Temperature Sensing with Thin Films of Flame-Formed Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
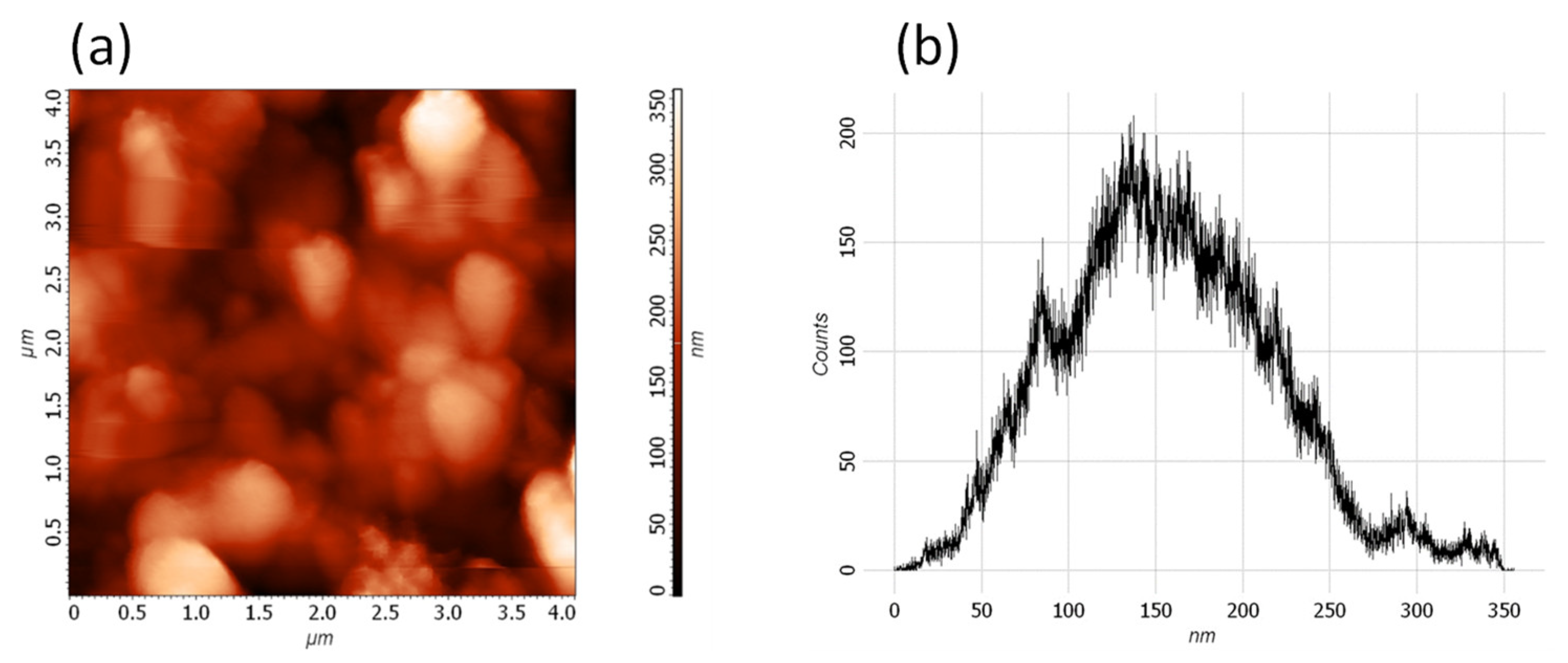
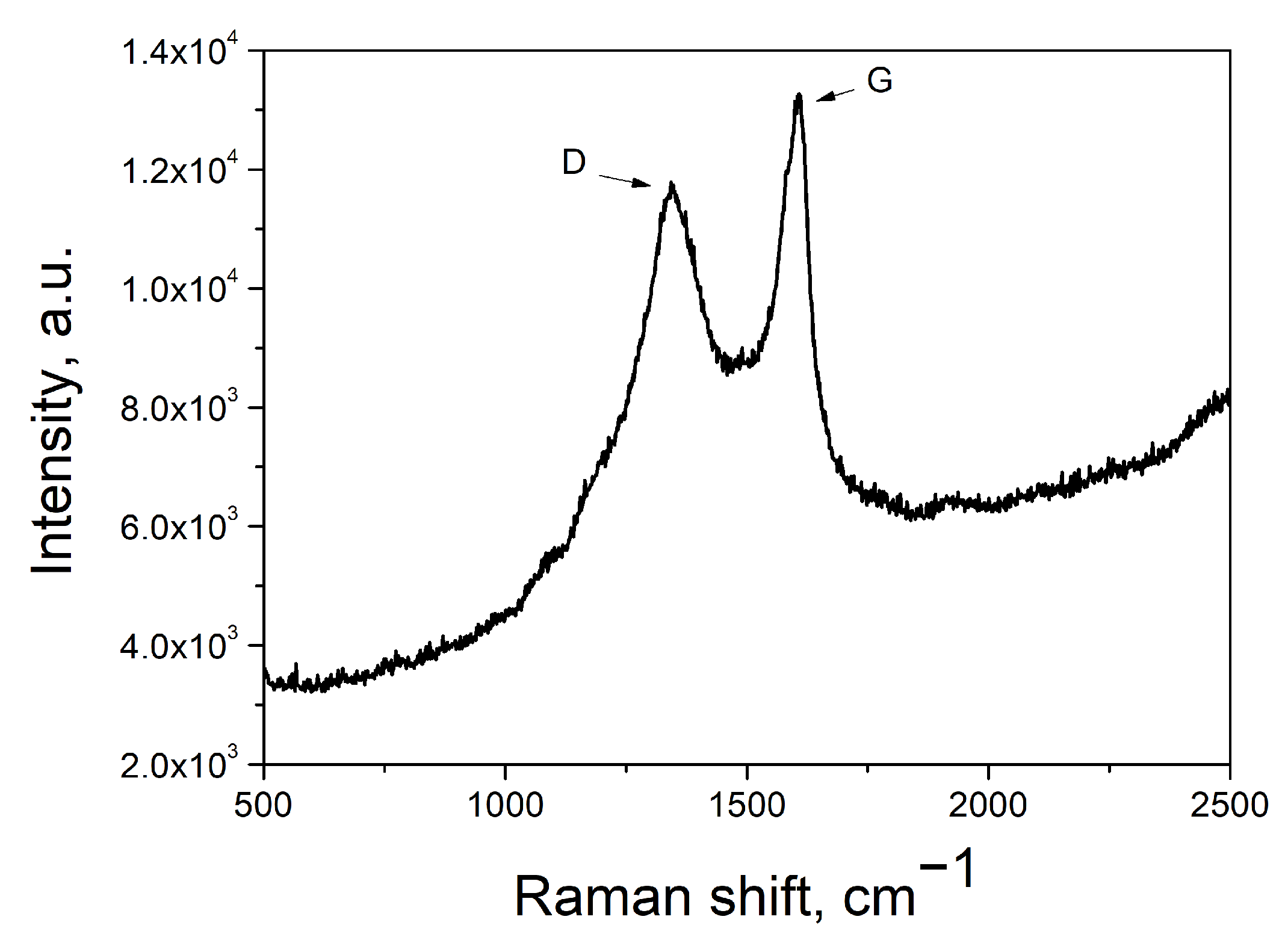
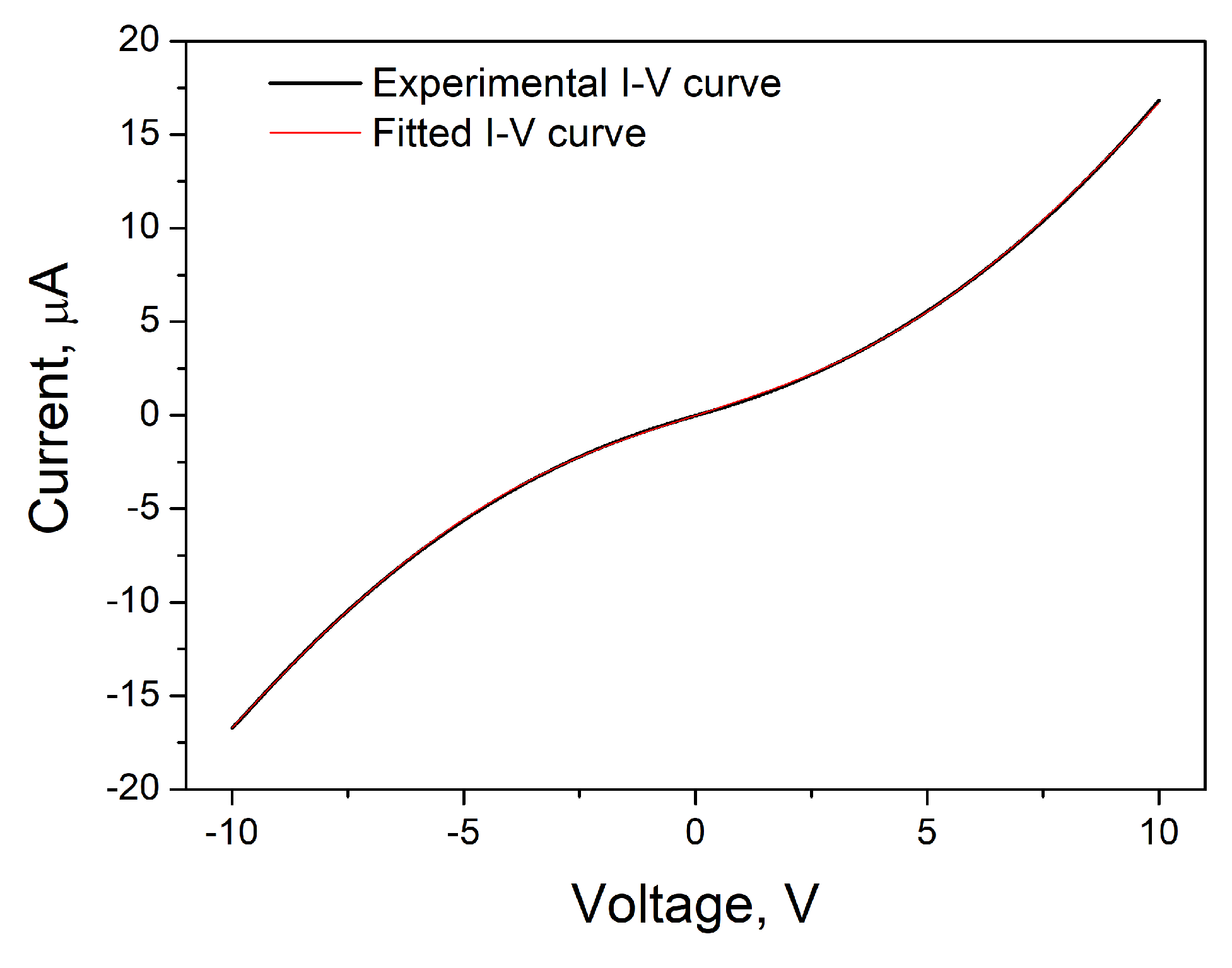
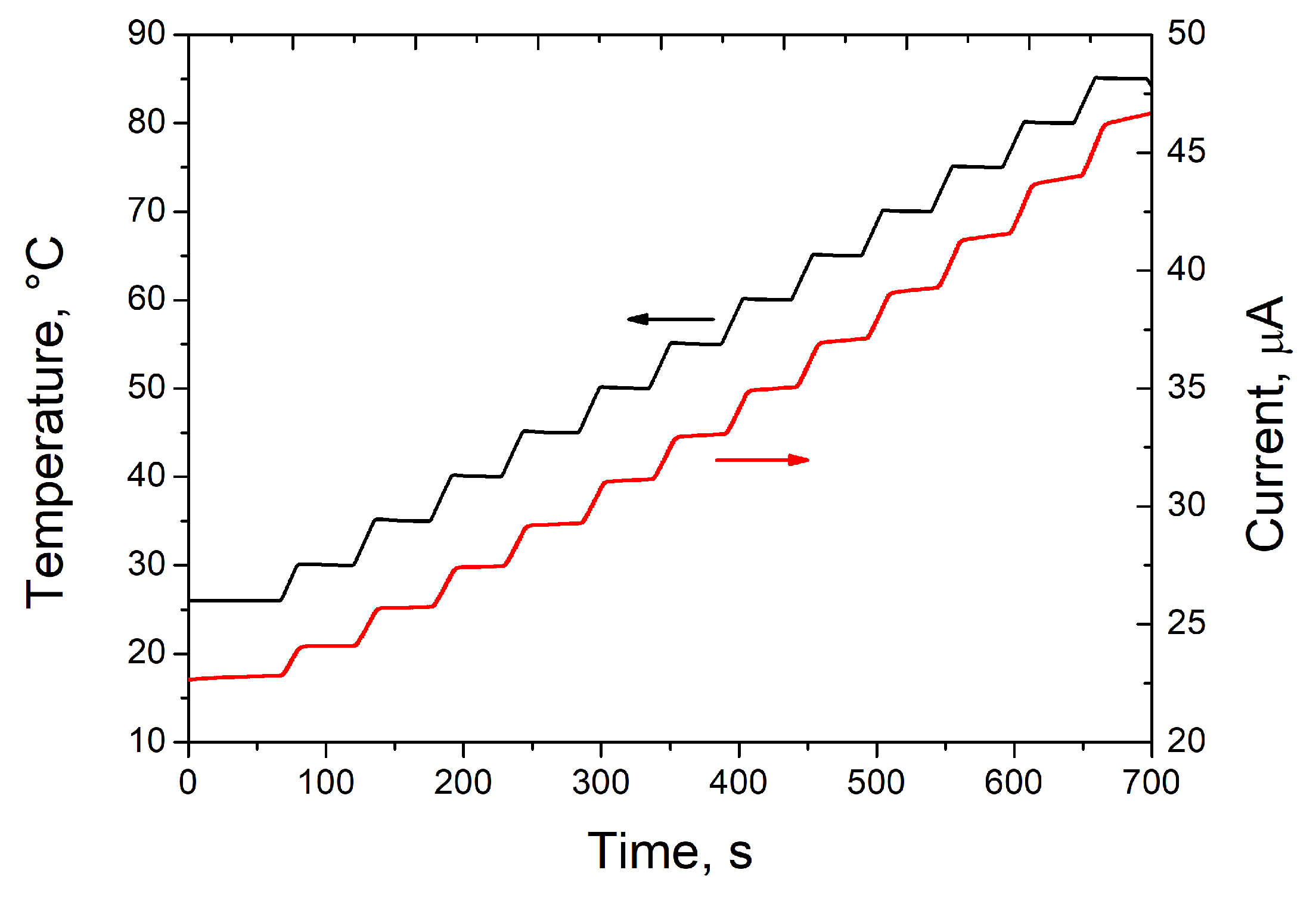
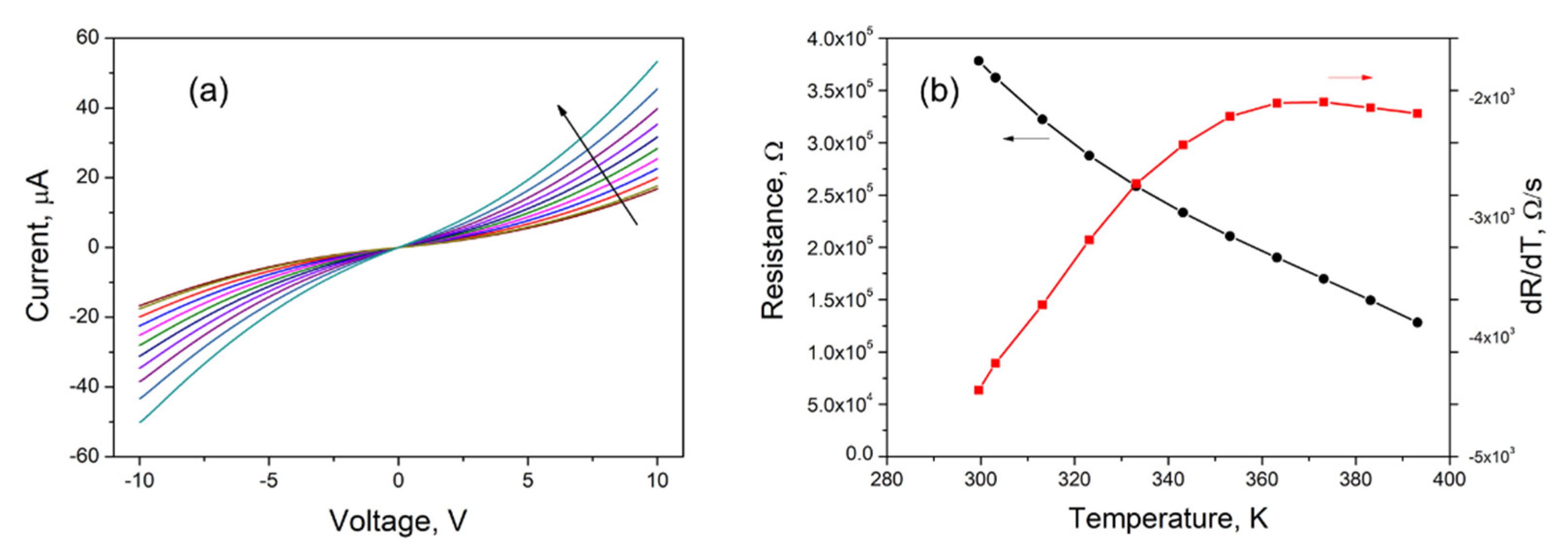
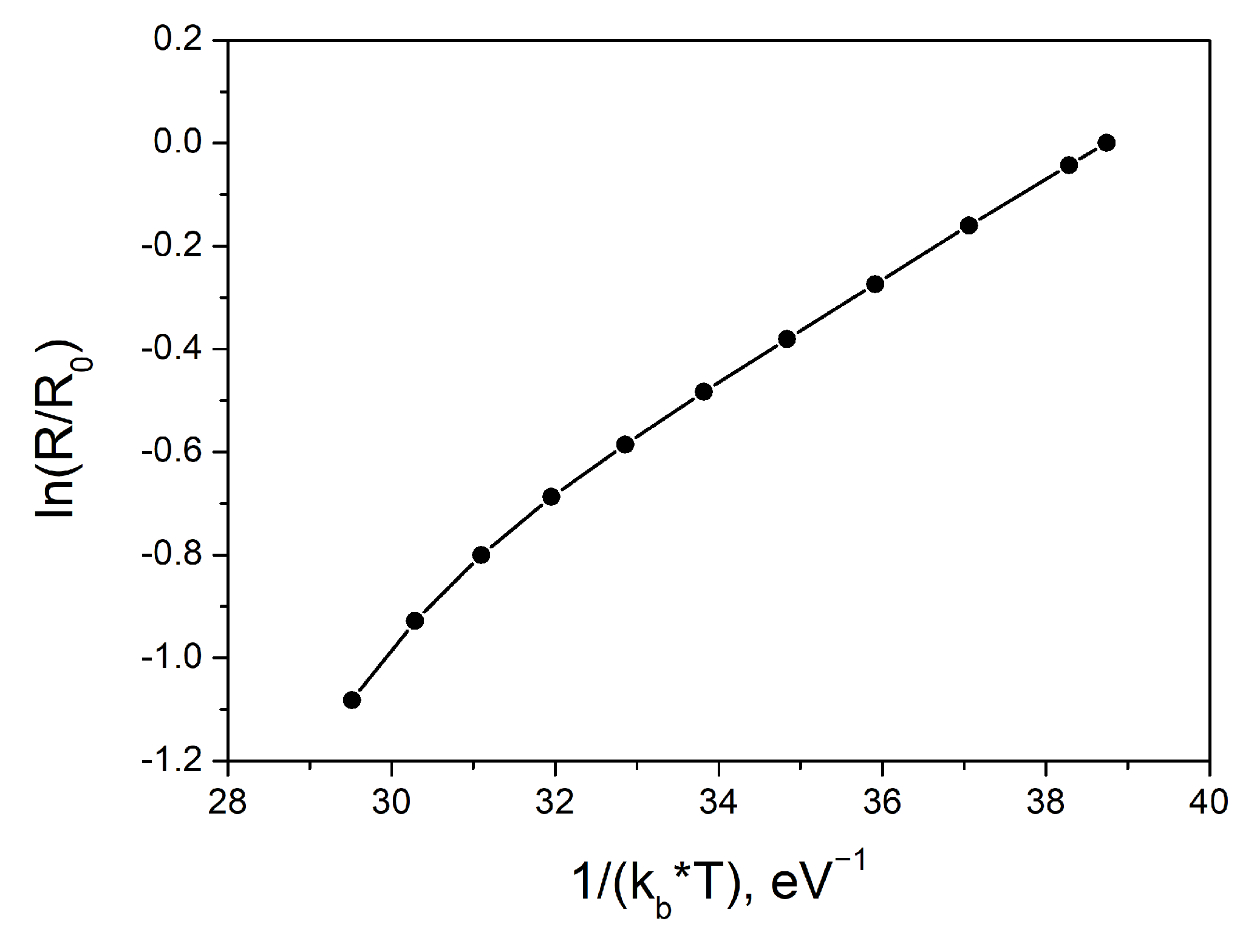
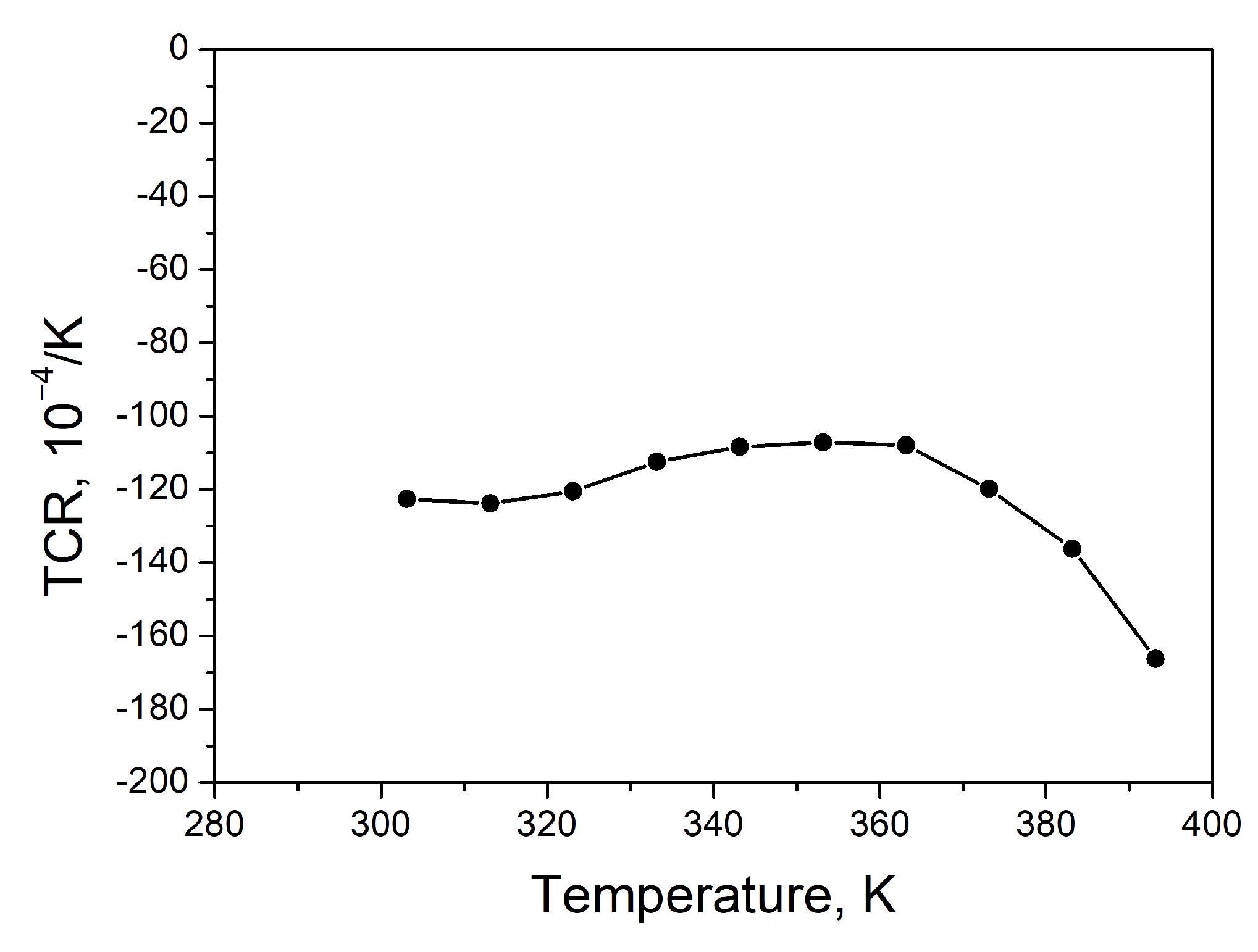
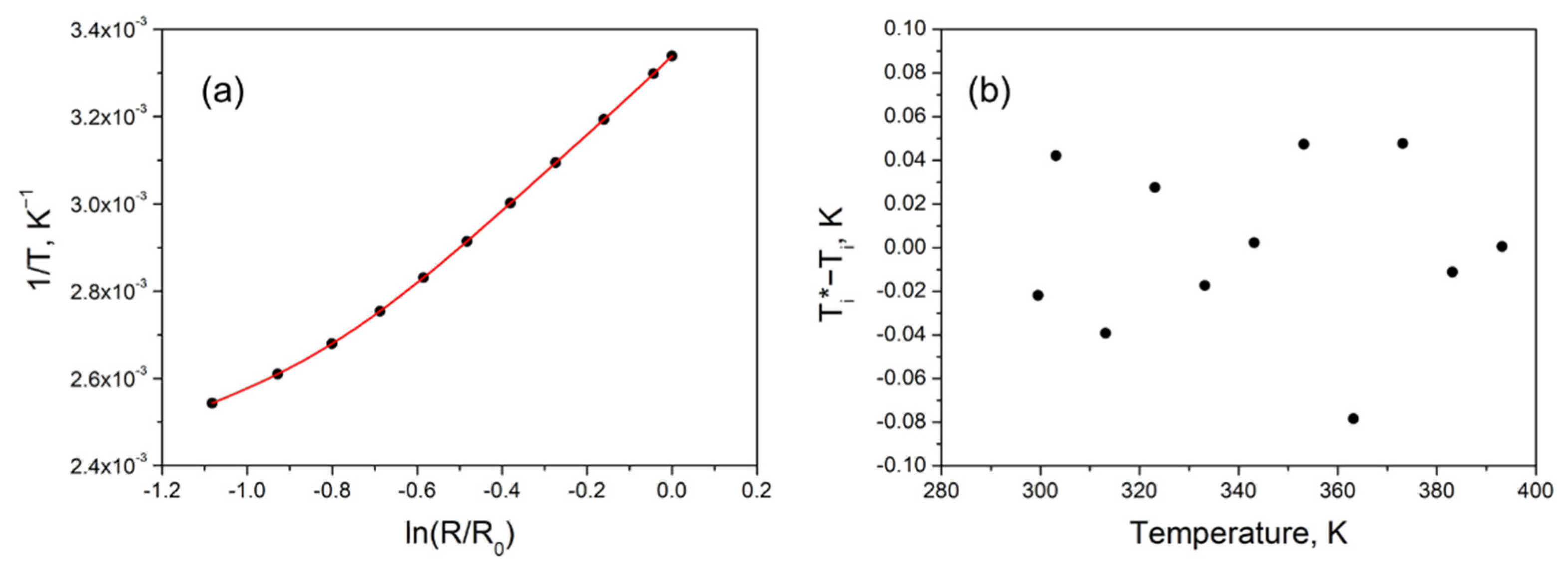
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trung, T.Q.; Ramasundaram, S.; Hwang, B.-U.; Lee, N.-E. An All-Elastomeric Transparent and Stretchable Temperature Sensor for Body-Attachable Wearable Electronics. Adv. Mater. 2016, 28, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Wang, X. Applications of Graphene-Based Materials in Sensors: A Review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Milcovich, G.; Chen, T.-Y.; Durack, E.; Mallen, S.; Ruan, Y.; Weng, X.; Hudson, S.P. Co-reductive fabrication of carbon nanodots with high quantum yield for bioimaging of bacteria. Beilstein J. Nanotechnol. 2018, 9, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Bartelmess, J.; Milcovich, G.; Maffeis, V.; D’Amora, M.; Bertozzi, S.M.; Giordani, S. Modulation of Efficient Diiodo-BODIPY in vitro Phototoxicity to Cancer Cells by Carbon Nano-Onions. Front. Chem. 2020, 8, 573211. [Google Scholar] [CrossRef]
- Li, W.; Fang, R.; Xia, Y.; Zhang, W.; Wang, X.; Xia, X.; Tu, J. Multiscale Porous Carbon Nanomaterials for Applications in Advanced Rechargeable Batteries. Batter. Supercaps 2019, 2, 9–36. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, H.; Dasappa, S.; Sabado, K.D.; Camacho, J. Production of Carbon Black in Turbulent Spray Flames of Coal Tar Distillates. Appl. Sci. 2021, 11, 10001. [Google Scholar] [CrossRef]
- Singh, M.; Gharpure, A.; Wal, R.L.V.; Kollar, J.; Herd, C.R. Effect of Fuel Composition on Carbon Black Formation Pathways. Appl. Sci. 2022, 12, 2569. [Google Scholar] [CrossRef]
- Richter, H.; Labrocca, A.J.; Grieco, W.J.; Taghizadeh, K.; Lafleur, A.A.L.; Howard, J.B. Generation of Higher Fullerenes in Flames. J. Phys. Chem. B 1997, 101, 1556–1560. [Google Scholar] [CrossRef]
- Height, M.J.; Howard, J.B.; Tester, J.W.; Sande, J.B.V. Flame synthesis of single-walled carbon nanotubes. Carbon 2004, 42, 2295–2307. [Google Scholar] [CrossRef]
- Khosravi, M.; Amini, M.K. Flame synthesis of carbon nanofibers on carbon paper: Physicochemical characterization and application as catalyst support for methanol oxidation. Carbon 2010, 48, 3131–3138. [Google Scholar] [CrossRef]
- Memon, N.; Tse, S.D.; Chhowalla, M.; Kear, B.H. Role of substrate, temperature, and hydrogen on the flame synthesis of graphene films. Proc. Combust. Inst. 2013, 34, 2163–2170. [Google Scholar] [CrossRef]
- Mulay, M.R.; Chauhan, A.; Patel, S.; Balakrishnan, V.; Halder, A.; Vaish, R. Candle soot: Journey from a pollutant to a func-tional material. Carbon 2019, 144, 684–712. [Google Scholar] [CrossRef]
- Lee, W.-J.; Kim, H.V.; Choi, J.-H.; Panomsuwan, G.; Lee, Y.-C.; Rho, B.-S.; Kang, J. Recycling Waste Soot from Merchant Ships to Produce Anode Materials for Rechargeable Lithium-Ion Batteries. Sci. Rep. 2018, 8, 5601. [Google Scholar] [CrossRef] [Green Version]
- Baldelli, A.; Esmeryan, K.D.; Popovicheva, O. Turning a negative into a positive: Trends, guidelines and challenges of developing multifunctional non-wettable coatings based on industrial soot wastes. Fuel 2021, 301, 121068. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.; Yu, B.; Zhou, F.; Liu, W. Candle soot as a supercapacitor electrode material. RSC Adv. 2014, 4, 2586–2589. [Google Scholar] [CrossRef]
- Campbell, D.J.; Andrews, M.J.; Stevenson, K.J. New Nanotech from an Ancient Material: Chemistry Demonstrations Involving Carbon-Based Soot. J. Chem. Educ. 2012, 89, 1280–1287. [Google Scholar] [CrossRef]
- D’Anna, A. Combustion-formed nanoparticles. Proc. Combust. Inst. 2009, 32, 593–613. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot inception: Carbonaceous nanoparticle formation in flames. Prog. Energy Combust. Sci. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Schulz, F.; Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Meyer, G.; D’anna, A.; Gross, L. Insights into incipient soot formation by atomic force microscopy. Proc. Combust. Inst. 2019, 37, 885–892. [Google Scholar] [CrossRef]
- Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Schulz, F.; D’Anna, A.; Gross, L. On the early stages of soot formation: Molecular structure elucidation by high-resolution atomic force microscopy. Combust. Flame 2019, 205, 154–164. [Google Scholar] [CrossRef]
- Vitiello, G.; De Falco, G.; Picca, F.; Commodo, M.; D’Errico, G.; Minutolo, P.; D’Anna, A. Role of radicals in carbon clustering and soot inception: A combined EPR and Raman spectroscopic study. Combust. Flame 2019, 205, 286–294. [Google Scholar] [CrossRef]
- Commodo, M.; De Falco, G.; Larciprete, R.; D’Anna, A.; Minutolo, P. On the hydrophilic/hydrophobic character of carbonaceous nanoparticles formed in laminar premixed flames. Exp. Therm. Fluid Sci. 2016, 73, 56–63. [Google Scholar] [CrossRef]
- Johansson, K.O.; Dillstrom, T.; Monti, M.; El Gabaly, F.; Campbell, M.F.; Schrader, P.E.; Popolan-Vaida, D.M.; Richards-Henderson, N.K.; Wilson, K.R.; Violi, A.; et al. Formation and emission of large furans and oxygenated hydrocarbons from flames. Proc. Natl. Acad. Sci. USA 2016, 113, 8374–8379. [Google Scholar] [CrossRef] [Green Version]
- Commodo, M.; D’Anna, A.; De Falco, G.; Larciprete, R.; Minutolo, P. Illuminating the earliest stages of the soot formation by photoemission and Raman spectroscopy. Combust. Flame 2017, 181, 188–197. [Google Scholar] [CrossRef]
- De Falco, G.; Mattiello, G.; Commodo, M.; Minutolo, P.; Shi, X.; D’Anna, A.; Wang, H. Electronic band gap of flame-formed carbon nanoparticles by scanning tunneling spectroscopy. Proc. Combust. Inst. 2021, 38, 1805–1812. [Google Scholar] [CrossRef]
- Liu, C.; Singh, A.V.; Saggese, C.; Tang, Q.; Chen, D.; Wan, K.; Vinciguerra, M.; Commodo, M.; De Falco, G.; Minutolo, P.; et al. Flame-formed carbon nanoparticles exhibit quantum dot behaviors. Proc. Natl. Acad. Sci. USA 2019, 116, 12692–12697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldelli, A.; Trivanovic, U.; Sipkens, T.A.; Rogak, S.N. On determining soot maturity: A review of the role of microscopy- and spectroscopy-based techniques. Chemosphere 2020, 252, 126532. [Google Scholar] [CrossRef] [PubMed]
- De Falco, G.; Commodo, M.; Bonavolontà, C.; Pepe, G.P.; Minutolo, P.; D’Anna, A. Optical and electrical characterization of carbon nanoparticles produced in laminar premixed flames. Combust. Flame 2014, 161, 3201–3210. [Google Scholar] [CrossRef]
- Dobbins, R.A.; Megaridis, C.M. Morphology of flame-generated soot as determined by thermophoretic sampling. Langmuir 1987, 3, 254–259. [Google Scholar] [CrossRef]
- Tricoli, A.; Bo, R. Nanoparticle-based biomedical sensors. Mater. Sci. Eng. R Rep. 2020, 15, 247–269. [Google Scholar] [CrossRef]
- Merchan-Breuer, D.A.; Murphy, E.; Berka, B.; Nova, L.C.M.; Liu, Y.; Merchan-Merchan, W. Synthesis of Carbonaceous Hydrophobic Layers through a Flame Deposition Process. Appl. Sci. 2022, 12, 2427. [Google Scholar] [CrossRef]
- Dhall, S.; Mehta, B. Room temperature hydrogen gas sensor using candle carbon soot. Int. J. Hydrogen Energy 2020, 45, 14997–15002. [Google Scholar] [CrossRef]
- Huth, M. Granular metals: From electronic correlations to strain-sensing applications. J. Appl. Phys. 2010, 107, 113709. [Google Scholar] [CrossRef]
- De Falco, G.; Commodo, M.; Barra, M.; Chiarella, F.; D’Anna, A.; Aloisio, A.; Cassinese, A.; Minutolo, P. Electrical characterization of flame-soot nanoparticle thin films. Synth. Met. 2017, 229, 89–99. [Google Scholar] [CrossRef]
- Sgro, L.; Basile, G.; Barone, A.; D’Anna, A.; Minutolo, P.; Borghese, A.; D’Alessio, A. Detection of combustion formed nanoparticles. Chemosphere 2003, 51, 1079–1090. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A guide to and review of the use of multiwavelength raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Bruschi, P.; Nannini, A. Current vs. voltage characteristics of ion-beam-grown polymer-metal granular thin films. Thin Solid Films 1991, 201, 29–38. [Google Scholar] [CrossRef]
- Balberg, I.; Wagner, N.; Goldstein, Y.; Weisz, S. Tunneling and Percolation Behavior in Granular Metals. MRS Proc. 1990, 195, 233–238. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; Taylor & Francis: London, UK, 1994. [Google Scholar]
- Obermeier, E.; Kopystynski, P.; NieBl, R. Characteristics of polysilicon layers and their application in sensors. In IEEE Solid-State Sensors Workshop; IEEE Press: New York, NY, USA, 1986. [Google Scholar]
- Kuo, J.T.W.; Yu, L.; Meng, E. Micromachined Thermal Flow Sensors—A Review. Micromachines 2012, 3, 550–573. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolomeo, A.; Sarno, M.; Giubileo, F.; Altavilla, C.; Iemmo, L.; Piano, S.; Bobba, F.; Longobardi, M.; Scarfato, A.; Sannino, D.; et al. Multiwalled carbon nanotube films as small-sized temperature sensors. J. Appl. Phys. 2009, 105, 064518. [Google Scholar] [CrossRef]
- Al-Mumen, H.; Rao, F.; Dong, L.; Li, W. Design, fabrication, and characterization of graphene thermistor. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 1135–1138. [Google Scholar] [CrossRef]
- Liu, G.; Guo, L.; Liu, C.; Wu, Q. Evaluation of different calibration equations for NTC thermistor applied to high-precision temperature measurement. Measurement 2018, 120, 21–27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minutolo, P.; De Falco, G.; Commodo, M.; Aloisio, A.; D’Anna, A. Temperature Sensing with Thin Films of Flame-Formed Carbon Nanoparticles. Appl. Sci. 2022, 12, 7714. https://doi.org/10.3390/app12157714
Minutolo P, De Falco G, Commodo M, Aloisio A, D’Anna A. Temperature Sensing with Thin Films of Flame-Formed Carbon Nanoparticles. Applied Sciences. 2022; 12(15):7714. https://doi.org/10.3390/app12157714
Chicago/Turabian StyleMinutolo, Patrizia, Gianluigi De Falco, Mario Commodo, Alberto Aloisio, and Andrea D’Anna. 2022. "Temperature Sensing with Thin Films of Flame-Formed Carbon Nanoparticles" Applied Sciences 12, no. 15: 7714. https://doi.org/10.3390/app12157714





