibits cell proliferation | Prostate carcinoma | [93] |
| miR-149-5p | RGS17/suppress cancer malignancy | Prostate carcinoma | [94] |
| miR-183, 205 | Prostate carcinoma | [96] | |
| miR-26a | Modulates the metastasis and tumor growth | Prostate carcinoma | [99] |
| miR-145 | suppressed cell proliferation | Prostate carcinoma | [100] |
| miR-205, 338-3p | BCL2/Regulates cell apoptosis | Prostate carcinoma | [102] |
| miR-188 | MARCKS / suppressed cell proliferation | Prostate carcinoma | [103] |
| miR-203 | Bladder carcinoma | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, T.; Uchiyama, T.; Takeda, M.; Shimada, K. Diagnostic Strategies for Urologic Cancer Using Expression Analysis of Various Oncogenic Surveillance Molecules—From Non-Coding Small RNAs to Cancer-Specific Proteins. Appl. Sci. 2022, 12, 7390. https://doi.org/10.3390/app12157390
Fujii T, Uchiyama T, Takeda M, Shimada K. Diagnostic Strategies for Urologic Cancer Using Expression Analysis of Various Oncogenic Surveillance Molecules—From Non-Coding Small RNAs to Cancer-Specific Proteins. Applied Sciences. 2022; 12(15):7390. https://doi.org/10.3390/app12157390
Chicago/Turabian StyleFujii, Tomomi, Tomoko Uchiyama, Maiko Takeda, and Keiji Shimada. 2022. "Diagnostic Strategies for Urologic Cancer Using Expression Analysis of Various Oncogenic Surveillance Molecules—From Non-Coding Small RNAs to Cancer-Specific Proteins" Applied Sciences 12, no. 15: 7390. https://doi.org/10.3390/app12157390






