The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland
Abstract
:1. Introduction
2. Material and Methods
2.1. Location of the Field Trials and Soil Conditions
2.2. Cropping Practice
2.3. Measurements
2.4. Weather Conditions
2.5. Statistical Methods
3. Results
3.1. Soybean Yield
3.2. Protein and Oil Contents
3.3. Tillage Versus No-Tillage Cultivation
3.4. Usefulness of Soybean Cultivars for the Cultivation System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hymowitz, T. Soybeans: The Success Story. In Advances in New Crops, Proceedings of the First National Symposium “New Crops: Research, Development, Economics”, Indianapolis, IN, USA, 23–26 October 1988; Timber Press: Portland, OR, USA, 1990; pp. 159–163. [Google Scholar]
- Helferich, W.G.; Andrade, J.E.; Hoagland, M.S. Phytoestrogens and Breast Cancer: A Complex Story. Inflammopharmacology 2008, 16, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Statistical Yearbook World Food and Agriculture. 2020. Available online: https://www.fao.org/3/cb1329en/online/cb1329en.html (accessed on 6 July 2022).
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops That Feed the World Soybean-Worldwide Production, Use, and Constraints Caused by Pathogens and Pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Twardowski, T.; Małyska, A.; Gracz, J.; Tyczewska, A. Soja Przyszłością Polskiego Rolnictwa? Nauka 2014, 4, 121–138. [Google Scholar]
- Lewandowska, S. Perspectives of soybean cultivation in Poland. In Proceedings of the Perspectives of Soybean Cultivation in Poland, Krzyżowa, Poland, 5 July 2016. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Hemingway, J.; Eskandari, M.; Rajcan, I. Genetic and Environmental Effects on Fatty Acid Composition in Soybeans with Potential Use in the Automotive Industry. Crop Sci. 2015, 55, 658–668. [Google Scholar] [CrossRef]
- Shaw, E.J.; Rajcan, I. Molecular Mapping of Soybean Seed Tocopherols in the Cross ‘OAC Bayfield’ × ‘OAC Shire’. Plant Breed 2017, 136, 83–93. [Google Scholar] [CrossRef]
- SOYSTAT. Available online: http://soystats.com/ (accessed on 6 July 2022).
- EC. Report from the Commission to the Council and the European Parliament on the Development of Plant Proteins in the European Union; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Brzóska, F.; Śliwa, J. Non-gm soybean–production and possible use in animal feeding in Poland. Part, I. Soy in the fodder balance and its cultivation in Poland. Wiadomości Zootech. 2016, 54, 98–100. [Google Scholar]
- Liu, K. Chemistry and Nutritional Value of Soybean Components. In Soybeans; Springer: Boston, MA, USA, 1997; pp. 25–113. ISBN 978-1-4613-5711-7. [Google Scholar]
- Sanders, T.A.B. Functional Dietary Lipids: Food Formulation, Consumer Issues and Innovation for Health; Woodhead Publishing: Sawston, UK, 2016; ISBN 978-1-78242-257-0. [Google Scholar]
- Polska Soja–Popularyzacja Uprawy Soi Na Terenie Polski. Available online: https://www.polskasoja.pl/asp/pl_start.asp?typ=13&menu=1&strona=1 (accessed on 6 July 2022).
- Jarecki, W.; Bobrecka-Jamro, D. Wpływ nawożenia dolistnego na plon i skład chemiczny nasion soi (Glycine max (L.) Merrill). Fragm. Agron. 2015, 32, 22–31. [Google Scholar]
- Jerzak, M.A.; Czerwińska-Kayzer, D.; Florek, J.; Śmiglak-Krajewska, M. Determinanty produkcji roślin strączkowych jako alternatywnego źródła białka-w ramach nowego obszaru polityki rolnej w Polsce. Rocz. Nauk. Rolniczych. Ser. G Ekon. Rol. 2012, 99, 113–120. [Google Scholar]
- Latawiec, A.E.; Koryś, A.; Koryś, K.A.; Kuboń, M.; Sadowska, U.; Gliniak, M.; Sikora, J.; Drosik, A.; Niemiec, M.; Klimek-Kopyra, A.; et al. Economic Analysis of Biochar Use in Soybean Production in Poland. Agronomy 2021, 11, 2108. [Google Scholar] [CrossRef]
- Ball, B.C.; Lang, R.W.; Robertson, E.A.G.; Franklin, M.F. Crop Performance and Soil Conditions on Imperfectly Drained Loams after 20–25 Years of Conventional Tillage or Direct Drilling. Soil Tillage Res. 1994, 31, 97–118. [Google Scholar] [CrossRef]
- López-Fando, C. Interactive Effects of Tillage and Crop Rotations on Yield and Chemical Properties of Soils in Semi-Arid Central Spain. Soil Tillage Res. 1995, 36, 45–57. [Google Scholar] [CrossRef]
- Kęsik, T.; Blazewicz-Wozniak, M.; Konopinski, M.; Wach, D.; Mitura, R. The effect of cover crop mulches and reduced soil tillage in onion cultivation on some features of soil. Rocz. Akad. Rol. Poznaniu. Ogrod. 2007, 517–521. [Google Scholar]
- Kang, Y.; Khan, S.; Ma, X. Climate Change Impacts on Crop Yield, Crop Water Productivity and Food Security–A Review. Prog. Nat. Sci. Mater. Int. 2009, 12, 1665–1674. [Google Scholar] [CrossRef]
- Hama, J.R.; Kolpin, D.W.; LeFevre, G.H.; Hubbard, L.E.; Powers, M.M.; Strobel, B.W. Exposure and Transport of Alkaloids and Phytoestrogens from Soybeans to Agricultural Soils and Streams in the Midwestern United States. Environ. Sci. Technol. 2021, 55, 11029–11039. [Google Scholar] [CrossRef] [PubMed]
- Żarski, J.; Kuśmierek-Tomaszewska, R.; Dudek, S. Trends of Changes in Climate Risk of Grain Maize Cultivation in the Bydgoszcz Region. Infrastrukt. Ekol. Teren. Wiej. 2016, III/1, 725–735. [Google Scholar]
- Leng, G.; Zhang, X.; Huang, M.; Asrar, G.R.; Leung, L.R. The Role of Climate Covariability on Crop Yields in the Conterminous United States. Sci. Rep. 2016, 6, 33160. [Google Scholar] [CrossRef] [PubMed]
- Stuczyński, T.; Demidowicz, G.; Deputat, T.; Górski, T.; Krasowicz, S.; Kuś, J. Adaptation Scenarios of Agriculture in Poland to Future Climate Changes. Environ. Monit. Assess. 2000, 61, 133–144. [Google Scholar] [CrossRef]
- Kozyra, J.; Doroszewski, A.; Nieróbca, A. Climate Change and Its Expected Impact on Agriculture in Poland; IUNG-PIB: Puławy, Poland, 2017. [Google Scholar]
- Żmudzka, E. Long-term changes of thermal resources in the vegetative period and the active growth of plants in Poland. Woda Środowisko Obszary Wiejskie 2012, 12, 377–389. [Google Scholar]
- Déqué, M.; Rowell, D.P.; Lüthi, D.; Giorgi, F.; Christensen, J.H.; Rockel, B.; Jacob, D.; Kjellström, E.; de Castro, M.; van den Hurk, B. An Intercomparison of Regional Climate Simulations for Europe: Assessing Uncertainties in Model Projections. Clim. Chang. 2007, 81, 53–70. [Google Scholar] [CrossRef]
- Wibig, J.; Glowicki, B. Trends of Minimum and Maximum Temperature in Poland. Clim. Res. 2002, 20, 123–133. [Google Scholar] [CrossRef]
- Anders, I.; Stagl, J.; Auer, I.; Pavlik, D. Climate Change in Central and Eastern Europe. In Managing Protected Areas in Central and Eastern Europe under Climate Change; Rannow, S., Neubert, M., Eds.; Advances in Global Change Research; Springer: Dordrecht, The Netherlands, 2014; pp. 17–30. ISBN 978-94-007-7960-0. [Google Scholar]
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic Improvement of U.S. Soybean in Maturity Groups II, III, and IV. Crop Sci. 2014, 54, 1419–1432. [Google Scholar] [CrossRef]
- De Bruin, J.; Pedersen, P. Growth, Yield, and Yield Component Changes among Old and New Soybean Cultivars. Agron. J. 2009, 101, 124–130. [Google Scholar] [CrossRef]
- Cober, E.R.; Morrison, M.J. Genetic Improvement Estimates, from Cultivar × Crop Management Trials, Are Larger in High-Yield Cropping Environments. Crop Sci. 2015, 55, 1425–1434. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Ziernicka-Wojtaszek, A.; Kopcińska, J. Variation in Atmospheric Precipitation in Poland in the Years 2001–2018. Atmosphere 2020, 11, 794. [Google Scholar] [CrossRef]
- EUR-Lex-C:2016:450:TOC-PL-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AC%3A2016%3A450%3ATOC (accessed on 6 July 2022).
- Centralny Ośrodek Badania Odmian Roślin Uprawnych, COBORU. Available online: http://www.coboru.gov.pl/ (accessed on 6 July 2022).
- Sudarić, A. Introductory Chapter. In Soybean—Quality and Utilization; IntechOpen: London, UK, 2020; ISBN 978-1-83881-019-1. [Google Scholar]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Europ. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Umburanas, R.C.; Kawakami, J.; Ainsworth, E.A.; Favarin, J.L.; Anderle, L.Z.; Dourado-Neto, D.; Reichardt, K. Changes in Soybean Cultivars Released over the Past 50 Years in Southern Brazil. Sci. Rep. 2022, 12, 508. [Google Scholar] [CrossRef]
- Śliwa, J.; Zajac, T.; Oleksy, A.; Klimek-Kopyra, A.; Lorenc-Kozik, A.; Kulig, B. Comparison of the Development and Productivity of Soybean (Glycine Max (L.) Merr.) Cultivated in Western Poland. Acta Sci. Pol. Agric. 2015, 14, 81–95. [Google Scholar]
- Salmerón, M.; Purcell, L.C. Simplifying the Prediction of Phenology with the DSSAT-CROPGRO-Soybean Model Based on Relative Maturity Group and Determinacy. Agric. Syst. 2016, 148, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Serafin-Andrzejewska, M.; Helios, W.; Jama-Rodzeńska, A.; Kozak, M.; Kotecki, A.; Kuchar, L. Effect of sowing date on soybean development in south-western Poland. Agriculture 2021, 11, 413. [Google Scholar] [CrossRef]
- Żarski, J.; Kuśmierek-Tomaszewska, R.; Dudek, S.; Kropkowski, M.; Kledzik, R. Identifying Climatic Risk to Soybean Cultivation in the Transitional Type of Moderate Climate in Central Poland. J. Cent. Eur. Agric. 2019, 20, 143–156. [Google Scholar] [CrossRef]
- Januszewska-Klapa, K. Tendencies of Changes in the Climatic Risk of Growing Crops in Selected Locations of the Kujawsko-Pomorskie Province. Ph.D. Thesis, University of Life Science and Technolgy in Bydgoszcz, Bydgoszcz, Poland, 2016. [Google Scholar]
- Starkel, L.; Kundzewicz, Z.W. Consequences of Climate Change for Spatial Organization of Poland. Nauka 2008, 1, 85–101. [Google Scholar]
- Novikova, L.Y.; Seferova, I.V.; Nekrasov, A.Y.; Perchuk, I.N.; Shelenga, T.V.; Samsonova, M.G.; Vishnyakova, M.A. Impact of weather and climate on seed protein and oil content of soybean in the North Caucasus. Vavilov. J. Genet.Breed. 2018, 22, 708–715. [Google Scholar] [CrossRef]
- Christensen, O.B.; Goodess, C.M.; Harris, I.; Watkiss, P. European and Global Climate Change Projections. Available online: https://www.weadapt.org/knowledge-base/economics-of-adaptation/climatecost-tpbn-1 (accessed on 6 July 2022).
- Faé, G.S.; Kemanian, A.R.; Roth, G.W.; White, C.; Watson, J.E. Soybean Yield in Relation to Environmental and Soil Properties. Eur. J. Agron. 2020, 118, 126070. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Q.; Patil, G.B.; Agarwal, G.; Deshmukh, R.K.; Lin, L.; Wang, B.; Wang, Y.; Prince, S.J.; Song, L.; et al. Identification and Comparative Analysis of Differential Gene Expression in Soybean Leaf Tissue under Drought and Flooding Stress Revealed by RNA-Seq. Front. Plant Sci. 2016, 7, 1044. [Google Scholar] [CrossRef] [Green Version]
- Xoconostle-Cázares, B.; Ramírez-Ortega, F.A.; Flores-Elenes, L.; Ruiz-Medrano, R. Drought Tolerance in Crop Plants. Am. J. Plant Physiol. 2010, 5, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic Profiling of Soybeans (Glycine max, L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.A.; Kitajima, K. Carbohydrate Storage Enhances Seedling Shade and Stress Tolerance in a Neotropical Forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Ferreira Neto, J.R.C.; Pandolfi, V.; Guimaraes, F.C.M.; Benko-Iseppon, A.M.; Romero, C.; Silva, R.L.D.O.; Rodrigues, F.A.; Abdelnoor, R.V.; Nepomuceno, A.L.; Kido, E.A. Early Transcriptional Response of Soybean Contrasting Accessions to Root Dehydration. PLoS ONE 2013, 8, e83466. [Google Scholar] [CrossRef] [Green Version]
- Borowska, M.; Prusiński, J. Effect of soybean cultivars sowing dates on seed yield and its correlation with yield parameters. Plant Soil Environ. 2021, 67, 360–366. [Google Scholar] [CrossRef]
- Michałek, S.; Borkowski, E. Yielding, oil, fatty acids and protein content in the seeds of polish soybean cultivars under drought conditions. Acta Agroph. 2006, 8, 459–471. [Google Scholar]
- Qin, X.; Feng, F.; Li, D.; Herbert, S.J.; Liao, Y.; Siddique, K.H.M.; Qin, X.; Feng, F.; Li, D.; Herbert, S.J.; et al. Changes in Yield and Agronomic Traits of Soybean Cultivars Released in China in the Last 60 Years. Crop Pasture Sci. 2017, 68, 973–984. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of Heat Stress during Seed Development on Soybean Seed Metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Gawęda, D.; Nowak, A.; Haliniarz, M.; Woźniak, A. Yield and Economic Effectiveness of Soybean Grown Under Different Cropping Systems. Int. J. Plant Prod. 2020, 14, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; van Groenigen, K.J.; Lee, J.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. When Does No-till Yield More? A Global Meta-Analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Hao, X.; Thelen, K.; Robertson, G.P. Agronomic Management System and Precipitation Effects on Soybean Oil and Fatty Acid Profiles. Crop Sci. 2009, 49, 1049–1057. [Google Scholar] [CrossRef]
- Di Mauro, G.; Borrás, L.; Rugeroni, P.; Rotundo, J.L. Exploring soybean management options for environments with contrasting water availability. J. Agron. Crop Sci. 2019, 205, 274–282. [Google Scholar] [CrossRef]

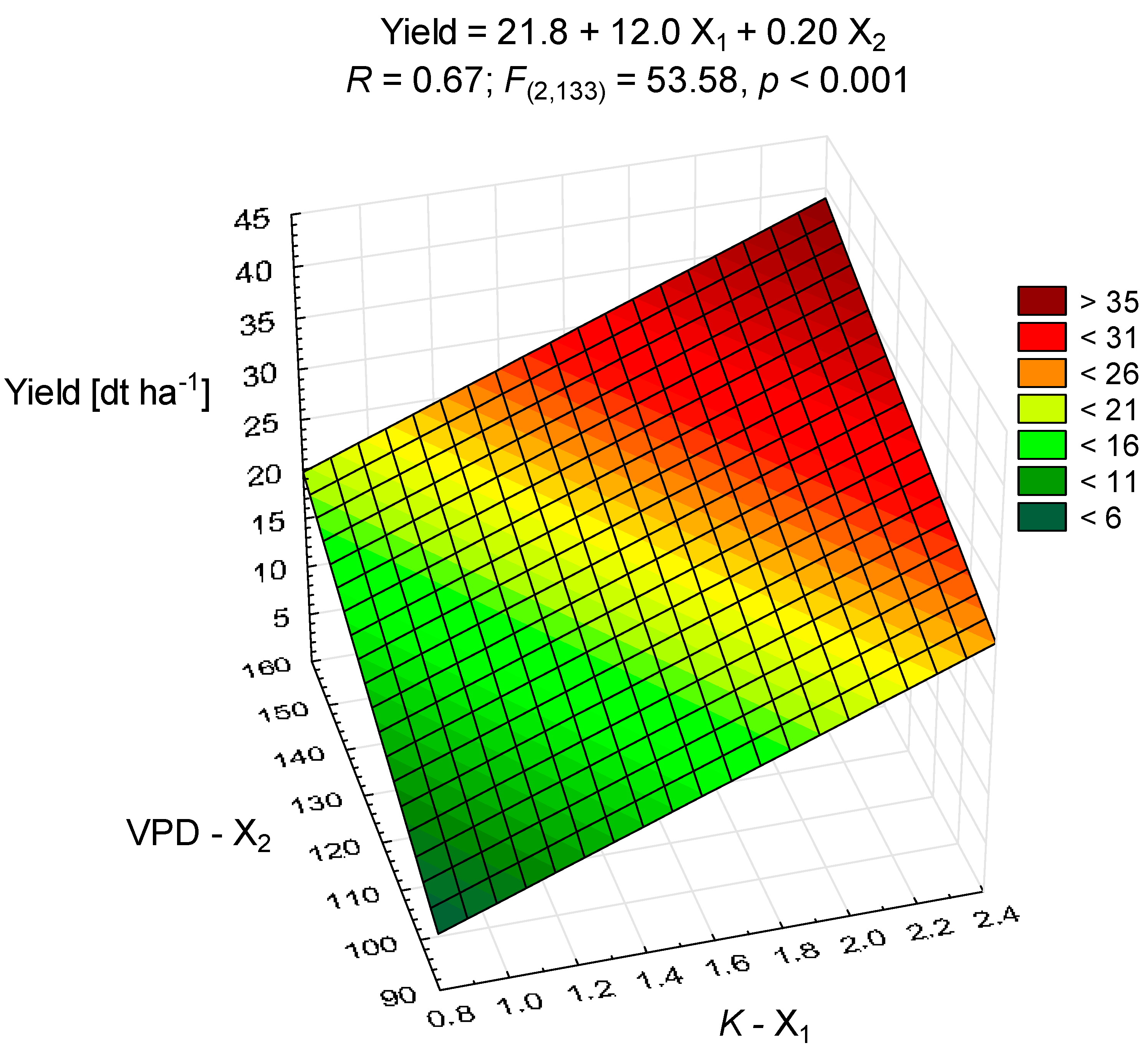
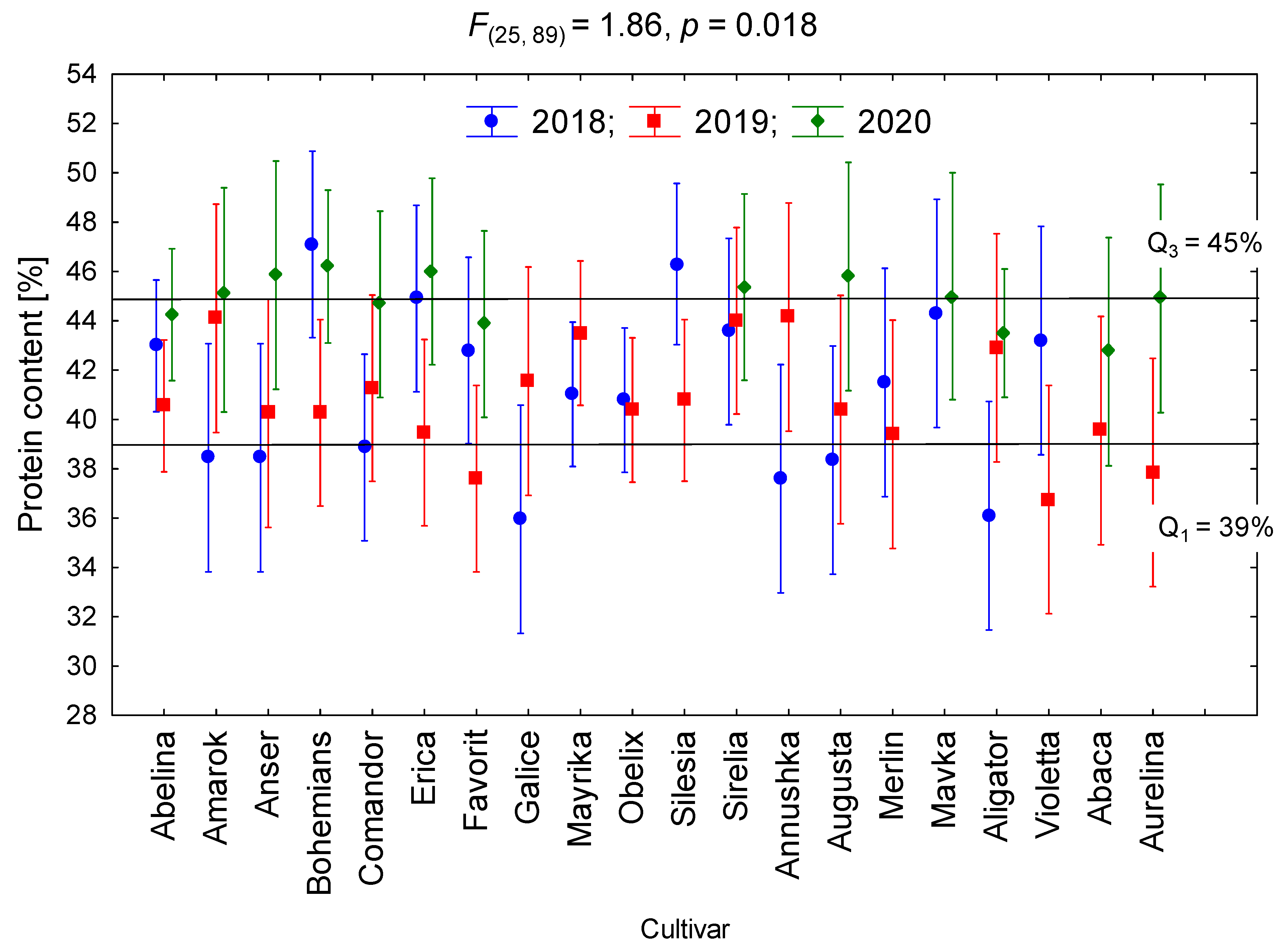

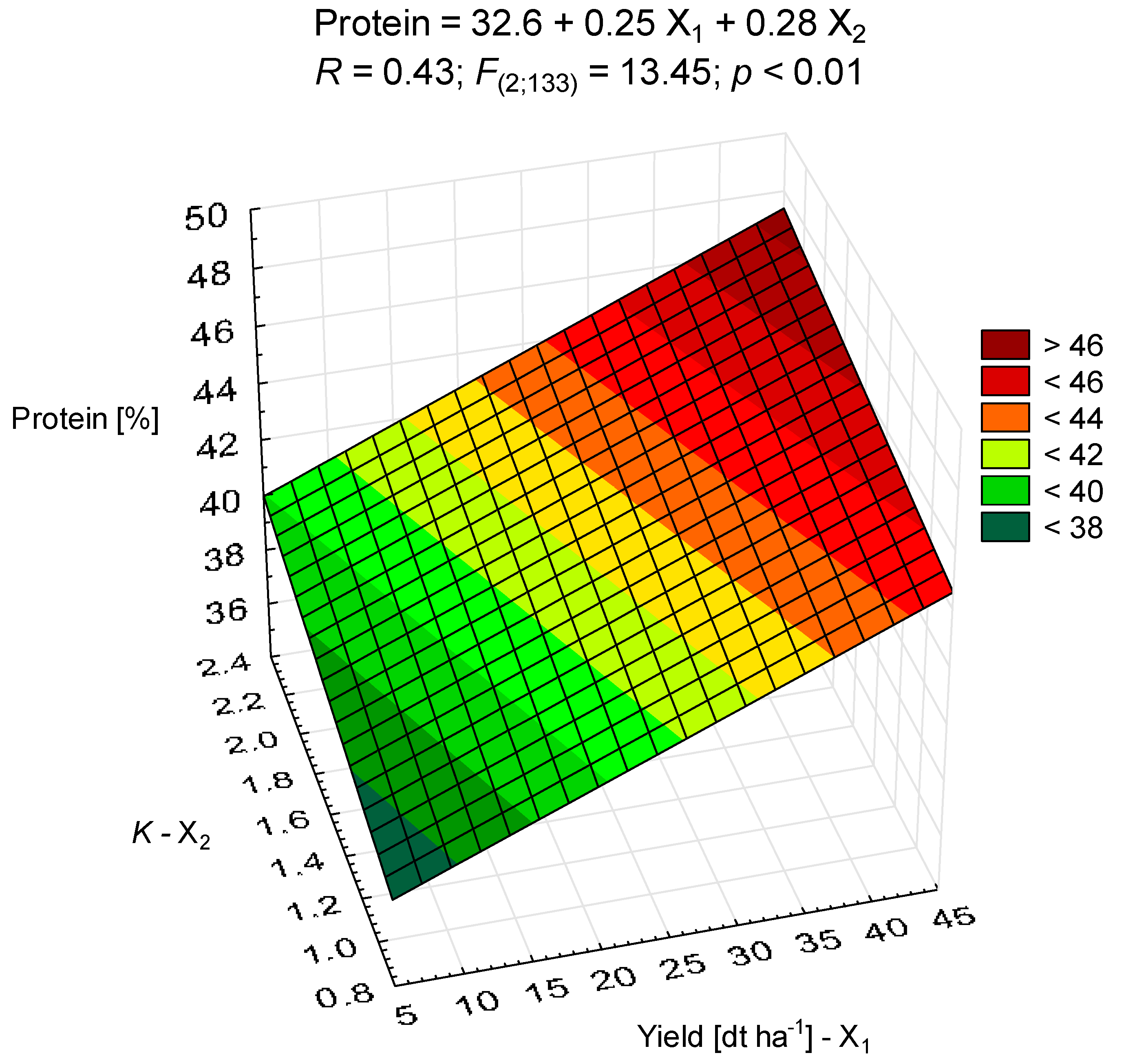
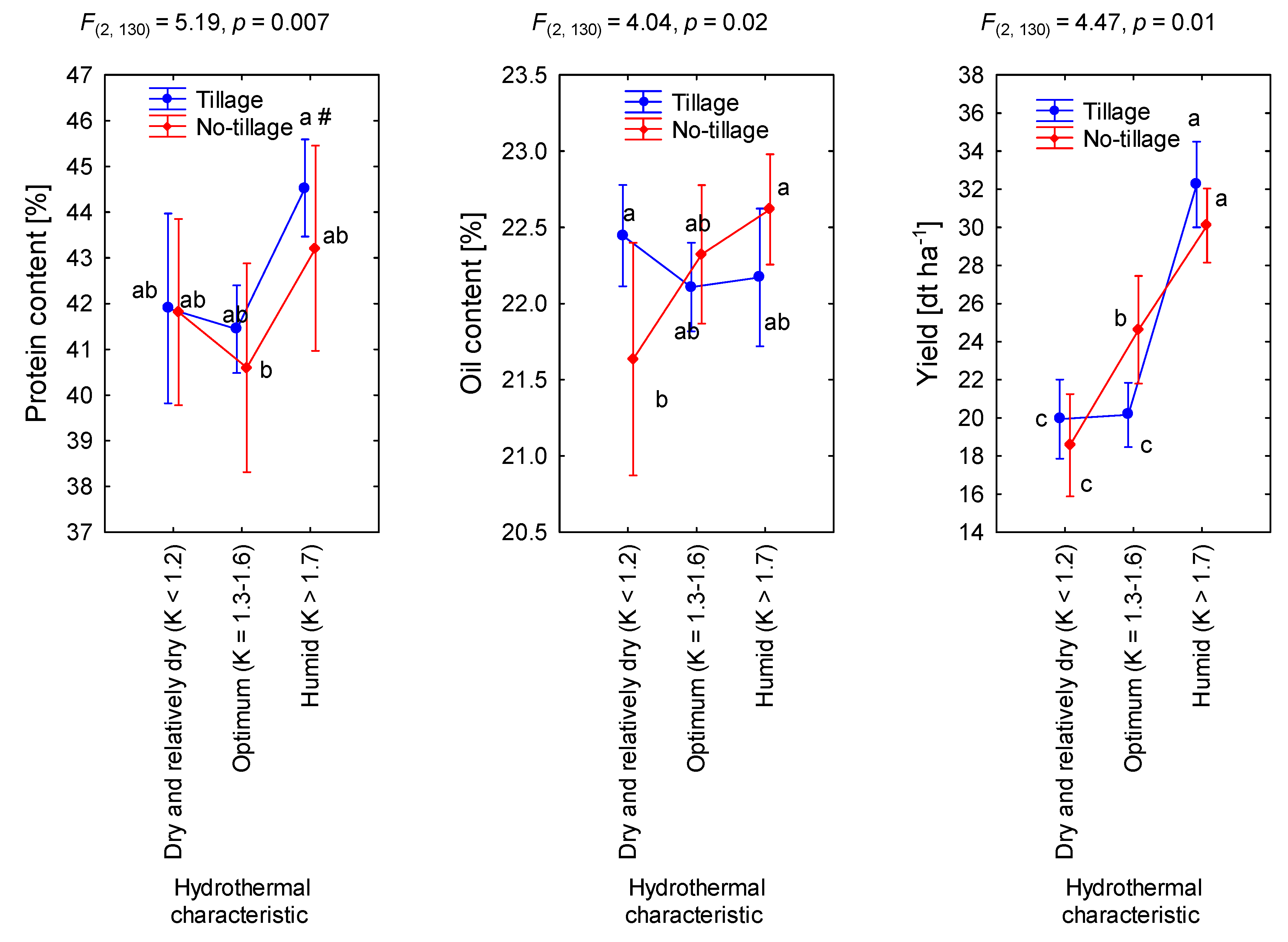

| Site | Latitude | Longitude | Particles (%) | Texture | N 1 | P | K | OM | pH | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silit | Clay | |||||||||
| Mochełek | 53°20′ N | 17°86′ E | 84.7 | 14.3 | 1.1 | Loamy sand | 0.17 | 238 | 216 | 1.8 | 6.6 |
| Minikowo | 52°21′ N | 16°56′ E | 80.5 | 17.6 | 1.9 | Loamy sand | 0.45 | 185 | 235 | 1.6 | 6.9 |
| Grocholin | 52°99′ N | 17°42′ E | 56.0 | 23.0 | 21.0 | Sandy (clay) loam | 0.70 | 182 | 197 | 1.2 | 6.2 |
| Rakowo | 53°52′ N | 19°20′ E | 64.1 | 33.5 | 2.4 | Sandy loam | 0.12 | 158 | 196 | 1.4 | 6.8 |
| Witrogoszcz | 53°15′ N | 17°15′ E | 82.7 | 15.5 | 2.8 | Loamy Sand | 0.54 | 178 | 216 | 1.4 | 6.7 |
| Jędrzejewo | 52°51′ N | 16°36′ E | 58.5 | 35.6 | 5.9 | Sandy loam | 0.32 | 167 | 234 | 2.2 | 6.8 |
| Cultivar | Origin | Years of Study | Earliness Group | Sites of Study # | Vegetation (VPD) |
|---|---|---|---|---|---|
| Abaca | AT | 2019, 2020 | 1st (0000) | G, Mo, J, R, W | 130–135 |
| Abelina | AT | 2018, 2019, 2020 | 2nd (000+) | G, J, Mo, Mn, R, W | 135–144 |
| Aligator | FR | 2018, 2019, 2020 | 4th (00) | J, R, W | 144–152 |
| Amarok | DE | 2018, 2019, 2020 | 3rd (000) | G, Mo | 140–146 |
| Annushka | UA | 2018, 2019 | 1st (0000) | W, Mo, G | 100–130 |
| Anser | RU | 2018, 2019, 2020 | 3rd (000) | G, Mn, R, W, J | 130–141 |
| Augusta | PL | 2018, 2019, 2020 | 1st (0000) | Mo, Mn, G | 120–127 |
| Aurelina | AT | 2019, 2020 | 4th (00) | Mo, Mn, R, W | 142–151 |
| Bohemians | CZ | 2018, 2019, 2020 | 3rd (000) | G, Mo, J, R, W | 135–142 |
| Comandor | FR | 2018, 2019, 2020 | 4th (00) | G, Mo, J | 140–151 |
| Erica | PL | 2018, 2019, 2020 | 2nd (000+) | G, Mo, Mn | 127–138 |
| Favorit | RU | 2018, 2019, 2020 | 4th (00) | G, Mo, Mn | 147–155 |
| Galice | CH | 2018, 2019 | 4th (00) | G, Mo | 145–154 |
| Mavka | PL | 2018, 2020 | 4th (00) | W, Mn | 142–145 |
| Mayrika | CZ | 2018, 2019 | 2nd (000+) | G, Mo, J, W, R | 130–143 |
| Merlin | AT | 2018, 2019 | 3rd (000) | J, Mo | 128–135 |
| Obelix | AT | 2018, 2019 | 4th (00) | G, J, Mo, W, R | 138–146 |
| Silesia | CZ | 2018, 2019 | 4th (00) | G, J, Mo, W | 140–150 |
| Sirelia | AT | 2018, 2019, 2020 | 4th (00) | G, Mo, Mn | 136–149 |
| Violetta | PL | 2018, 2019 | 3rd (000) | R, W | 134–140 |
| Site | Year | Pre-Crop | Soil Cultivation | Sowing Date | Emergency DAS # | Herbicides 1 | Fungicide 2 | Insecticide 3 |
|---|---|---|---|---|---|---|---|---|
| Mochełek | 2018 | Triticale | Tillage | 05/04 | 20 | I-Met., II-Bent. + Imaz. | - | - |
| 2019 | Wheat | Tillage | 05/05 | 23 | I-Met. + Fluf. | Thioph.-methyl | Acet. | |
| 2020 | Triticale | Tillage | 05/02 | 24 | I-Pend., Bent. + Imaz. | - | Acet. | |
| Minikowo | 2018 | Seed corn | Tillage | 05/07 | 19 | I-Met., II-Bent. + Imaz. | - | - |
| 2019 | Seed corn | Tillage | 05/02 | 24 | I-Met., II-Bent. + Imaz. | Thioph.-methyl | Acet. | |
| 2020 | Triticale | Tillage | 05/08 | 24 | I-Pend., II- Bent. + Imaz. | Thioph.-methyl | Acet. | |
| Grocholin | 2018 | Seed corn | Tillage | 05/02 | 18 | I-Met., II-Bent. + Imaz. | Thioph.-methyl | Acet. |
| 2019 | Wheat | Tillage | 04/30 | 22 | I-Met., II-Bent. + Imaz. | Thioph.-methyl | Acet. | |
| 2020 | Wheat | Tillage | 04/31 | 16 | I-Pend., II- Bent. + Imaz. | Thioph.-methyl | Acet. | |
| Rakowo | 2018 | Seed corn | No-tillage | 05/09 | 16 | I-Met., II-Bent. + Imaz. | - | - |
| 2019 | Seed corn | No-tillage | 05/07 | 24 | I-Met. + Fluf., II-Clet. +Bent. + Imaz. | Thioph.-methyl | Acet. | |
| 2020 | Seed corn | No-tillage | 05/08 | 14 | I-Pend., II- Bent. + Imaz. | Thioph.-methyl | Acet. | |
| Witrogoszcz | 2018 | Barley | No-tillage | 05/08 | 25 | I-Met., II-Clet. + Bent. + Imaz. | - | - |
| 2019 | Silage corn | No-tillage | 05/07 | 15 | I-Met. + Fluf., II-Clet. +Bent. + Imaz. | Thioph.-methyl | Acet. | |
| 2020 | Triticale | No-tillage | 05/05 | 13 | I-Pend., II- Bent. + Imaz. | - | - | |
| Jędrzejewo | 2018 | Rape | No-tillage | 05/04 | 16 | I-Met., II-Bent. + Imaz. | - | - |
| 2019 | Barley | No-tillage | 04/30 | 30 | I-Met. + Fluf., II- II-Clet. +Bent. + Imaz. | - | Acet. | |
| 2020 | Wheat | No-tillage | 05/02 | 14 | I-Pend., II- Bent. + Imaz. | - | - |
| Month | Precipitation (mm) | Temperature (°C) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mochełek | Minikowo | Grocholin | Rakowo | Witrogoszcz | Jędrzejewo | Mochełek | Minikowo | Grocholin | Rakowo | Witrogoszcz | Jędrzejewo | |||
| 2018 | ||||||||||||||
| April | 40.0 | 36.4 | 71.0 | 46.0 | 15.0 | 46.0 | 12.0 | 12.0 | 11.1 | 9.9 | 11.8 | 11.2 | ||
| May | 14.2 | 9.8 | 5.5 | 30.0 | 7.0 | 8.0 | 16.9 | 17.0 | 17.0 | 16.8 | 16.5 | 16.8 | ||
| June | 26.7 | 19.2 | 21.0 | 40.0 | 16.0 | 38.0 | 18.4 | 18.3 | 17.8 | 18.5 | 17.6 | 19.2 | ||
| July | 86.0 | 34.6 | 44.0 | 15.0 | 70.0 | 86.0 | 20.5 | 20.2 | 20.7 | 20.7 | 20.2 | 20.1 | ||
| August | 23.7 | 20.8 | 31.5 | 28.0 | 15.0 | 42.0 | 19.9 | 20.2 | 20.6 | 18.6 | 19.3 | 19.5 | ||
| September | 17.0 | 11.4 | 32.5 | 34.5 | 26.5 | 76.5 | 15.6 | 15.5 | 16.7 | 16.0 | 16.3 | 15.8 | ||
| Sum | 207.6 | 132.2 | 205.5 | 193.5 | 149.5 | 296.5 | Mean | 17.2 | 17.2 | 17.3 | 16.8 | 17.0 | 17.1 | |
| 2019 | ||||||||||||||
| April | 1.5 | 4.2 | 9.0 | 30.0 | 45.0 | 5.0 | 9.3 | 9.3 | 9.1 | 8.5 | 8.7 | 8.9 | ||
| May | 89.2 | 58.2 | 86.5 | 95.0 | 55.0 | 39.0 | 12.1 | 12.0 | 13.5 | 14.1 | 15.0 | 14.6 | ||
| June | 17.7 | 19.2 | 33.5 | 65.0 | 75.0 | 21.0 | 21.9 | 21.6 | 22.0 | 21.5 | 22.0 | 22.3 | ||
| July | 22.4 | 16.8 | 45.0 | 50.0 | 15.0 | 52.5 | 18.6 | 18.5 | 19.0 | 19.3 | 19.2 | 18.9 | ||
| August | 37.7 | 36.2 | 35.5 | 61.0 | 1.5 | 45.0 | 19.7 | 19.7 | 20.2 | 20.3 | 19.9 | 20.5 | ||
| September | 98.5 | 72.6 | 76.5 | 45.0 | 19.5 | 87.0 | 13.5 | 13.2 | 14.2 | 14.2 | 13.9 | 14.6 | ||
| Sum | 267.0 | 207.2 | 286.0 | 346.0 | 211.0 | 249.5 | Mean | 15.9 | 15.7 | 16.3 | 16.3 | 16.5 | 16.6 | |
| 2020 | ||||||||||||||
| April | 0.7 | 3 | 13.4 | 56.5 | 50.6 | 26.7 | 8.2 | 8.2 | 7.9 | 7.5 | 8.0 | 8.1 | ||
| May | 34.6 | 23.8 | 35.6 | 76.8 | 67.4 | 98.6 | 10.9 | 10.8 | 10.8 | 10.9 | 11.0 | 10.8 | ||
| June | 153.9 | 114 | 123.0 | 95.4 | 87.6 | 123 | 17.9 | 17.7 | 17.6 | 17.5 | 18.0 | 17.9 | ||
| July | 85.1 | 78.6 | 65.0 | 75.6 | 55.4 | 65.7 | 18.0 | 18.0 | 17.9 | 18.1 | 17.7 | 18.1 | ||
| August | 90.0 | 60.4 | 76.8 | 65.4 | 78.8 | 59.5 | 19.2 | 19.3 | 19.5 | 19.3 | 19.0 | 19.1 | ||
| September | 71.2 | 70.4 | 67.5 | 56.9 | 67.7 | 89 | 14.4 | 14.6 | 15.3 | 14.2 | 14.3 | 14.4 | ||
| Sum | 435.5 | 347.2 | 367.9 | 370.1 | 356.9 | 435.8 | Mean | 16.1 | 16.1 | 16.2 | 16.0 | 16.0 | 16.1 | |
| Study Site | 2018 | Classification | 2019 | Classification | 2020 | Classification |
|---|---|---|---|---|---|---|
| Mochełek | 0.8 | Dry | 1.5 | Optimal humid | 2.2 | Humid |
| Minikowo | 0.4 | Dry | 1.2 | Rel. dry | 1.7 | Optimal humid |
| Grocholin | 0.6 | Dry | 1.5 | Optimal humid | 1.9 | Humid |
| Rakowo | 0.7 | Dry | 1.5 | Optimal humid | 2.0 | Humid |
| Witrogoszcz | 0.6 | Dry | 0.8 | Dry | 1.9 | Humid |
| Jędrzejewo | 1.1 | Rel. dry | 1.2 | Rel. dry | 2.4 | Humid |
| Source of Variation | df | Yield | Protein | Oil |
|---|---|---|---|---|
| Year | 2 | 4446 *** | 130.1 *** | 0.98 |
| Site | 5 | 430.9 *** | 19.4 * | 1.33 |
| Cultivar | 19 | 151.4 *** | 25.6 * | 1.72 * |
| Year × site | 10 | 33.1 *** | 12.8 | 0.75 |
| Year × cultivar | 25 | 33.3 *** | 29.7 * | 1.19 |
| Site × cultivar | 32 | 46.3 *** | 1.26 | 0.84 |
| Residual | 314 | 4.07 | 12.6 | 0.68 |
| Cultivar | Yield (dt ha−1) | Protein (%) | Oil (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |
| Earliness group 1st (0000) | |||||||||
| Abaca | - | 22.4 ± 1.75 | 32.6 ± 1.30 | - | 39.6 ± 3.95 | 42.8 ± 1.45 | - | 21.3 ± 0.10 | 21.8 ± 1.30 |
| Annushka | 9.25 ± 1.58 | 12.8 ± 0.45 | - | 37.6 ± 0.24 | 44.1 ± 1.45 | - | 20.7 ± 0.85 | 22.4 ± 0.45 | - |
| Augusta | 13.7 ± 1.77 | 12.7 ± 0.73 | 24.1 ± 0.72 | 38.4 ± 0.25 | 40.4 ± 2.80 | 45.8 ± 0.60 | 21.7 ± 1.77 | 23.5 ± 1.05 | 21.5 ± 0.45 |
| Earliness group 2nd (000+) | |||||||||
| Abelina | 24.8 ± 2.96 | 22.6 ± 1.74 | 33.1 ± 0.44 | 43.0 ± 1.64 | 40.6 ± 1.93 | 44.3 ± 0.59 | 23.1 ± 0.22 | 22.9 ± 0.32 | 22.1 ± 0.20 |
| Erica | 19.8 ± 2.06 | 13.5 ± 2.72 | 28.5 ± 1.52 | 44.9 ± 2.23 | 39.5 ± 0.83 | 46.0 ± 0.40 | 21.3 ± 0.12 | 22.0 ± 1.17 | 22.8 ± 0.23 |
| Mayrika | 25.6 ± 0.93 | 19.2 ± 1.16 | - | 41.0 ± 1.18 | 43.5 ± 0.94 | - | 21.9 ± 0.93 | 21.7 ± 0.26 | - |
| Earliness group 3rd (000) | |||||||||
| Amarok | 22.8 ± 3.80 | 16.8 ± 1.40 | 32.2 ± 1.05 | 38.4 ± 0.50 | 44.1 ± 1.20 | 45.1 ± 1.20 | 21.9 ± 0.05 | 20.5 ± 0.50 | 21.8 ± 0.50 |
| Anser | 20.7 ± 1.70 | 16.3 ± 0.52 | 33.1 ± 1.56 | 38.5 ± 0.50 | 40.3 ± 2.45 | 45.9 ± 0.65 | 22.1 ± 0.10 | 21.7 ± 0.45 | 22.2 ± 0.90 |
| Bohemians | 24.5 ± 3.36 | 20.1 ± 3.32 | 34.0 ± 0.95 | 47.1 ± 2.55 | 40.3 ± 2.67 | 46.2 ± 0.95 | 22.7 ± 0.70 | 22.9 ± 0.96 | 22.4 ± 0.65 |
| Merlin | 22.6 ± 2.87 | 18.9 ± 5.20 | - | 41.5 ± 0.55 | 39.4 ± 2.80 | - | 22.6 ± 0.50 | 23.1 ± 0.85 | - |
| Violetta | 22.9 ± 1.15 | 15.1 ± 0.25 | - | 43.2 ± 1.45 | 36.8 ± 1.15 | - | 21.9 ± 0.56 | 22.2 ± 0.95 | - |
| Earliness group 4rd (00) | |||||||||
| Aligator | 31.3 ± 0.87 | 28.7 ± 3.25 | 35.5 ± 0.95 | 42.3 ± 0.80 | 42.9 ± 2.60 | 43.5 ± 1.30 | 23.5 ± 0.10 | 22.3 ± 1.05 | 22.2 ± 1.00 |
| Aurelina | - | 15.5 ± 0.50 | 30.9 ± 2.85 | - | 37.9 ± 0.10 | 44.9 ± 0.70 | - | 22.9 ± 0.95 | 22.3 ± 0.80 |
| Comandor | 22.4 ± 1.10 | 19.0 ± 3.52 | 33.4 ± 0.79 | 38.9 ± 0.57 | 41.3 ± 1.69 | 44.7 ± 1.47 | 21.7 ± 0.20 | 22.6 ± 0.67 | 22.6 ± 0.27 |
| Favorit | 21.5 ± 0.96 | 16.4 ± 4.42 | 34.7 ± 3.63 | 42.8 ± 3.26 | 37.6 ± 0.10 | 43.9 ± 0.33 | 22.2 ± 0.60 | 21.2 ± 0.61 | 21.9 ± 0.59 |
| Galice | 23.3 ± 1.78 | 16.1 ± 0.18 | - | 36.0 ± 0.55 | 41.6 ± 1.65 | - | 23.1 ± 0.30 | 22.9 ± 0.45 | - |
| Mavka | 22.2 ± 1.80 | - | 31.2 ± 1.15 | 44.3 ± 1.10 | - | 44.9 ± 0.95 | 19.9 ± 1.25 | - | 19.5 ± 0.65 |
| Obelix | 22.2 ± 2.00 | 19.6 ± 1.56 | - | 40.8 ± 1.97 | 40.4 ± 1.66 | - | 22.4 ± 0.20 | 21.6 ± 0.41 | - |
| Silesia | 24.4 ± 0.91 | 16.2 ± 2.80 | - | 46.3 ± 2.08 | 40.8 ± 1.90 | - | 22.5 ± 0.55 | 22.6 ± 0.29 | - |
| Sirelia | 26.3 ± 2.93 | 17.1 ± 2.34 | 31.5 ± 0.92 | 43.6 ± 3.44 | 44.0 ± 0.80 | 45.4 ± 1.13 | 23.4 ± 0.25 | 21.3 ± 0.23 | 22.7 ± 0.32 |
| Characteristic | Tillage | No-Tillage | ||||
|---|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | p | Cluster 1 | Cluster 2 | p | |
| VPD (day) | 128.3 ± 3.33 | 143.8 ± 1.31 | <0.01 | 131.2 ± 6.55 | 140.2 ± 2.06 | <0.05 |
| Yield (dt ha−1) | 19.5 ± 2.07 | 22.0 ± 0.84 | <0.01 | 18.5 ± 2.49 | 27.3 ± 1.34 | <0.01 |
| Oil (%) | 22.1 ± 0.15 | 22.3 ± 0.17 | 0.42 | 21.2 ± 0.45 | 22.6 ± 0.10 | <0.01 |
| Protein (%) | 41.2 ± 0.55 | 42.2 ± 0.53 | 0.23 | 42.3 ± 1.33 | 41.9 ± 0.66 | 0.83 |
| Cultivars | Abaca Annushka, Anser, Augusta, Erica, Mayrika, Merlin, | Abelina, Amarok, Aurelina Bohemians, Comandor, Favorit, Galice, Mavka, Obelix, Silesia, Sirelia, | Annushka, Mavka, Mayrika, Violetta, | Abelina, Aligator Bohemians, Comandor, Merlin, Obelix, Silesia, | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenda-Piesik, A.; Ambroziak, K. The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland. Appl. Sci. 2022, 12, 7830. https://doi.org/10.3390/app12157830
Wenda-Piesik A, Ambroziak K. The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland. Applied Sciences. 2022; 12(15):7830. https://doi.org/10.3390/app12157830
Chicago/Turabian StyleWenda-Piesik, Anna, and Krystian Ambroziak. 2022. "The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland" Applied Sciences 12, no. 15: 7830. https://doi.org/10.3390/app12157830
APA StyleWenda-Piesik, A., & Ambroziak, K. (2022). The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland. Applied Sciences, 12(15), 7830. https://doi.org/10.3390/app12157830






