Effects of Ocular Hypertension on Cytoskeleton and Stiffness of Trabecular Meshwork Cells in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microspheres Injection
2.3. HE Staining
2.4. Cell Isolation and Culture
2.5. Immunohistochemical Analyses
2.6. Cytoskeleton Staining
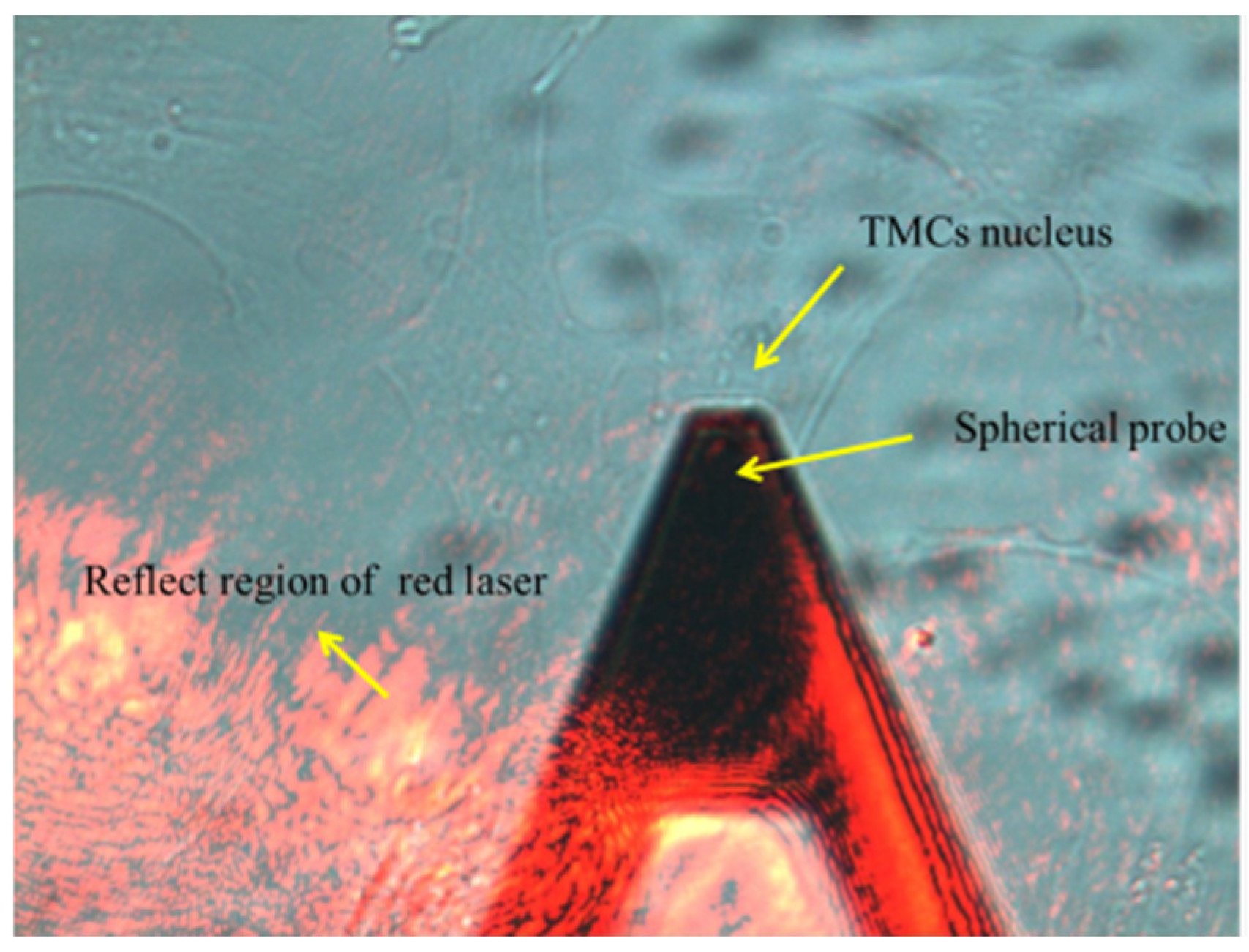
2.7. Atomic Force Microscopy
2.8. Western Blot
2.9. Statistics
3. Results
3.1. IOP Elevation
3.2. Characteristics of TM Cells
3.3. Effects of OHT on Cytoskeleton Morphology in TM Cells
3.4. Increase of Elastic Moduli of TM Cells Induced by OHT
3.5. The Expression of Vimentin in TM Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F. The cell and molecular biology of glaucoma: Biomechanical factors in glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2473–2475. [Google Scholar] [CrossRef]
- Zhu, W.; Hou, F.; Fang, J.; Fard, M.R.B.; Liu, Y.; Ren, S.; Wu, S.; Qi, Y.; Sui, S.; Read, A.T.; et al. The role of Piezo1 in conventional aqueous humor outflow dynamics. iScience 2021, 24, 102042. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Goel, M.; Pacciani, R.G.; Lee, R.K.; Battacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patteson, A.E.; Carroll, R.J.; Iwamoto, D.V.; A Janmey, P. The vimentin cytoskeleton: When polymer physics meets cell biology. Phys. Biol. 2020, 18, 011001. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Read, A.T.; Chan, D.W.-H.; Ethier, C.R. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp. Eye Res. 2007, 84, 214–226. [Google Scholar] [CrossRef]
- Dos Santos, Á.; Fili, N.; Pearson, D.S.; Hari-Gupta, Y.; Toseland, C.P. High-throughput mechanobiology: Force modulation of ensemble biochemical and cell-based assays. Biophys. J. 2021, 120, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Sun, Y.Y.; Peters, D.M.; Keller, K.E. The effects of mechanical stretch on integrins and filopodial-associated proteins in normal and glaucomatous trabecular meshwork cells. Front. Cell Dev. Biol. 2022, 10, 886706. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Zelenak, D. Experimental glaucoma in the primate induced by latex microspheres. J. Neurosci. Methods. 2001, 111, 39–48. [Google Scholar] [CrossRef]
- Pang, I.-H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Cone, F.E.; Gelman, S.E.; Son, J.L.; Pease, M.E.; Quigley, H.A. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp. Eye Res. 2010, 91, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Rho, S.; Park, I.; Seong, G.J.; Lee, N.; Lee, C.K.; Hong, S.; Kim, C.Y. Chronic ocular hypertensive rat model using microbead injection: Comparison of polyurethane, polymethylmethacrylate, silica and polystyene microbeads. Curr. Eye Res. 2014, 39, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Samsel, P.A.; Kisiswa, L.; Erichsen, J.; Cross, S.D.; Morgan, J.E. A novel method for the induction of experimental glaucoma using magnetic microspheres. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1671–1675. [Google Scholar] [CrossRef] [Green Version]
- Sappington, R.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Mao, W.; Liu, Y.; Wordinger, R.J.; Clark, A.F. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3600–3606. [Google Scholar] [CrossRef] [Green Version]
- Eastlake, K.; Jayaram, H.; Luis, J.; Hayes, M.; Khaw, P.T.; Limb, G.A. Strain specific responses in a microbead rat model of experimental glaucoma. Curr. Eye Res. 2021, 46, 387–397. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kanamori, A.; Nakamura, M.; Negi, A. Rat chronic glaucoma model induced by intracameral injection of microbeads suspended in sodium sulfate-sodium hyaluronate. Jpn J. Ophthalmol. 2014, 58, 290–297. [Google Scholar] [CrossRef]
- Ge, P.; Navarro, I.D.; Kessler, M.M.; Bernier, S.G.; Perl, N.R.; Sarno, R.; Masferrer, J.; Hannig, G.; Stamer, W.D. The soluble guanylate cyclase stimulator IWP-953 increases conventional outflow facility in mouse eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Khaw, P.T.; Occleston, N.L.; Schultz, G.; Grierson, I.; Sherwood, M.B.; Larkin, G. Activation and suppression of fibroblast function. Eye 1994, 8 Pt 2, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, L.; Liu, Z. Experimental research on the relationship between the stiffness and the expressions of fibronectin proteins and adaptor proteins of rat trabecular meshwork cells. BMC Ophthalmol. 2017, 17, 268. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Utsunomiya, T.; Ishibazawa, A.; Song, Y.-S.; Udo, M.S.B.; Tasaki, Y.; Yoshida, A. Extracellular matrix gene expression in human trabecular meshwork cells following mechanical fluid flow stimulation. Int. J. Ophthalmol. 2022, 15, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Nettesheim, A.; Hirt, J.; Liton, P.B. The autophagic protein LC3 translocates to the nucleus and localizes in the nucleolus associated to NUFIP1 in response to cyclic mechanical stress. Autophagy 2020, 16, 1248–1261. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Tan, Y.; Wang, Y. Effects of mechanical compression on cell morphology and function in human corneal fibroblasts. Curr. Eye Res. 2021, 46, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Tirendi, S.; Saccà, S.C.; Vernazza, S.; Traverso, C.; Bassi, A.M.; Izzotti, A. A 3D model of human trabecular meshwork for the research study of glaucoma. Front. Neurol. 2020, 11, 591776. [Google Scholar] [CrossRef]
- Osmond, M.; Bernier, S.M.; Pantcheva, M.B.; Krebs, M.D. Collagen and collagen-chondroitin sulfate scaffolds with uniaxially aligned pores for the biomimetic, three dimensional culture of trabecular meshwork cells. Biotechnol. Bioeng. 2017, 114, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Smith, R.; Sundberg, J.; John, S. The anterior segment and ocular adnexae. In Systematic Evaluation of the Mouse Eye; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Cameron, M.J.; Clark, A.F.; Pang, I.H. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 685. [Google Scholar]
- Millar, J.C.; Pang, I.H. Non-continuous measurement of intraocular pressure in laboratory animals. Exp. Eye Res. 2015, 141, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; McCulloch, C.A. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007, 9, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overby, D.R.; Bertrand, J.; Schicht, M.; Paulsen, F.; Stamer, W.D.; Lütjen-Drecoll, E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3727–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.F.; Brotchie, D.; Read, A.T.; Hellberg, P.; English-Wright, S.; Pang, I.-H.; Ethier, C.R.; Grierson, I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskelet. 2005, 60, 83–95. [Google Scholar] [CrossRef]
- Zhang, X.; Ognibene, C.M.; Clark, A.F.; Yorio, T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp. Eye Res. 2007, 84, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, T.; Inoue, T.; Inoue-Mochita, M.; Tanihara, H. Live cell imaging of actin dynamics in dexamethasone-treated porcine trabecular meshwork cells. Exp. Eye Res. 2016, 145, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Raghunathan, V.; Stamer, W.D.; Ganapathy, P.S.; Herberg, S. Extracellular matrix stiffness and TGFβ2 regulate YAP/TAZ activity in human trabecular meshwork cells. Front. Cell Dev. Biol. 2022, 10, 844342. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Qian, X.; Li, L.; Zhang, H.; Liu, Z. RhoA/ROCK-YAP/TAZ axis regulates the fibrotic activity in dexamethasone-treated human trabecular meshwork cells. Front. Mol. Biosci. 2021, 8, 728932. [Google Scholar] [CrossRef]
- Uray, I.P.; Uray, K. Mechanotransduction at the plasma membrane-cytoskeleton interface. Int. J. Mol. Sci. 2021, 22, 11566. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; McNally, S.; Kilpatrick, J.I.; Jarvis, S.P.; O’Brien, C.J. Aging and ocular tissue stiffness in glaucoma. Surv. Ophthalmol. 2018, 63, 56–74. [Google Scholar] [CrossRef]
- McKee, C.T.; Wood, J.A.; Shah, N.M.; Fischer, M.E.; Reilly, C.M.; Murphy, C.J.; Russell, P. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials 2011, 32, 2417–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katta, S.; Krieg, M.; Goodman, M.B. Feeling force: Physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 2015, 31, 347–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyckmans, J.; Boudou, T.; Yu, X.; Chen, C.S. A hitchhiker’s guide to mechanobiology. Dev. Cell 2011, 21, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.T.; Raghunathan, V.K.; Chang, Y.-R.; Murphy, C.J.; Russell, P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 2015, 6, 15362–15374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, S.Y.; Fu, J.; Chen, C.S.; Janmey, P.A. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011, 100, L25–L27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranka, J.A.; Staverosky, J.A.; Reddy, A.P.; Wilmarth, P.A.; David, L.L.; Acott, T.S.; Russell, P.; Raghunathan, V.K. Biomechanical rigidity and quantitative proteomics analysis of segmental regions of the trabecular meshwork at physiologic and elevated pressures. Investig. Ophthalmol. Vis. Sci. 2018, 59, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Schaedel, L.; Lorenz, C.; Schepers, A.V.; Klumpp, S.; Köster, S. Vimentin intermediate filaments stabilize dynamic microtubules by direct interactions. Nat. Commun. 2021, 12, 3799. [Google Scholar] [CrossRef]
- Jiu, Y.; Peränen, J.; Schaible, N.; Cheng, F.; Eriksson, J.E.; Krishnan, R.; Lappalainen, P. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci. 2017, 130, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yin, L.; Song, X.; Yang, H.; Ren, X.; Gong, X.; Wang, F.; Yang, L. Effects of vimentin disruption on the mechanoresponses of articular chondrocyte. Biochem. Biophys. Res. Commun. 2016, 469, 132–137. [Google Scholar] [CrossRef]
- Sharma, P.; Bolten, Z.T.; Wagner, D.R.; Hsieh, A.H. Deformability of human mesenchymal stem cells is dependent on vimentin intermediate filaments. Ann. Biomed. Eng. 2017, 45, 1365–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladilin, E.; Gonzalez, P.; Eils, R. Dissecting the contribution of actin and vimentin intermediate filaments to mechanical phenotype of suspended cells using high-throughput deformability measurements and computational modeling. J. Biomech. 2014, 47, 2598–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathje, L.-S.Z.; Nordgren, N.; Pettersson, T.; Rönnlund, D.; Widengren, J.; Aspenström, P.; Gad, A.K.B. Oncogenes induce a vimentin filament collapse mediated by HDAC6 that is linked to cell stiffness. Proc. Natl. Acad. Sci. USA 2014, 111, 1515–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Loosdregt, I.A.E.W.; Weissenberger, G.; van Maris, M.P.F.H.L.; Oomens, C.W.J.; Loerakker, S.; Stassen, O.M.J.A.; Bouten, C.V.C. The mechanical contribution of vimentin to cellular stress generation. J. Biomech. Eng. 2018, 140, 061006. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Cleland, M.M.; Murthy, S.P.; Mahammad, S.; Kuczmarski, E.R. Inroads into the structure and function of intermediate filament networks. J. Struct. Biol. 2012, 177, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenbeck, P.J.; Bershadsky, A.D.; Pletjushkina, O.Y.; Tint, I.S.; Vasiliev, J.M. Intermediate filament collapse is an ATP-dependent and actin-dependent process. J. Cell Sci. 1989, 92 Pt 4, 621–631. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Araújo, H.C.; Lautenschläger, F.; Gad, A.K.B. Vimentin diversity in health and disease. Cells. 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Grin, B.; Mendez, M.G.; Kuczmarski, E.R. Intermediate filaments: Versatile building blocks of cell structure. Curr. Opin. Cell Biol. 2008, 20, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Yu, Y.; Li, L.; Li, S.; Liu, Z. Effects of Ocular Hypertension on Cytoskeleton and Stiffness of Trabecular Meshwork Cells in Rats. Appl. Sci. 2022, 12, 7862. https://doi.org/10.3390/app12157862
Huang S, Yu Y, Li L, Li S, Liu Z. Effects of Ocular Hypertension on Cytoskeleton and Stiffness of Trabecular Meshwork Cells in Rats. Applied Sciences. 2022; 12(15):7862. https://doi.org/10.3390/app12157862
Chicago/Turabian StyleHuang, Shan, Yang Yu, Lin Li, Shanshan Li, and Zhicheng Liu. 2022. "Effects of Ocular Hypertension on Cytoskeleton and Stiffness of Trabecular Meshwork Cells in Rats" Applied Sciences 12, no. 15: 7862. https://doi.org/10.3390/app12157862
APA StyleHuang, S., Yu, Y., Li, L., Li, S., & Liu, Z. (2022). Effects of Ocular Hypertension on Cytoskeleton and Stiffness of Trabecular Meshwork Cells in Rats. Applied Sciences, 12(15), 7862. https://doi.org/10.3390/app12157862





