Liver Disease Assessment in Children with Fontan and Glenn Surgeries for Univentricular Hearts—The Role of Elastography and Biochemical Fibrosis Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample Selection
2.2. Ethics
2.3. Elastography
2.4. Laboratory
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Groups
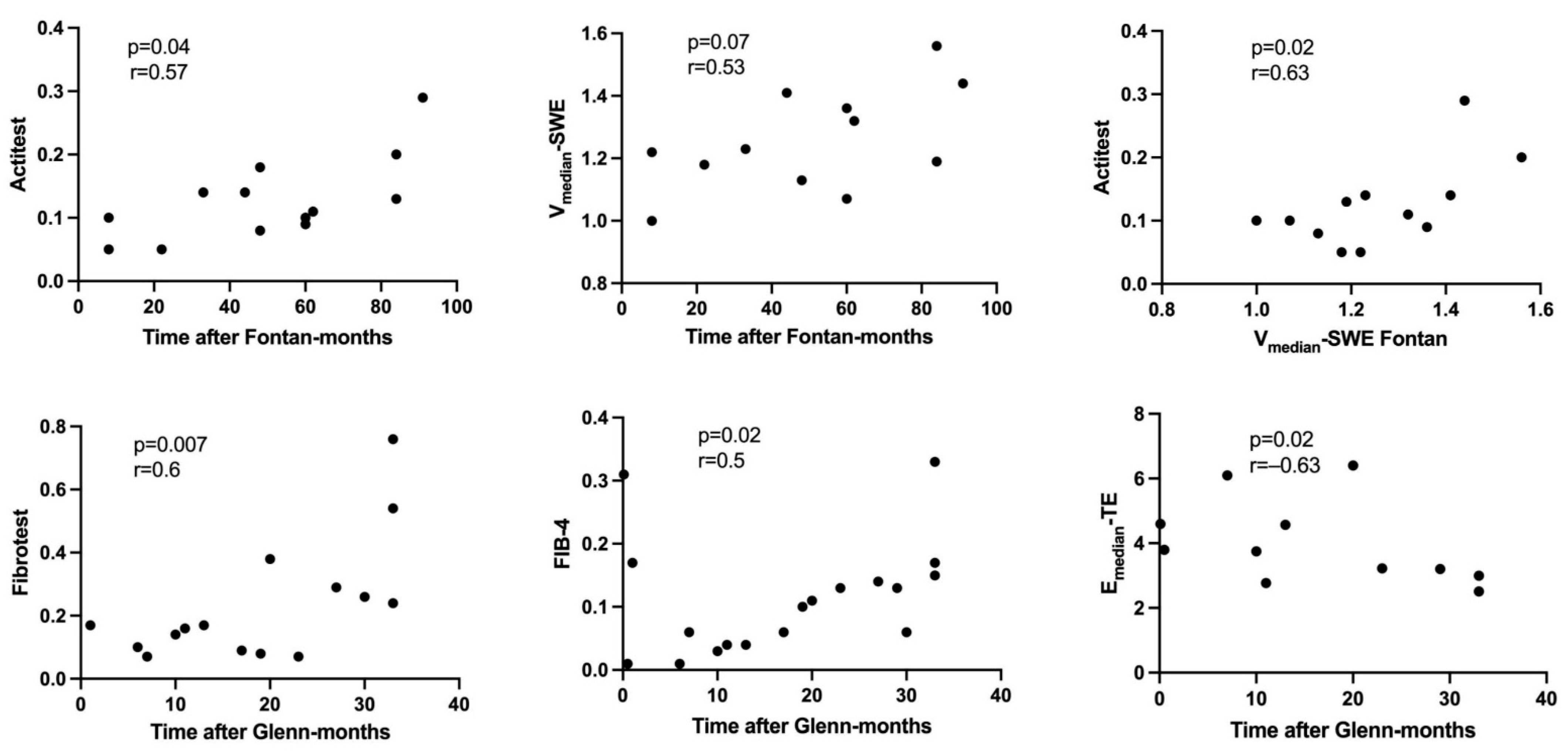
3.2. Fontan Group
3.3. Glenn Group
3.4. Fontan Group vs. Glenn Group
3.5. Operated Patients vs. Controls
4. Discussion
4.1. Liver Elastography
4.2. Biochemical Markers
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Emedian | median stiffness |
| Vmedian | median velocity |
| TE | transient elastography |
| ARFI | acoustic radiation force impulse |
| SWE | shear wave elastography |
| APRI | AST-to platelet ratio index |
| PLT | platelet |
| GGT | Gamma glutamyl transpeptidase |
| n | number |
| ApoA1 | apolipoprotein A1 |
| A2MG | alpha 2 macroglobulin |
| TB | total bilirubin |
References
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soufi, A.; Gouton, M.; Metton, O.; Mitchell, J.; Bernard, Y.F.; Bozio, A.; Ninet, J.; Di Filippo, S. Quality of life of adult Fontan patients. Cardiol. Young 2021, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Fernandes, S.M.; Mayer, J.E., Jr.; Triedman, J.K.; Walsh, E.P.; Lock, J.E.; Landzberg, M.J. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008, 117, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamaullee, J.; Khan, S.; Weaver, C.; Goldbeck, C.; Yanni, G.; Kohli, R.; Genyk, Y.; Zhou, S.; Shillingford, N.; Sullivan, P.M.; et al. Non-invasive biomarkers of Fontan-associated liver disease. JHEP Rep. 2021, 3, 100362. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Surrey, L.F.; Glatz, A.C.; Dodds, K.; O’Byrne, M.L.; Lin, H.C.; Fogel, M.; Rome, J.J.; Rand, E.B.; Russo, P.; et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J. Am. Heart Assoc 2017, 6, e004809. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez De Santiago, E.; Téllez, L.; Guerrero, A.; Albillos, A. Hepatocellular carcinoma after Fontan surgery: A systematic review. Hepatol. Res. 2021, 51, 116–134. [Google Scholar] [CrossRef]
- Ohuchi, H. Adult patients with Fontan circulation: What we know and how to manage adults with Fontan circulation? J. Cardiol. 2016, 68, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Mares, R.C.; Marginean, C.O. Noninvasive diagnosis of liver fibrosis in the complex cardiac malformation survivors—A review of the literature. Med. Ultrason 2021, 23, 453–462. [Google Scholar] [CrossRef]
- Gordon-Walker, T.T.; Bove, K.; Veldtman, G. Fontan-associated liver disease: A review. J. Cardiol. 2019, 74, 223–232. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [Green Version]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Săsăran, M.O. Reference values of normal liver stiffness in healthy children by two methods: 2D shear wave and transient elastography. Sci. Rep. 2020, 10, 7213. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Săsăran, M.O. The assessment of liver fibrosis in children with obesity on two methods: Transient and two dimensional shear wave elastography. Sci. Rep. 2019, 9, 19800. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Babu, A.; Wells, M.L.; Teytelboym, O.M.; Mackey, J.E.; Miller, F.H.; Yeh, B.M.; Ehman, R.L.; Venkatesh, S.K. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics 2016, 36, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Shear wave liver elastography. Abdom. Radiol. 2018, 43, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.T.; Pan, J.; Qian, J.D.; Cheng, H.; Wang, Y.; Wang, G.Q. Shear wave elastography may be sensitive and more precise than transient elastography in predicting significant fibrosis. World J. Clin. Cases 2020, 8, 3730–3742. [Google Scholar] [CrossRef]
- Fang, C.; Sidhu, P.S. Ultrasound-based liver elastography: Current results and future perspectives. Abdom. Radiol. 2020, 45, 3463–3472. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Koch, C.; Rentzsch, A.; Sarrazin, C.; Schwarz, P.; Herrmann, E.; Lindinger, A.; Sarrazin, U.; Poynard, T.; Schäfers, H.J.; et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J. Thorac. Cardiovasc. Surg. 2008, 135, 60–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poynard, T.; Munteanu, M.; Deckmyn, O.; Ngo, Y.; Drane, F.; Castille, J.M.; Housset, C.; Ratziu, V.; Imbert-Bismut, F. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J. Hepatol. 2012, 57, 541. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, M.; Rusu, M.; Neculoiu, D.; Radu, C.; Serban, A.; Catanas, M.; Grigorescu, M.D. The FibroTest value in discriminating between insiginificant and significant fibrosis in chronic hepatitis C patients. The Romanian experience. J. Gastrointestin Liver Dis. 2007, 16, 31–37. [Google Scholar]
- Halfon, P.; Munteanu, M.; Poynard, T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol. Clin. Biol. 2008, 32, 22–39. [Google Scholar] [CrossRef]
- Poynard, T.; Ngo, Y.; Munteanu, M.; Thabut, D.; Ratziu, V. Noninvasive markers of hepatic fibrosis in chronic hepatitis B. Curr. Hepat. Rep. 2011, 10, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Lassailly, G.; Caiazzo, R.; Hollebecque, A.; Buob, D.; Leteurtre, E.; Arnalsteen, L.; Louvet, A.; Pigeyre, M.; Raverdy, V.; Verkindt, H.; et al. Validation of non-invasive biomarkers (FibroTest, SteatoTest and NashTest) for prediction of liver injury in patients with morbid obesity. Eur. J. Gastroenterol. Hepatol. 2011, 23, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; SSulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Safak, A.A.; Simsek, E.; Bahcebasi, T. Sonographic assessment of the normal limits and percentile curves of liver, spleen, and kidney dimensions in healthy school-aged children. J. Ultrasound Med. 2005, 24, 1359–1364. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Schwartz, M.C.; Glatz, A.C.; Daniels, K.; Goldberg, D.J.; Rand, E.; Epelman, M.S.; Cohen, M.S. Hepatic Abnormalities Are Present Before and Early After the Fontan Operation. Ann. Thorac. Surg. 2015, 100, 2298–2304. [Google Scholar] [CrossRef]
- Evans, W.N.; Acherman, R.J.; Ciccolo, M.L.; Carrillo, S.A.; Galindo, A.; Rothman, A.; Mayman, G.A.; Adams, E.A.; Reardon, L.C.; Winn, B.J.; et al. A composite noninvasive index correlates with liver fibrosis scores in post-Fontan patients: Preliminary findings. Congenit Heart Dis. 2018, 13, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Kutty, S.S.; Peng, Q.; Danford, D.A.; Fletcher, S.E.; Perry, D.; Talmon, G.A.; Scott, C.; Kugler, J.D.; Duncan, K.F.; Quiros-Tejeira, R.E.; et al. Increased hepatic stiffness as consequence of high hepatic afterload in the Fontan circulation: A vascular Doppler and elastography study. Hepatology 2014, 59, 251–260. [Google Scholar] [CrossRef]
- Kutty, S.S.; Zhang, M.; Danford, D.A.; Hasan, R.; Duncan, K.F.; Kugler, J.D.; Quiros-Tejeira, R.E.; Kutty, S. Hepatic stiffness in the bidirectional cavopulmonary circulation: The Liver Adult-Pediatric-Congenital-Heart-Disease Dysfunction Study group. J. Thorac. Cardiovasc. Surg. 2016, 151, 678–684. [Google Scholar] [CrossRef] [Green Version]
- DiPaola, F.W.; Schumacher, K.R.; Goldberg, C.S.; Friedland- Little, J.; Parameswaran, A.; Dillman, J.R. Effect of Fontan operation on liver stiffness in children with single ventricle physiology. Eur. Radiol. 2017, 27, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Deorsola, L.; Aidala, E.; Cascarano, M.T.; Valori, A.; Agnoletti, G.; Pace Napoleone, C. Liver stiffness modifications shortly after total cavopulmonary connection. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, B.W.; Choi, J.Y.; Eun, L.Y.; Park, H.K.; Park, Y.H.; Kim, S.U. Congestive hepatopathy after Fontan operation and related factors assessed by transient elastography. J. Thorac. Cardiovasc. Surg. 2014, 148, 1498–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samir, A.E.; Dhyani, M.; Vij, A.; Bhan, A.K.; Halpern, E.F.; Méndez-Navarro, J.; Corey, K.E.; Chung, R.T. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: Determining accuracy and ideal site for measurement. Radiology 2015, 274, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Fraquelli, M.; Rigamonti, C.; Casazza, G.; Conte, D.; Donato, M.F.; Ronchi, G.; Colombo, M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007, 56, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.M.; Opotowsky, A.R.; Raza, R.; Harney, S.; Ukomadu, C.; Landzberg, M.J.; Valente, A.M.; Breitbart, R.E.; Singh, M.N.; Gauvreau, K.; et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit. Heart Dis. 2014, 9, 438–447. [Google Scholar] [CrossRef]
- Talwar, S.; Gudala, V.; Joshi, R.; Shalimar, D.M.; Madhusudhan, K.S.; Kalaivani, M.; Choudhary, S.K. Noninvasive Assessment of Liver Stiffness in Patients Undergoing the Fontan Procedure. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 572–577. [Google Scholar] [CrossRef]
- Ferraioli, G.; De Silvestri, A.; Lissandrin, R.; Maiocchi, L.; Tinelli, C.; Filice, C.; Barr, R.G. Evaluation of inter system variability in liver stiffness measurements. Ultraschall Med. 2019, 40, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.S.; Bae, E.J.; Ko, J.S.; Kim, G.B.; Kwon, B.S.; Lee, S.Y.; Noh, C.I.; Park, E.A.; Lee, W. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart 2010, 96, 1750–1755. [Google Scholar] [CrossRef]
- Kiesewetter, C.H.; Sheron, N.; Vettukattill, J.J.; Hacking, N.; Stedman, B.; Millward-Sadler, H.; Haw, M.; Cope, R.; Salmon, A.P.; Sivaprakasam, M.C.; et al. Hepatic changes in the failing Fontan circulation. Heart 2007, 93, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Schleiger, A.; Salzmann, M.; Kramer, P.; Danne, F.; Schubert, S.; Bassir, C.; Müller, T.; Müller, H.P.; Berger, F.; Ovroutski, S. Severity of Fontan-Associated Liver Disease Correlates with Fontan Hemodynamics. Pediatr. Cardiol. 2020, 41, 736–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, I.; Johnson, J.; Everitt, M.D.; Hoffman, J.; Yetman, A.T. Impact of liver disease after the Fontan operation. Am. J. Cardiol. 2015, 115, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Salama, H.; Ahmed, R.; Hamdy, S.; Al-Akel, W.; Shafi, S.A.; Mahgoub, A.; Hareedy, A.; Fathy, W. Non-invasive fibrosis seromarkers as a predictor of liver fibrosis in chronic hepatitis C and/or non-alcoholic steatohepatitis. Arab J. Gastroenterol. 2009, 10, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fidai, A.; Dallaire, F.; Alvarez, N.; Balon, Y.; Clegg, R.; Connelly, M.; Dicke, F.; Fruitman, D.; Harder, J.; Myers, K.; et al. Non-invasive Investigations for the Diagnosis of Fontan-Associated Liver Disease in Pediatric and Adult Fontan Patients. Front. Cardiovasc. Med. 2017, 4, 15. [Google Scholar] [CrossRef]
- Raizner, A.; Shillingford, N.; Mitchell, P.D.; Harney, S.; Raza, R.; Serino, J.; Jonas, M.M.; Lee, C.K. Hepatic Inflammation May Influence Liver Stiffness Measurements by Transient Elastography in Children and Young Adults. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.S.; Cheng, C.C.; Lee, S.C.; Liu, M.L.; Lee, J.Y.; Wang, W.M.; Wang, C.C. Novel biomarkers predict liver fibrosis in hepatitis C patients: Alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J. Biomed. Sci 2010, 17, 58. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, G.; Castera, L.; Halfon, P.; Pol, S.; Mangia, A.; Di Marco, V.; Pirisi, M.; Voiculescu, M.; Bourliere, M.; Alberti, A. The impact of liver disease etiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: An international study of 2411 cases. Aliment. Pharmacol. Ther. 2011, 34, 1202–1216. [Google Scholar] [CrossRef]
- Egbe, A.; Miranda, W.R.; Connolly, H.M.; Khan, A.R.; Al-Otaibi, M.; Venkatesh, S.K.; Simonetto, D.; Kamath, P.; Warnes, C. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. Int. J. Cardiol. 2018, 258, 299–304. [Google Scholar] [CrossRef]



| Patient Characteristics | Fontan N/Mean ± SD | Glenn N/Mean ± SD | Control N/Mean ± SD |
|---|---|---|---|
| Demographics | n = 13 | n = 21 | n = 32 |
| Male/Female | 9/4 | 11/10 | 17/15 |
| Age (years) | 10 ± 3.83 | 5.24 ± 4.7 | 8.39 ± 5.48 |
| Time interval since operation (months) | 50 ± 27 | 25.2 ± 36 | - |
| Ultrasound evaluation | |||
| Univentricular left heart | 8 | 9 | - |
| Univentricular right heart | 5 | 12 | - |
| Extracardiac tunnel | 13 | - | |
| Fenestration | 9 | - | |
| Hepatomegaly | 5 | 7 | 0 |
| Splenomegaly | 3 | 1 | 0 |
| Clinical characteristics | |||
| BMI (kg/m2) | 15.33 ± 2.67 | 15.71 ± 3.59 | 17.70 ± 4.01 |
| SO2 (%) | 93.8 ± 2.39 | 82.8 ± 5.6 | 98.7 ± 0.2 |
| Investigation | Mean—Fontan | n | Mean—Glenn | n | Mean—Control | n | p— Fontan vs. Control | p— Glenn vs. Control | p— Fontan vs. Glenn |
|---|---|---|---|---|---|---|---|---|---|
| Emedian-SWE (kPa) | 4.85 ± 0.99 | 13 | 3.99 ± 0.74 | 20 | 3.91 ± 0.39 | 32 | 0.0049 | 0.96 | 0.01 |
| Vmedian-SWE (m/s) | 1.25 ± 0.16 | 12 | 1.16 ± 0.16 | 12 | 1.12 ± 0.16 | 32 | 0.01 | 0.53 | 0.08 |
| Fibrotest | 0.35 ± 0.19 | 13 | 0.24 ± 0.18 | 17 | 0.14 ± 0.07 | 30 | 0.0015 | 0.037 | 0.1 |
| Actitest | 0.12 ± 0.06 | 13 | 0.08 ± 0.03 | 17 | 0.05 ± 0.03 | 30 | 0.0001 | 0.0013 | 0.1 |
| AST (U/L) | 34.1 ± 14.6 | 13 | 33.2 ± 10.5 | 18 | 26.6 ± 11.9 | 28 | 0.05 | 0.04 | 0.61 |
| ALT (U/L) | 25.8 ± 9 | 13 | 21.34 ± 8.7 | 21 | 17.1 ± 7.7 | 28 | 0.003 | 0.01 | 0.09 |
| Fib-4 | 0.28 ± 0.1 | 12 | 0.14 ± 0.13 | 21 | 0.19 ± 0.1 | 26 | 0.03 | 0.18 | 0.001 |
| GGT (U/L) | 35.8 ± 16 | 12 | 16.57 ± 10 | 21 | 16 ± 18.6 | 30 | <0.0001 | 0.25 | 0.0004 |
| TB (mg/dL) | 0.58 ± 0.36 | 10 | 0.44 ± 0.24 | 18 | 0.93 ± 2.4 | 30 | 0.6 | 0.64 | 0.3 |
| A2MG (g/L) | 3.27 ± 0.53 | 13 | 3.34 ± 0.74 | 17 | 3.14 ± 0.58 | 30 | 0.5 | 0.34 | 0.8 |
| Haptoglobin (g/L) | 0.76 ± 0.38 | 13 | 0.77 ± 0.65 | 17 | 1.03 ± 0.658 | 30 | 0.22 | 0.06 | 0.6 |
| ApoA1 (g/L) | 1.22 ± 0.1 | 13 | 1.2 ± 0.25 | 17 | 1.4 ± 0.27 | 30 | 0.04 | 0.01 | 0.24 |
| Right liver lobe (mm) | 112.39 ± 29.3 | 12 | 91.51 ± 29.3 | 12 | 101.84 ± 20.3 | 31 | 0.39 | 0.08 | 0.15 |
| Left liver lobe (mm) | 44.7 ± 10.1 | 12 | 37.85 ± 11.34 | 31 | 42.84 ± 9.5 | 31 | 0.4 | 0.14 | 0.06 |
| APRI | 0.39 ± 0.29 | 12 | 0.39 ± 0.12 | 20 | 0.21 ± 0.13 | 28 | 0.0016 | 0.0017 | 0.86 |
| Thrombocyte count (×103/uL) | 241.6 ± 59 | 12 | 271.38 ± 87.56 | 21 | 290.72 ± 64.4 | 32 | 0.03 | 0.21 | 0.59 |
| Investigation | Mean—Fontan + Glenn | n | Mean—Control | n | p |
|---|---|---|---|---|---|
| Emedian-SWE (kPa) | 4.33 ± 0.93 | 33 | 3.91 ± 0.39 | 32 | 0.12 |
| Vmedian-SWE (m/s) | 1.2 ± 0.1 | 30 | 1.12 ± 0.16 | 32 | 0.08 |
| Fibrotest | 0.29 ± 0.19 | 30 | 0.14 ± 0.07 | 30 | 0.0018 |
| Actitest | 0.1 ± 0.05 | 30 | 0.05 ± 0.03 | 30 | <0.0001 |
| AST (U/L) | 33.62 ± 12.1 | 33 | 26.68 ± 11.9 | 28 | 0.0076 |
| ALT (U/L) | 23.06 ± 9 | 34 | 17.35 ± 7.54 | 30 | 0.0049 |
| Fib-4 | 0.19 ± 0.13 | 33 | 0.19 ± 0.13 | 26 | 0.92 |
| GGT (U/L) | 23.57 ± 15.75 | 33 | 16 ± 18 | 30 | 0.0045 |
| TB (mg/dL) | 0.49 ± 0.29 | 28 | 0.9 ± 2.48 | 30 | 0.98 |
| A2MG (g/L) | 3.31 ± 0.65 | 30 | 3.14 ± 0.58 | 30 | 0.32 |
| Haptoglobin (g/L) | 0.77 ± 0.54 | 30 | 1.03 ± 0.58 | 30 | 0.05 |
| ApoA1 (g/L) | 1.21 ± 0.2 | 30 | 1.41 ± 0.27 | 30 | 0.0053 |
| Right liver lobe (mm) | 102.06 ± 25.4 | 32 | 101.83 ± 20.3 | 31 | 0.57 |
| Left liver lobe (mm) | 41.6 ± 8.8 | 31 | 42.8 ± 9.5 | 31 | 0.68 |
| APRI | 0.35 ± 0.2 | 32 | 0.21 ± 0.13 | 32 | 0.0001 |
| Thrombocyte count (×103/uL) | 260.5 ± 65 | 33 | 290.72 ± 64.4 | 34 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mareș, R.-C.; Meliț, L.E.; Togănel, R.; Mareș, R.-G.; Mărginean, C.O. Liver Disease Assessment in Children with Fontan and Glenn Surgeries for Univentricular Hearts—The Role of Elastography and Biochemical Fibrosis Markers. Appl. Sci. 2022, 12, 7481. https://doi.org/10.3390/app12157481
Mareș R-C, Meliț LE, Togănel R, Mareș R-G, Mărginean CO. Liver Disease Assessment in Children with Fontan and Glenn Surgeries for Univentricular Hearts—The Role of Elastography and Biochemical Fibrosis Markers. Applied Sciences. 2022; 12(15):7481. https://doi.org/10.3390/app12157481
Chicago/Turabian StyleMareș, Roxana-Cristina, Lorena Elena Meliț, Rodica Togănel, Răzvan-Gheorghiță Mareș, and Cristina Oana Mărginean. 2022. "Liver Disease Assessment in Children with Fontan and Glenn Surgeries for Univentricular Hearts—The Role of Elastography and Biochemical Fibrosis Markers" Applied Sciences 12, no. 15: 7481. https://doi.org/10.3390/app12157481
APA StyleMareș, R.-C., Meliț, L. E., Togănel, R., Mareș, R.-G., & Mărginean, C. O. (2022). Liver Disease Assessment in Children with Fontan and Glenn Surgeries for Univentricular Hearts—The Role of Elastography and Biochemical Fibrosis Markers. Applied Sciences, 12(15), 7481. https://doi.org/10.3390/app12157481








