Abstract
Application of biostimulant in the form of a spraying requires appropriate selection of technical parameters. One of the key factors in ensuring that the liquid is sprayed correctly is the choice of suitable spray nozzles. This study investigated selected technical parameters of seaweed biostimulant spray applications as a factor for plant biometrics, crop quality and yield, and the economic viability of production. This objective was achieved by conducting a two-year field experiment involving spraying soybean plants with a biostimulant containing seaweed extract. The spraying was carried out with two types of nozzles (standard and air-injector), for which the droplet size and the degree of plant coverage were tested at 4.0 MPa. The surface tension of the spray liquid and the area and volume of the droplets were tested. The height of soybean plants, the height and the position of the first pod, the number of pods, seed yield, and the weight of 1000 seeds were measured. The physical properties of the liquid sprayed on the plants and the type of nozzles used were factors affecting the height of the first pods, plant height, and protein content. The application of biostimulants using standard nozzles is more economically advantageous compared to nozzles with an air-injector.
1. Introduction
Crop spraying is one of the most commonly used treatments in crop production. The selection of appropriate technical parameters of the spray treatment is very important and should be matched to the substance we apply to the plants [1,2,3]. This is vital especially during development of sustainable food production systems and is a crucial issue for protecting the environment while providing healthy food. As agricultural land shrinks due to climate change and increasing urbanisation, and as we approach the limits of the genetic potential of crops, research has been carried out to find new cultivation technologies and make existing ones more effective [4]. One way to improve the economic viability of crop production is to use plant-based biostimulants to enhance plant protection against biotic and abiotic stress. The application of biostimulants has been shown to improve—or at least stabilise, in adverse conditions—crop quality and yield [5].
Under Regulation (EU) 2019/1009, biostimulants are defined as “products that stimulate plant nutritional processes regardless of the nutrient content of the product, whose sole purpose is to improve one or more of the following characteristics of the plant or its rhizosphere: nutrient use efficiency, abiotic stress tolerance, quality characteristics, availability of limited nutrients in the soil or rhizosphere” [6].
According to the new definition, biostimulants are considered to be new modulators of plant life processes, adding to the effects provided by fertilizers, growth regulators, and pesticides. The underlying mechanisms of biostimulants and their bioactive compounds consist of simple, direct correlations between nutrient uptake and the effects of single plant hormones. The defining feature of these products is that they eliminate the nutritious effect in plants. It involves using a low dosage of the preparation, which is usually higher for most nutrients [7].
Extensive research has been conducted into the use of biostimulants in agriculture and horticulture. Numerous studies have found that there are differences in how individual plant species, and even varieties, respond to biostimulants [8,9,10,11,12,13]. The use of seaweed biostimulants has been investigated extensively in recent years. Field and pot experiments have confirmed the positive effects of seaweed extract on metabolism and morphological traits, as well as plant yields [1,14,15,16,17,18,19,20]. Biostimulant studies rely primarily on soil or foliar application. It is usually more advisable to employ the latter method since soil application requires substantial amounts of both the biostimulant and water.
Application in the form of spraying requires the proper selection of technical parameters [21]. One of the key factors in ensuring that the liquid is sprayed correctly is the choice of suitable spray nozzles [22]. Spray nozzles—as well as their condition and working pressure—are critical for spraying quality, which, in turn, plays a fundamental role in the effectiveness of various agrochemicals, including biostimulants, and—in the case of pesticides—on the degree of environmental pollution [2,23,24,25,26]. While natural biostimulants cause no environmental pollution concerns, they pose significant problems with spray drift, which affects the amount and distribution uniformity of the liquid [27,28,29]. Spray nozzle type is a considerable factor in spray drift [30,31,32]. Spray drift reduces active substance coverage expressed as droplet count and weight per unit of plant surface area, or as plant surface coverage (%). Active substance coverage is a determining factor in the biological efficacy of spray application [33,34]. One way to reduce spray drift is to use air-injector nozzles. Unlike standard flat-spray nozzles, they provide a significant reduction in drift. However, these nozzles also have a negative impact on plant coverage [35,36]. Air-injector nozzles may cause runoff from plants, compromising the biological efficacy of the preparation. Flowing natural biostimulants from plants do not cause soil pollution. However, it may reduce their effectiveness. Therefore, it was hypothesised that the technical parameters of the biostimulant spray are determinants of soybean seeds’ yield and biometric traits of plants. Accordingly, this study investigated selected technical parameters of seaweed biostimulant spray application as a factor for plant biometrics, crop quality and yield, and the economic viability of production.
2. Materials and Methods
2.1. Field Experiments
Field experiments were conducted at the Experimental Farm of the University of Life Sciences in Lublin, which is located in Czesławice, Lublin Province. Soybean Abelina cv. was cultivated in 2020 and 2021. The experiment makes use of randomised-block design with four repeated measures on 20 m2 experimental plots. Seeds were sown on 10 May 2020 and 10 May 2021, with row spacing of 30 cm and in-row spacing of 4.0 cm. The Kelpak SL biostimulant was applied to the plants in each growing season by double spray application in phases BBCH 14-15, i.e., a minimum of three-leaf stage, and BBCH 61, i.e., at the beginning of blooming. The biostimulant was dissolved in tap water at a concentration of 1%, with an operating dose of 300 L·ha−1. Soybean plants sprayed with water were used as controls. Spraying was performed with an AgroMax P161 tractor sprayer at a pressure of 4.0 bar and with an operating speed of 4.8 km h−1. Two types of spray nozzles were employed: Agroplast 120 03 (AP12003) standard nozzle (Figure 1a) and Agroplast 6MSC (6MS03C) air-injector nozzle (Figure 1b).

Figure 1.
Nozzles tested: (a) AP12003; (b): 6MS03C.
2.2. Droplet Size and Coverage
Plant coverage was measured with water-sensitive papers placed on soybean plant leaves. The coverage analysis was performed with the use of water-sensitive papers and Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD, USA). The degree of plant coverage was measured during the treatments. The droplet size and spectrum were measured with a HELOS/R laser diffractometer (Sympatec GmbH, Clausthal-Zellerfeld, Germany) with a measuring range of R3 to R7 (0.1–8750 μm).
2.3. Physical Properties of the Spray Liquid
The surface tension was tested on a Drop Shape Analyzer (DSA30 KRÜSS GmbH, Hamburg, Germany) using the hanging drop method. Drops were dispensed by the device controlling the program. Then, using the input data, the program automatically determined the contours of the hanging drop shape and calculated the surface tension according to the Young–Laplace equation. For each liquid sample analysed, 40 measurements were taken at 25 °C.
2.4. Yield, Biometrics, and Economic Viability
In phase BBCH 89 (mature pods) (Figure 2), the plants were harvested, and plant height, first pod height, pod count, seed yield, and 1000 seed weight were determined. Protein content and fat content were measured. Protein content was determined using the Kjedahl method by multiplying the percentage of nitrogen by a factor 6.25 [37], and fat content was determined using acid hydrolysis (Extraction System B-811, BÜCHI Labortechnik AG, Flawil, Switzerland) [37].

Figure 2.
Soybeans at harvest stage BBCH 89.
The economic effect of biostimulant application was calculated based on the value of the resulting yield increase and the cost of biostimulant application [38]. The value of the yield increase (EUR·ha−1) was evaluated as a product of the average price of soybean seeds in a given study year and the difference between the seed yield from the combination with the biostimulant and the seed yield from the control combination. The costs of the treatment with the biostimulant (EUR·ha−1) were computed as a sum cost of purchase of the extracts, cost of water used for the treatment, and cost of performing the treatment. The average price for the seeds was determined from market offers (363.64 EUR·t−1 in 2020 and 510.64 EUR·t−1 in 2021). The cost of Kelpak biostimulant (12.50 EUR·L−1 in 2020 and 12.77 EUR·L−1 in 2021) was taken as the average price from the 3 stores offering it. The cost of water was the average price of 1 m3 (2.35 EUR·m−3) in Lublin Province. The cost of the procedure was the average price of the plant spraying service (14.15 EUR·ha−1). Revenue from cultivation was calculated as the product of the yield and the price of soybeans in a given research year. The change in revenue was calculated as the ratio of the revenue from the cultivation with biostimulant application to the revenue from the control.
2.5. Statistical Analysis
The results were subjected to statistical treatment using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). The Shapiro–Wilk test was performed to test for normal distribution. Tukey’s range test was employed to estimate the significance of differences between mean values, with a significance level of p < 0.05.
3. Results and Discussion
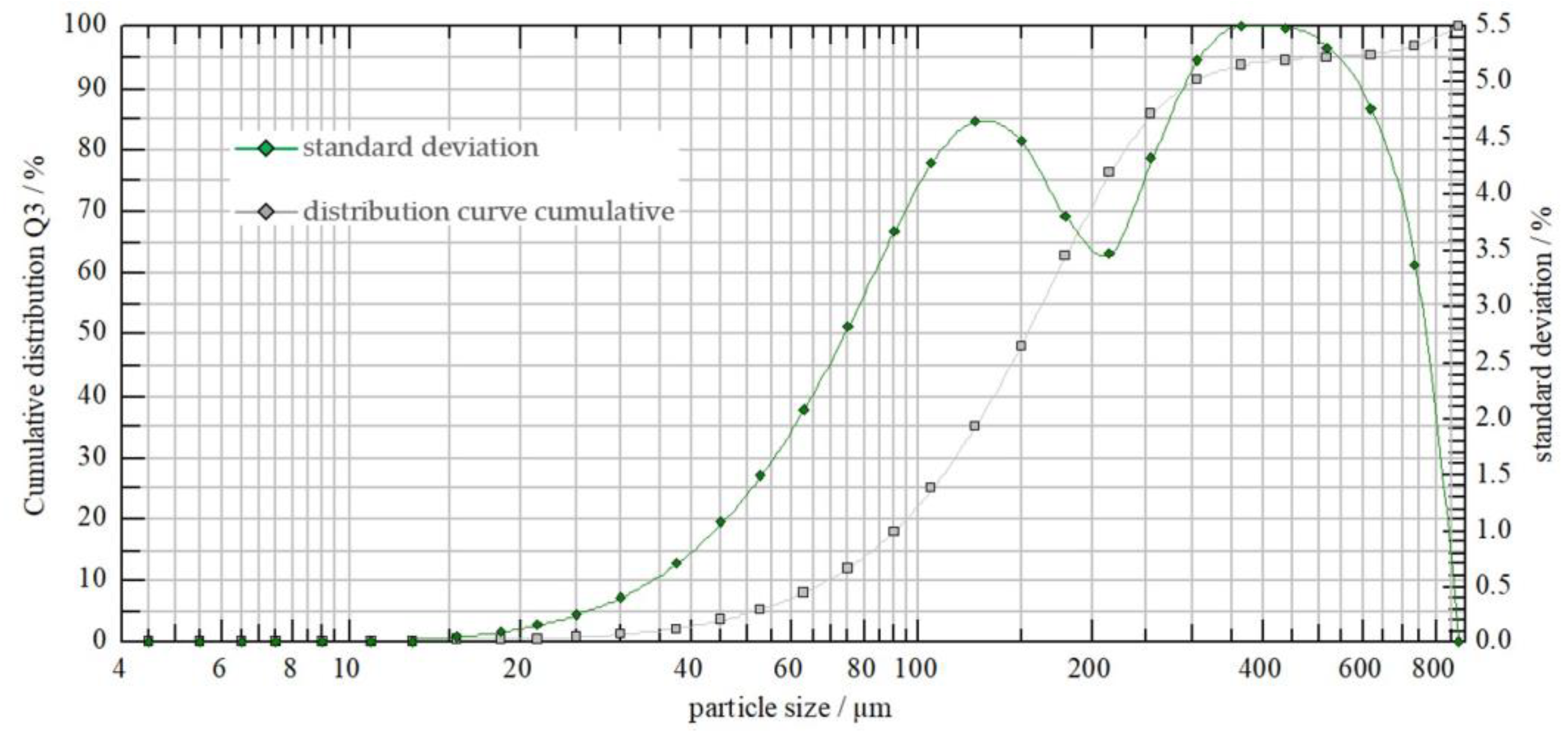
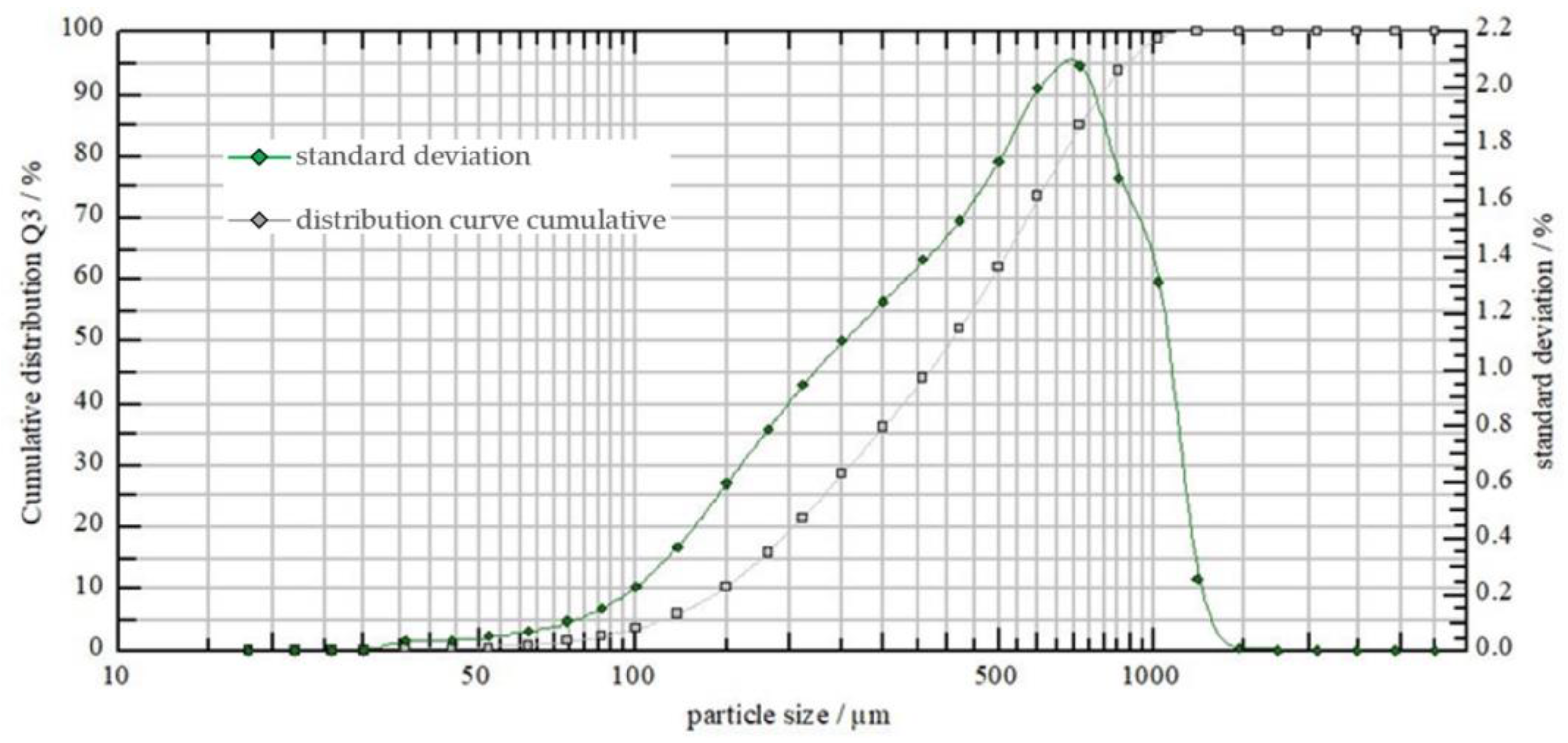
Consistent with the assumptions, droplet spectrum measurements demonstrated differences in droplet count and size between the two types of nozzles (Figure 3 and Figure 4). At a liquid pressure of 0.40 MPa, the AP12003 sprayer produced finer droplets compared to the 6MS03C sprayer.
Figure 3.
AP12003 sprayer’s droplet spectrum at a pressure of 0.40 MPa.
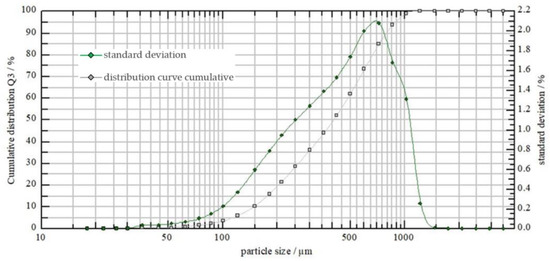
Figure 4.
6MS03C sprayer’s droplet spectrum at a pressure of 0.40 MPa.
Significant differences were found between both concentrations in plant coverage with the seaweed extract biostimulant (Table 1). Air-injector nozzle sprayers provided smaller plant coverage. Plants sprayed with tap water had a smaller liquid coverage compared to those sprayed with the biostimulant. Although no significant coverage differences were identified between concentrations, a higher biostimulant concentration could be seen to increase plant coverage. This is attributable to the reduced surface tension caused by the biostimulant’s presence in the water. Significant differences in surface tension were identified between tap water and the mixture of water and seaweed biostimulant (Table 1). Differences were also noted in droplet area and droplet volume (not the dosed volume). The surface tension is a factor in the droplet spectrum, which, in turn, plays a role in plant coverage. The role of the surface tension, among other factors, in plant coverage was investigated by Yu et al. [39]. They found that a reduction in the surface tension could increase plant wetness. Xu et al. [40] came to a similar conclusion, demonstrating that the surface tension influenced plant wetness. The positive effects of the reducing surface tension noted in these studies have been corroborated by other studies [41], which found changes in droplet behaviour on the leaf surface, potentially improving plant coverage and distribution uniformity [42].

Table 1.
Physical properties of the working liquid and plant coverage.
An important factor in the mechanical harvesting of soybean is the first pod height. Harvester-caused losses are smaller when the first pod is higher. The present study involving the application of a seaweed biostimulant with two types of spray nozzles revealed that both standard (AP12003) and air-injector (6MS03C) nozzles had a positive effect on first pod height (Table 2). Although no significant differences in first pod height were found between the two types of nozzles, it was noted that, with standard-nozzle application, the height was 19% greater, on average, than with air-injector nozzle application. Studies by Szparaga et al. [10] showed that first-pod height is influenced by plant height, probably due to the increased internode distances on the stem. Tanase et al. [43] had similar observations. They found that improved soybean height could be attributed to the application of biostimulants containing, among others, phenolic compounds.

Table 2.
Soybean biometrics (mean ± SD).
The two-year study described here has demonstrated that there is a significant correlation between the biostimulant application technique and plant height. Plants sprayed with the biostimulant using standard nozzles (AP12003) were 9 percent taller, on average, than plants sprayed using air-injector nozzles. It is important to stress here that both types of application led to a substantial increase in plant height relative to the control combination (spraying with tap water). Research corroborating the positive effects of extracts from various seaweeds on soybean growth spans more than a dozen years. Multiple studies have proven that the application of seaweed extracts increases plant height by a dozen to several dozen percent, mainly as a function of concentration [34,35,36,37,38,39,40,41,42,43,44,45,46].
The two-year study presented here found that biostimulant application led to an increase in pod count regardless of the nozzle used. Conversely, 1000 seed weight was found to differ between biostimulant applications using a standard nozzle and an air-injector nozzle (Table 2). These differences could be attributed to the larger quantity of pods and, consequently, seeds. Studies by Kocira et al. [47] have demonstrated that while a higher pod and seed count causes 1000 seed weight to decrease, it nevertheless has a beneficial effect on yield. The present study has corroborated this—the yield was significantly higher after biostimulant application despite a lower 1000 seed weight.
Protein concentration in soybean seeds was higher after biostimulant application using standard nozzles (Table 3). Biostimulant application with air-injector nozzles did not have a significant effect on protein content in soybean seeds. No significant differences were found in fat content between soybean seeds from plants treated with the biostimulant and plants treated with tap water (Table 3). Basile et al. [48] and Rouphael and Colla [49] have noted that this effect could be due to the modification of plant physiological processes by biostimulants.

Table 3.
Protein and fat content in soybean seeds (mean ± SD).
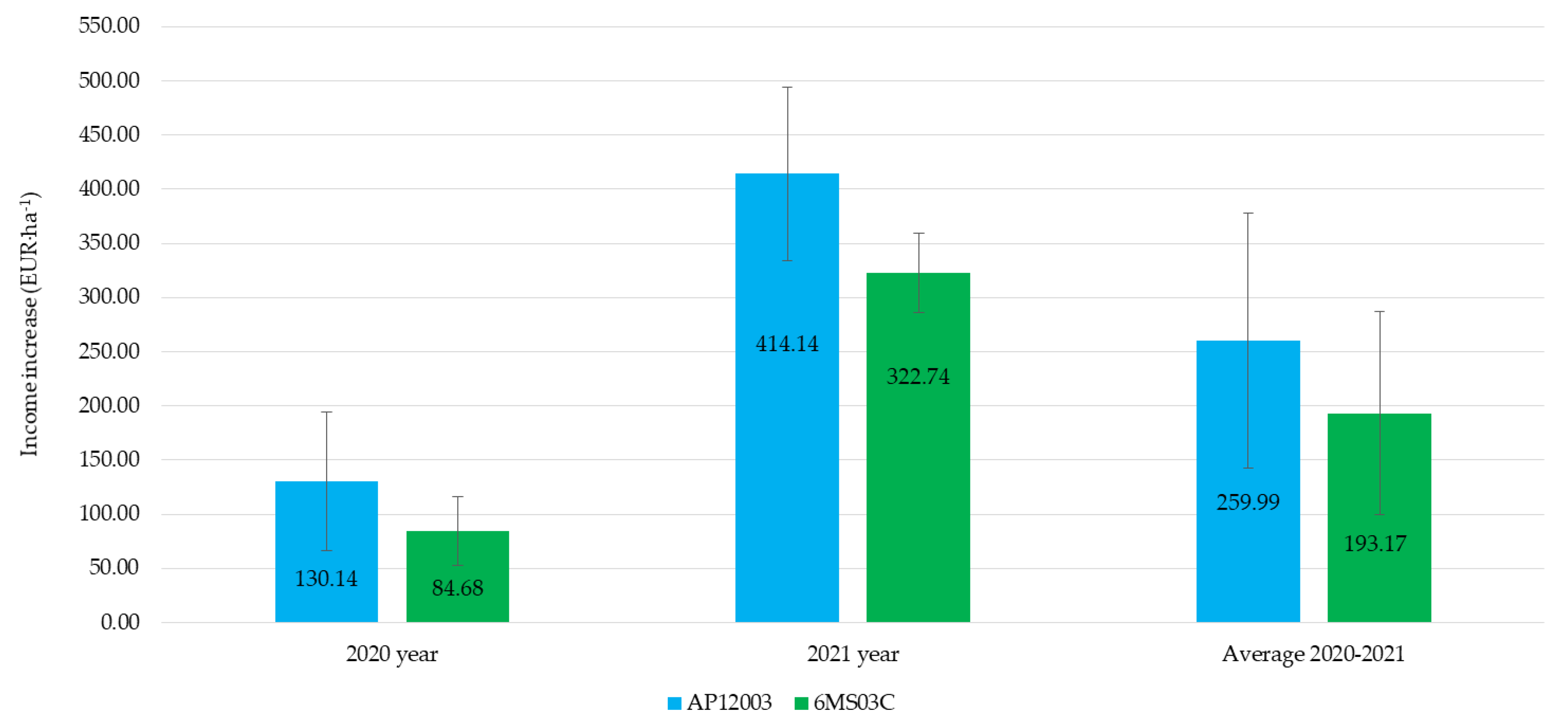
The application of a seaweed-extract biostimulant had a positive effect on economic viability (Figure 5). The average annual increase in income from soybean sales, resulting from biostimulant application using standard nozzles, in comparison to air-injector nozzle application, was 34% higher. It should be noted that the application with both types of nozzles led to an increase in income from soybean sales in each year of the study. The economic aspects of using biostimulants were addressed by Nowosad et al. [20], who concluded that the increased yield and the relatively low cost of biostimulant application were the critical advantages of this solution. Santoso et al. [50] claimed that the use of biostimulants would allow farmers to profit more from higher yields. Previous research by the authors of this study has also proven that biostimulant application in soybean and bean cultivation is an economically viable solution [10,17]. Literature on the economic effects of biostimulant application is scarce. This is probably due to the focus being more on yield quality as a factor offsetting expenditures from the use of biostimulants. Some elements of economic aspects are discussed in Zarzecka et al. [51] and Mystkowska [52].
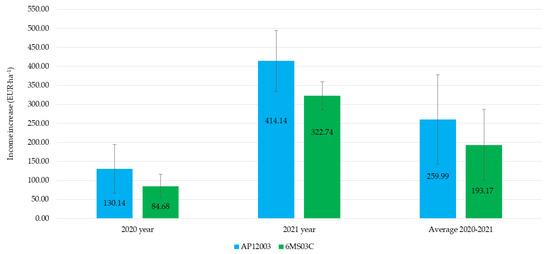
Figure 5.
The economic effects of biostimulant application using selected sprayers.
4. Conclusions
The results of the two-year experiment indicate that the application of a seaweed-extract biostimulant has a positive effect on soybean plant biometrics, seed yield, and protein content. The study found that the physical properties of the liquid sprayed on plants and the type of sprayer used were both factors for first-pod height, plant height, and protein content. Furthermore, biostimulant application using standard nozzles proved to be more economically beneficial compared to air-injector nozzles. We have concluded from our study that the physical properties of the working liquid and the type of spray nozzle—allowing the best possible plant coverage—are critical factors for plant biometrics and seed yield.
Author Contributions
Conceptualization, A.K., B.H., F.R., S.P., A.P. and S.K.; methodology, A.K., B.H., S.P., A.P. and S.K.; software, A.K., M.K., S.P. and S.K.; validation, A.K., B.H., S.P., A.P., E.L. and S.K.; formal analysis, A.K., B.H., F.R., S.P., A.P., E.L. and S.K; data curation, A.K., S.P., A.P. E.L. and S.K.; writing—original draft preparation, A.K., B.H., F.R., S.P., A.P., E.L. and S.K.; writing—review and editing, A.K., B.H., F.R., S.P., A.P., E.L. and S.K.; visualization, S.P., M.K. and S.K.; project administration, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The project is co-financed by the Polish National Agency for Academic Exchange, grant number PPN/BWA/2019/1/00011. Bilateral exchange program of scientists between the Republic of Poland and Belgium Wallonia-Brussels.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, C.; Li, S.; Wu, X.; Wang, Y.; Kang, F. Analysis of Droplet Size Uniformity and Selection of Spray Parameters Based on the Biological Optimum Particle Size Theory. Environ. Res. 2022, 204, 112076. [Google Scholar] [CrossRef]
- Griesang, F.; Ferreira, M.D.C.; Spadoni, A.B.; Della Vechia, J.F.; Santos, R.T.D.S.; dos Santos, C.A. How do the Droplet Spectrum Uniformity and Spray Volume of Flat-Fan Nozzles Influence Fungicide Spray Distribution Quality in Soybeans? Eng. Agríc 2022, 42, e20210122. [Google Scholar] [CrossRef]
- Sayinci, B.; Demir, B.; Açik, N. Comparison of Spray Nozzles in Terms of Spray Coverage and Drop Distribution Uniformity at Low Volume. Turkish J. Agric. For. 2020, 44, 262–270. [Google Scholar] [CrossRef]
- Hoek, A.C.; Malekpour, S.; Raven, R.; Court, E.; Byrne, E. Towards environmentally sustainable food systems: Decision-making factors in sustainable food production and consumption. Sustain. Prod. Consum. 2021, 26, 610–626. [Google Scholar] [CrossRef]
- Kaur, H.; Chahal, S.; Jha, P.; Pandey, D.K.; Arencibia, A.D.; Kumar, V. Biostimulants, the cinderella for plant development. In Biostimulants for Crops from Seed Germination to Plant Development; Academic Press: London, UK, 2021; pp. 61–72. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 11 December 2022).
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs). In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Gupta, S.; Hrdlička, J.; Ngoroyemoto, N.; Nemahunguni, N.K.; Gucký, T.; Novák, O.; Kulkarni, M.G.; Doležal, K.; van Staden, J. Preparation and Standardisation of Smoke-Water for Seed Germination and Plant Growth Stimulation. J. Plant Growth Regul. 2020, 39, 338–345. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological Activity of Vegetal Extracts Containing Phenols on Plant Metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Prototyping extracts from Artemisia absinthium L. for their biostimulating properties yield-enhancing, and farmer income-increasing properties. Ind. Crop. Prod. 2021, 160, 113125. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef]
- Kocira, A.; Kocira, S.; Stryjecka, M. Effect of Asahi SL application on common bean yield. Agric. Agric. Sci. Proc. 2015, 7, 103–107. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; and Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Godlewska, A.; Ciepiela, G. Carbohydrate and lignin contents in perennial ryegrass (Lolium perenne L.) treated with sea bamboo (Ecklonia maxima) extract against the background of nitrogen fertilisation regime. Appl. Ecol. Environ. Res. 2020, 18, 6087–6097. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Lucini, L. Plant biostimulants from seaweeds or vegetal proteins enhance the salinity tolerance in greenhouse lettuce by modulating plant metabolism in a distinctive manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Kocira, A.; Lamorska, J.; Kornas, R.; Nowosad, N.; Tomaszewska, M.; Leszczyńska, D.; Kozłowicz, K.; Tabor, S. Changes in Biochemistry and Yield in Response to Biostimulants Applied in Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 189. [Google Scholar] [CrossRef]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Nowosad, N.; Kocira, A.; Kornas, R. Profitability of using biostimulants in cultivation of bean (Phaseolus vulgaris L.) ‘Orzeł’. Agron. Sci. 2020, 75, 17–28. [Google Scholar] [CrossRef]
- Lodwik, D.; Pietrzyk, J.; Malesa, W. Analysis of Volume Distribution and Evaluation of the Spraying Spectrum in Terms of Spraying Quality. Appl. Sci. 2020, 10, 2395. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Parafiniuk, S. Postęp techniczny w zakresie aplikacji pestycydów. Stud. Rap. IUNG-PIB 2019, 60, 83–102. [Google Scholar]
- Nuyttens, D.; Baetens, K.; De Schampheleire, M.; Sonck, B. Effect of nozzle type, size and pressure on spray droplet characteristics. Biosyst. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- Krawczuk, A.; Parafiniuk, S.; Przywara, A.; Huyghebaert, B.; Rabier, F.; Limbourg, Q.; Mostade, O.; Kocira, S. Technical Parameters of Biostimulant Spraying a Determinant of Biometric Traits and Yield of Soybean Seeds. Agric. Eng. 2021, 25, 171–179. [Google Scholar] [CrossRef]
- Wawrzosek, J.; Parafiniuk, S. Optimization of the Opening Shape in Slot Spray Nozzles in a Field Boom Sprayer. Sustainability 2021, 13, 3291. [Google Scholar] [CrossRef]
- Griesang, F.; Spadoni, A.B.D.; Urah Ferreira, P.H.; da Costa Ferreira, M. Effect of Working Pressure and Spacing of Nozzles on the Quality of Spraying Distribution. Crop Prot. 2022, 151, 105818. [Google Scholar] [CrossRef]
- Ozkan, H.E. Reducing Spray Drift; Ohio State University Extension Service, Publication AEX: Columbus, OH, USA, 2000; pp. 816–900. [Google Scholar]
- Lodwik, D.; Pietrzyk, J. Evaluation of resistance to spray drift of selected sprayer nozzles in the aspect of non-uniformity in transverse distribution of liquid. Teka. Comm. Mot. Power Ind. Agric. 2017, 17, 65–70. [Google Scholar]
- Ozkan, H.E.; Derksen, R.C. Effectiveness of TurboDrop and Turbo TeeJet Nozzles in Drift Reduction; Ohio State University Extension Service, Publication AEX: Columbus, OH, USA, 1998; pp. 524–598. [Google Scholar]
- Nuyttens, D.; Taylor, W.A.; de Schampheleire, M.; Verboven, P.; Dekeyser, D. Influence of nozzle type and size on drift potential by means of different wind tunnel evaluation methods. Biosyst. Eng. 2009, 103, 271–280. [Google Scholar] [CrossRef]
- Bai, G.; Nakano, K.; Mizukami, T.; Miyahara, S.; Ohashi, S.; Kubota, Y.; Takizawa, K.-I.; Yan, H. Characteristics and classification of Japanese nozzles based on relative spray drift potential. Crop. Prot. 2013, 46, 88–93. [Google Scholar] [CrossRef]
- Lipiński, A.J.; Lipiński, S.; Burg, P.; Sobotka, S.M. Influence of the Instability of the Field Crop Sprayer Boom on the Spraying Uniformity. J. Agric. Food Res. 2022, 10, 100432. [Google Scholar] [CrossRef]
- Gajtkowski, A. Technika Ochrony Roślin; Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego: Poznań, Poland, 2000; p. 257. [Google Scholar]
- Milanowski, M.; Subr, A.; Parafiniuk, S.; Różańska-Boczula, M. The Effect of Adjuvant Concentration on Changes of Spray Characteristics and Spraying Parameters for Selected Types of Nozzles. Agric. Eng. 2022, 26, 119–131. [Google Scholar] [CrossRef]
- Baetens, K.; Ho, Q.T.; Nuyttens, D.; de Schampheleire, M.; Endalew, A.M.; Hertog, M.L.A.T.M.; Nicolaï, B.; Ramon, H.; Verboven, P. A validated 2-D diffusion–advection model for prediction of drift from ground. Atmos. Environ. 2009, 43, 1674–1682. [Google Scholar] [CrossRef]
- Subr, A.; Parafiniuk, S.; Milanowski, M.; Krawczuk, A.; Kachel, M. Study of deposited spray quality of spraying agents with different physical properties. Plant Arch. 2020, 20, 6109–6114. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists (AOAC) International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kocira, S.; Szparaga, A.; Krawczuk, A.; Bartoš, P.; Zaguła, G.; Plawgo, M.; Černý, P. Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L. Materials 2021, 14, 6661. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, H.; Ozkan, H.E.; Derksen, R.C.; Krause, C.R. Evaporation and deposition coverage area of droplets containing insecticides and spray additives on hydrophilic, hydrophobic, and crabapple leaf surfaces. Trans. ASAE 2009, 52, 39–49. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Bagley, W.E.; Krause, C.R. Droplet evaporation and spread on waxy and hairy leaves associated with type and concentration of adjuvants. Pest Manag. Sci. 2011, 67, 842–851. [Google Scholar] [CrossRef]
- He, Y.; Xiao, S.; Wu, J.; Fang, H. Influence of Multiple Factors on the Wettability and Surface Free Energy of Leaf Surface. Appl. Sci. 2019, 9, 593. [Google Scholar] [CrossRef]
- Lopes, D.L.; dos Reis, E.F. Spectrum of spray droplets with different nozzles and adjuvants. Rev. Fac. Cien. Agrar. 2020, 15, e6552. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.-C.; Popa, V.I. Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- Kumar, N.A.; Vanlalzarzova, B.; Sridhar, S.; Baluswami, M. Effect of liquid seaweed fertilizer of Sargassum wightii Grev. on the growth and biochemical content of green gram (Vigna radiata (L.) R. Wilczek). Recent Res. Sci. Technol. 2012, 4, 40–45. [Google Scholar]
- Latique, S.; Chernane, H.; Mansori, M.; El Kaoua, M. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris variety Paulista) under hydroponic system. Eur. Sci. J. 2013, 9, 174–191. [Google Scholar]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling Biometric Traits, Yield and Nutritional and Antioxidant Properties of Seeds of Three Soybean Cultivars Through the Application of Biostimulant Containing Seaweed and Amino Acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-Based Biostimulant as Sustainable Alternative to Synthetic Growth Regulators in Two Sweet Cherry Cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Santoso, D.; Gunawan, A.; Budiani, A.; Sari, D.A. Priyono Plant biostimulant to improve crops productivity and planters profit. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 183, p. 012017. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Głuszczak, B.; Mystkowska, I. Ekonomiczne uzasadnienie stosowania herbicydów i biostymulatorów w uprawie ziemniaków jadalnych. Rocz. Nauk. Stow. Ekon. Rol. Agrobiz. 2018, 20, 169–173. [Google Scholar]
- Mystkowska, I. Wpływ zróżnicowanej techniki odchwaszczania i stosowania biostymulatorów na efektywność ekonomiczną uprawy ziemniaków jadalnych. Rocz. Nauk. Stow. Ekon. Rol. Agrobiz. 2017, 19, 190–193. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).