Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films
Abstract
:1. Introduction
2. Metal Sulfide Thin Films
2.1. Silver Sulfide Films
2.2. Copper Zinc Tin Sulfide Films
2.3. Tin Sulfide Films
2.4. Copper Tin Sulfide Films
2.5. Zinc Sulfide Films
2.6. Lanthanum Sulfide Films
2.7. Copper Sulfide Films
2.8. Cadmium Sulfide Films
2.9. Copper Aluminum Sulfide Films
2.10. Nickel-Doped Indium Sulfide Films
2.11. Indium Sulfide Films
2.12. Cobalt Sulfide Films
2.13. Nickel Sulfide Films
2.14. Arsenic Sulfide Films
2.15. Manganese Sulfide Films
2.16. Iron Sulfide Films
2.17. Copper Indium Disulfide Films
2.18. Lead Sulfide Films
2.19. Molybdenum Sulfide Films
2.20. Antimony Sulfide Films
2.21. Bismuth Sulfide Films
2.22. Cobalt Tin Sulfide Films
3. Metal Selenide Thin Films
3.1. Cadmium Selenide Films
3.2. Bismuth Selenide Films
3.3. Zinc Selenide Films
3.4. Cobalt Selenide Films
3.5. Zinc Indium Selenide Films
3.6. Copper Indium Diselenide Films
3.7. Copper Selenide Films
3.8. Lead Selenide Films
3.9. Dysprosium Selenide Films
3.10. Indium Selenide Films
3.11. Boron-Doped Indium Selenide Films
3.12. Antimony Selenide Films
3.13. Lanthanum Selenide Films
4. Metal Telluride Thin Films
4.1. Cadmium Telluride Films
4.2. Lanthanum Telluride Films
4.3. Zinc Telluride Films
4.4. Copper Telluride Films
4.5. Samarium Telluride Films
5. Metal Oxide Thin Films
5.1. Zinc Oxide Films
5.2. Titanium Oxide Films
5.3. Cadmium Oxide Films
5.4. Iron Oxide Films
5.5. Tin Oxide Films
5.6. Nickel Oxide Films
5.7. Zirconium-Doped Cadmium Oxide Films
5.8. Copper Nickel Oxide Films
5.9. Tungsten-Doped Zinc Oxide Films
5.10. Copper-Doped Zinc Oxide Films
5.11. Strontium-Doped Zinc Oxide Films
5.12. Nickel-Doped Zinc Oxide Films
5.13. Cobalt-Doped Nickel Oxide Films
5.14. Cobalt-Doped Manganese Oxide Films
5.15. Manganese-Doped Copper Oxide Films
5.16. Manganese Oxide Films
5.17. Tungsten Oxide Films
5.18. Silver-Doped Zinc Oxide Films
5.19. Indium-Doped Zinc Oxide Films
5.20. Aluminum Oxide Films
5.21. Aluminum-Doped Zinc Oxide Films
5.22. Bismuth Oxide Films
5.23. Copper Oxide and Cuprous Oxide Films
5.24. Cobalt Oxide Films
5.25. Zinc Iron Oxide Films
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harif, N.; Halina, M.; Hasrul, R.; Kazi, S.; Camellia, D.; Sopian, K.; Amin, N. The role of deposition temperature in the photovoltaic proeprties of RF sputtered CdSe thin films. Crystals 2021, 11, 73. [Google Scholar] [CrossRef]
- Megan, J.; Kreiml, P.; Mitterer, C. Materials engineering for flexible metallic thin film applications. Materials 2022, 15, 926. [Google Scholar] [CrossRef]
- Ho, S.M.; Mandati, S.; Chandran, R.; Mallik, A.; Arif, S.; Deepa, K. preparation of CuInSe2 thin films by using various methods (a short review). Orient. J. Chem. 2019, 35, 1–13. [Google Scholar]
- Bin, W.; Huang, W.; Lin, W.; Lee, H. Study on optical and electrical properties of thermally evaporated tin oxide thin films for perovskite solar cells. Crystals 2021, 11, 1380. [Google Scholar] [CrossRef]
- Zaman, M.; Taru, C.; Dehury, K.; Rajaram, P. Synthesis and characterization of spin coated ZnS thin films. AIP Conf. Proc. 2018, 1953, 100066. [Google Scholar] [CrossRef]
- Ho, S.M. Studies of power conversionefficiency and optical properties of Ni3Pb2S2 thin films. Makara J. Sci. 2017, 21, 119–124. [Google Scholar]
- Mikayal, A.; Zarine, S.; Gohar, S.; Ladimir, A.; Artak, S. Gas senor based on ZnO nanostructured film for the detection of ethanol vapor. Chemosensors 2022, 10, 245. [Google Scholar] [CrossRef]
- Istrate, A.; Vulpe, S.; Florin, N.; Lucia, M.; Cosmin, R.; Oana, T. Ca-doped ZnO:Al thin films: Synthesis and characterization. Coatings 2021, 11, 1023. [Google Scholar] [CrossRef]
- Agustin, L.; Rosa, M.; Cesia, G.; Isaac, R.; Felipe, C.; Edith, O.; Ricardo, A. Automated instrument for the deposition of thin films using successive ionic layer adsorption and reaction. Processes 2022, 10, 492. [Google Scholar] [CrossRef]
- Jose, O.; Jorge, S.; Acros, M.; Omar, R.; Bustos, E.; Gonzalez, M. Design and development of software for the SILAR control process using a lost-cost embedded system. Processes 2021, 9, 967. [Google Scholar] [CrossRef]
- Sundhar, A. Development of ZnS thin film with Co, Cu and Ag dopingusing SILAR method. Mater. Today Proc. 2022, 48, 377–381. [Google Scholar] [CrossRef]
- Mahesh, K.; Send, K.; Kuar, M.; Panda, B. Chlorophyll a functionalized ZnCdS thin flm fabricated by SILAR technique for dye sensitized solar cells. Inorg. Chem. Commun. 2022, 142, 109670. [Google Scholar] [CrossRef]
- Saba, F.; Sabah, I.; Salim, A.; AlOgaili, H. Influence of deposition time on absorption and electrical characteristics of ZnS thin films. Optik 2022, 260, 169056. [Google Scholar] [CrossRef]
- Pathan, M.; Salunkhe, V.; Sankapal, B.; Lokhande, D. Photoelectrochemical investigation of Ag2S thin films deposited by SILAR method. Mater. Chem. Phys. 2001, 72, 105–108. [Google Scholar] [CrossRef]
- Vijendra, A.; Dargad, S.; Rajesh, A.; Govind, K. Fabrication of Highly Conducting Ag2S Thin Films on FTO Substrate by Using SILAR. Adv. Mater. Res. 2022. [Google Scholar] [CrossRef]
- Lokhande, C.; Sankapal, B.; Mane, R. A new chemical method for the preparation of Ag2S thin films. Mater. Chem. Phys. 2000, 63, 226–229. [Google Scholar]
- Kakade, B.; Nikam, P.; Gosavi, R. Effect of immersion cycles on structural, morphology and optoelectronic properties of nanocrystalline Ag2S thin films deposited by SILAR technique. IOSR J. Appl. Phys. 2014, 6, 6–12. [Google Scholar]
- Gosavi, R.; Patel, S.; Patil, R.; Jadhav, M. Studies on Characterization of Nanocrystalline Silver Sulphide Thin Films Deposited by Chemical Bath Deposition (CBD) and Successive Ionic Layer Adsorption and Reaction (SILAR) method. Arch. Phys. Res. 2011, 2, 27–35. [Google Scholar]
- Sadovnikov, S.; Gusev, A.; Rempel, A. Nanocrystalline silver sulfide Ag2S. Rev. Adv. Mater. Sci. 2015, 41, 7–19. [Google Scholar]
- Shen, H.; Jiao, X.; Oron, D.; Lin, H.; Li, J. Efficient electron injection in non-toxic silver sulfide (Ag2S) sensitized solar cells. J. Power Sources 2013, 240, 8–13. [Google Scholar] [CrossRef]
- Ambily, K.; Ali, K.; Geetha, V.; Kannan, P. Towards phase pure CZTS thin films by SILAR method with augmented Zn adsorption for photovoltaic application. Mater. Renew. Sustain. Energy 2019, 8, 16. [Google Scholar] [CrossRef]
- Kumar, G.; Balaji, P.; Gnana, P.; Kaushik, R.; Shriram, M. Investigations on SILAR coated CZTS thin films for solar cells applications. Phase Transit. 2021, 94, 556–566. [Google Scholar] [CrossRef]
- Gayatri, B.; Das, S.; Ghosh, S. Optical Properties of Cu2ZnSnS4 (CZTS) Made By SILAR Method. Mater. Today Proc. 2019, 18, 494–500. [Google Scholar]
- Sawanta, M.; Patil, P.; Oh, W.; Betty, A.; Shinde, S. Synthesis and characterization of Cu2ZnSnS4 thin films by SILAR method. J. Phys. Chem. Solids 2012, 3, 735–740. [Google Scholar]
- Suryawanshi, P.; Patil, S.; Shin, S.; Gurav, K.; Agawane, L.; Gang, G. The synergistic influence of anionic bath immersion time on the photoelectrochemical performance of CZTS thin films prepared by a modified SILAR sequence. RSC Adv. 2014, 4, 18537–18540. [Google Scholar] [CrossRef]
- Mane, S.; Lokhande, D.; Sankapal, B. Successive ionic layer adsorption and reaction (SILAR) method for the deposition of large area (∼10 cm2) tin disulfide (SnS2) thin films. Mater. Res. Bull. 2000, 35, 2027–2035. [Google Scholar]
- Mani, P.; Manikandan, K.; Prince, J. Influence of molar concentration on triethanolamine (TEA) added tin sulfide (SnS) thin films by SILAR method. J. Mater. Sci. Mater. Electron. 2016, 27, 9255–9264. [Google Scholar] [CrossRef]
- Mitra, P.; Mukherjee, A. Structural and optical characteristics of SnS thin film prepared by SILAR. Mater. Sci. 2015, 33, 847–851. [Google Scholar]
- Biswajit, G.; Das, M.; Pushan, B.; Das, S. Fabrication and optical properties of SnS thin films by SILAR method. Appl. Surf. Sci. 2008, 254, 6436–6440. [Google Scholar]
- Qachaou, Y.; Daoudi, O.; Raidou, A.; Fahoume, M.; Lharch, M. The influence of solution temperature of SnS thin films grown by successive ionic layer adsorption and reaction (SILAR) method. IOP Conf. Ser. J. Phys. 2019, 1292, 012022. [Google Scholar] [CrossRef]
- Mani, P.; Joseph, P.; Manikandan, K. Influence of molar concentration on ethylene diamine tetra acetic acid (EDTA) added to tin sulfide (SnS) thin films grown by silar method. J. Mater. Sci. Mater. Electron. 2017, 28, 13602–13612. [Google Scholar] [CrossRef]
- Hao, G.; Shen, H.; Chao, G.; He, X. The influence of annealing atmosphere on the phase formation of Cu–Sn–S ternary compound by SILAR method. J. Mater. Sci. Mater. Electron. 2013, 24, 3195–3198. [Google Scholar]
- Harshad, D.; Lokhande, A.; Raut, V.; Patil, M.; Kim, H. Facile synthesis of Cu2SnS3 thin films grown by SILAR method: Effect of film thickness. J. Mater. Sci. Mater. Electron. 2017, 28, 7912–7921. [Google Scholar]
- Aykut, A. Structural and optical properties of Cu2SnS3 thin films obtained by SILAR method. Sakarya Univ. J. Sci. 2017, 21, 505–510. [Google Scholar]
- Shelke, H.; Patil, A.; Lokhande, C.; Kim, H. Electrochemical impedance analysis of SILAR deposited Cu2SnS3 (CTS) thin film. Int. J. Eng. Res. Technol. 2017, 10, 578–586. [Google Scholar]
- Kahraman, S.; Samed, C.; Haci, M.; Ali, C.; Salih, G. Cu2SnS3 absorber thin films prepared via successive ionic layer adsorption and reaction method. Int. J. Mater. Res. 2013, 104, 1020–1027. [Google Scholar] [CrossRef]
- Su, Z.; Sun, K.; Han, Z.; Liu, F.; Lai, Y.; Jie, L.; Liu, Y. Fabrication of ternary Cu–Sn–S sulfides by a modified successive ionic layer adsorption and reaction (SILAR) method. J. Mater. Chem. 2012, 22, 16346–16352. [Google Scholar] [CrossRef]
- Ashith, K.; Rao, K. Structural and Optical Properties of ZnS Thin Films by SILAR Technique obtained by acetate Precursor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 360, 012058. [Google Scholar] [CrossRef]
- Haneefa, M.; Gani, M.; Fareed, S.; Raja, J. The studies on optical and structural properties of zinc sulfide thin films deposited by SILAR method. J. Chem. Pharm. Res. 2015, 7, 141–145. [Google Scholar]
- Mana, M.; Pankaj, K.; Habib, M. Fabrication and Characterization of ZnS based Photoelectrochemical Solar Cell. ES Energy Environ. 2020, 12, 77–85. [Google Scholar] [CrossRef]
- Geetha, V.; Rajan, V. Studies of Structural and Optical Properties of Zinc Sulphide Thin Films. Int. J. Mater. Phys. 2014, 5, 5–14. [Google Scholar]
- Roy, S.; Mitra, P. Preparation of ZnS thin film by Silar. Mater. Sci. Res. India 2008, 5, 447–452. [Google Scholar]
- Laukaitis, G.; Lindroos, S.; Leskela, M.; Rackaitis, M. Stress and surface studies of SILAR grown ZnS thin films on(100)GaAs substrates. Mater. Sci. Eng. A 2000, 288, 223–230. [Google Scholar] [CrossRef]
- Lindroos, S.; Bonnin, C.; Leskea, A.; Charreire, Y. Growth and Characterization of Zinc Sulfide Thin Films Deposited by the Successive Ionic Layer Adsorption and Reaction (Silar) Method Using Complexed Zinc Ions As the Cation Precursor. Mater. Res. Bull. 1998, 33, 453–459. [Google Scholar] [CrossRef]
- Trupti, T.; Abhishek, C.; Rahul, B.; Lokhande, D. Lanthanum sulfide/graphene oxide composite thin films and their supercapacitor application. SN Appl. Sci. 2018, 1, 110. [Google Scholar] [CrossRef]
- Sartale, D.; Lokhande, C. Growth of copper sulphide thin films by successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2000, 65, 63–67. [Google Scholar] [CrossRef]
- Obed, Y.; Dalia, A.; Zeuz, M. Deposition of Highly Crystalline Covellite Copper Sulphide Thin Films by SILAR. Phys. Status Solidi A Appl. Mater. Sci. 2017, 214, 1700500. [Google Scholar] [CrossRef]
- Seppo, L.; Arnold, A.; Markku, L. Growth of CuS thin films by the successive ionic layer adsorption and reaction method. Appl. Surf. Sci. 2000, 158, 75–80. [Google Scholar]
- Mani, P.; Janaki, A.; Seelan, A.; Prince, J.; Hameed, S.; Syed, S. Influence of Molar Concentrations on Optical Properties of Copper Sulphide Thin Films by Silar Method. Int. J. ChemTech Res. 2014, 214, 3573–3578. [Google Scholar]
- Ravindra, N.; Lee, Y.; Shm, J.; Roh, C.; Thi, T.; Sumanta, S. Chemical synthesis of 3D copper sulfide with different morphologies for high performance supercapacitors application. RSC Adv. 2016, 6, 14844–14851. [Google Scholar]
- Fouad, B.; Raidou, A.; Sall, T.; Larbi, L.; Ahmed, Q. Structural and optical properties of CdS thin films prepared by SILAR method. In Proceedings of the 2013 International Renewable and Sustainable Energy Conference (IRSEC), Ouarzazate, Morocco, 7–9 March 2013. [Google Scholar] [CrossRef]
- Dipalee, J.; Shaeed, S.; Farha, S.; Birajdar, R.; Anil, G.; Sharma, R. Effect of annealing on structural and optoelectronic properties of CdS thin film by SILAR method. Adv. Appl. Sci. Res. 2011, 2, 417–425. [Google Scholar]
- Hua, S.; Mu, J. SILAR Deposition of CdS Thin Films on Glass Substrates Modified with 3-Mercaptopropyltrimethoxysilane. J. Dispers. Sci. Technol. 2005, 26, 719–722. [Google Scholar] [CrossRef]
- Kester, O.; Tochukwu, M.; Kenneth, I.; Blssing, N. Influence of Dip Cycles on the Structural, Optical and Morphological Properties of CdS-SILAR Deposited Thin Films. J. NanoSci. NanoEng. Appl. 2016, 6, 19–26. [Google Scholar]
- Loreta, T.; Birute, S.; Albina, Z.; Leonas, N.; Selskis, A.; Norkus, E. Investigation of CdS Thin Films Deposition by the SILAR Method Using Cd(II) Organic Salt as Precursor. ECS Meet. Abstr. 2014, MA2014-02, 837. [Google Scholar] [CrossRef]
- Garadkar, K.; Patil, A.; Krake, P.; Hankare, P. Characterization of CdS thin films synthesized by SILAR method at room temperature. Arch. Appl. Sci. Res. 2010, 2, 429–437. [Google Scholar]
- Mitra, P.; Ayan, M.; Protim, P. Synthesis of Nanocrystalline CdS by SILAR and Their Characterization. J. Mater. 2014, 2014, 138163. [Google Scholar] [CrossRef]
- Joseph, O.; Ngozi, E.; Kelechi, N. Optical Properties of CuAl2S Alloyed Thin Films Prepared Using Enhanced Successive Ionic Layer Adsorption and Reaction Method. Int. J. Res. Innov. Appl. Sci. 2021, 6, 95–99. [Google Scholar]
- Fatma, G.; Serdar, U. Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR. Open Chem. 2018, 16, 757–762. [Google Scholar]
- Anita, R.; Teny, J.; Sudha, K.; Vijayakumar, P. structural and Optical Properties of Indium Sulfide Thin Films Prepared by Silar Technique. Open Condens. Matter Phys. J. 2009, 2, 9–14. [Google Scholar]
- Pathan, M.; Han, S.; Seth, T.; Kulkarni, S.; Lokhande, C. Some studies on successive ionic layer adsorption and reaction (SILAR) grown indium sulphide thin films. Mater. Res. Bull. 2005, 40, 1018–1023. [Google Scholar] [CrossRef]
- Ranjith, R.; John, T.; Sudha, C.; Ae, T.; Kashiwaba, Y. Post-deposition annealing effect on In2S3 thin films deposited using SILAR technique. Mater. Sci. Semicond. Process. 2007, 10, 49–55. [Google Scholar] [CrossRef]
- Jingjing, Z.; Hou, Z.; Yuan, X.; Zeng, G.; Tu, W.; Wang, S. Tailred indium sulfide based materials for solar energy conversion and utilization. J. Photochem. Photobiol C Photochem. Rev. 2019, 38, 1–26. [Google Scholar]
- Sartale, D.; Lokhande, D. Deposition of cobalt sulphide thin films by successive ionic layer adsorption and reaction (SILAR) method and their characterization. Indian J. Pure Appl. Phys. 2000, 38, 48–52. [Google Scholar]
- Kester, O.; Blessing, N.; Agbogu, A.; Nwanya, C.; Obi, D.; Botha, S.; Malik, M.; Fabian, I. The effect of deposition cycles on intrinsic and electrochemical properties of metallic cobalt sulfide by Simple chemical route. Mater. Sci. Semicond. Process. 2019, 101, 16–27. [Google Scholar]
- Mitkari, A.; Ubale, U. Thickness Dependent Physical Properties of SILAR Deposited Nanostructured CoS Thin Films. ES Mater. Manuf. 2019, 5, 49–56. [Google Scholar] [CrossRef]
- Sartale, D.; Lokhande, D. Preparation and characterization of nickel sulphide thin films using successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2001, 72, 101–104. [Google Scholar] [CrossRef]
- Ofeliya, O.; Azizov, A.; Mustafa, B.; Abel, M.; Goncha, M.; Rasim, M.; Zamin, M. β-NiS and Ni3S4 nanostructures: Fabrication and characterization. Mater. Res. Bull. 2016, 75, 155–161. [Google Scholar]
- Sathya, D. NiS thin films for solar cell application by successive ionic layer adsorption and reaction (SILAR) method. GIS Sci. J. 2022, 9, 89–95. [Google Scholar]
- Sartale, D.; Lokhande, C. Preparation and characterization of As2S3 thin films deposited using successive ionic layer adsorption and reaction (SILAR) method. Mater. Res. Bull. 2000, 35, 1345–1353. [Google Scholar] [CrossRef]
- Yildirim, M.; Tuna, Y.; Ates, A.; Cavanmirza, I. Chemically synthesis and characterization of MnS thin films by SILAR method. Chem. Phys. Lett. 2016, 647, 73–78. [Google Scholar] [CrossRef]
- Pathan, M.; Kale, S.; Han, S.; Joo, O. Preparation and characterization of amorphous manganese sulfide thin films by SILAR method. Mater. Res. Bull. 2007, 42, 1565–1569. [Google Scholar] [CrossRef]
- Admuthe, A.; Sambaji, S.; Susmita, K.; Ganesh, N. Synthesis and Characterization of MnS Thin Film at Room Temperature for Supercapacitor Application. Macromol. Symp. 2020, 392, 2000186. [Google Scholar] [CrossRef]
- Manikandan, K.; Mani, P.; Dilip, S.; Fermi, H.; Joseph, P. Effect of complexing agent TEA: The structural, morphological, topographical and optical properties of FexSx nano thin films deposited by SILAR technique. Appl. Surf. Sci. 2014, 288, 76–82. [Google Scholar] [CrossRef]
- Mahesari, B.; Dhanam, M. Optimization of deposition temperature of SILAR Cu-rich CuInS2 thin films. Mater. Sci. Poland 2013, 31, 193–200. [Google Scholar] [CrossRef]
- Wei, P.; Gong, J.; Ge, C.; Li, J. Preparation and Properties of CuInS2 Thin-Film by Successive Ionic Layer Adsorption and Reaction (SILAR) Method. Key Eng. Mater. 2005, 280-283, 877–880. [Google Scholar] [CrossRef]
- Xueqing, X.; Wan, Q.; Luan, C.; Mei, F.; Zhao, Q.; An, P.; Liang, Z.; Juan, Z. Fabrication of CuInS2-Sensitized Solar Cells via an Improved SILAR Process and Its Interface Electron Recombination. Appl. Mater. Interfaces. 2013, 5, 10605–10613. [Google Scholar]
- Fanghong, X.; Yong, S.; Zhao, Q.; Li, C.; Li, X. Effects of hydrothermal annealing on characteristics of CuInS2 thin films by SILAR method. Appl. Surf. Sci. 2012, 258, 7465–7469. [Google Scholar]
- Zhengguo, J.; Yong, S.; Li, C.; Qiu, J.; An, H. Effect of [Cu]/[In] ratio on properties of CuInS2 thin films prepared by successive ionic layer absorption and reaction method. Appl. Surf. Sci. 2006, 252, 3737–3743. [Google Scholar]
- Mutlu, K. Characterization of CuInS2 Thin Films with Different Cu/In Ratio. Chin. J. Chem. Phys. 2010, 23, 582–586. [Google Scholar]
- Tapio, K.; Seppo, L.; Jarkko, I. Growth of strongly orientated lead sulfide thin films by successive ionic layer adsorption and reaction (SILAR) technique. J. Mater. Chem. 1996, 6, 161–164. [Google Scholar]
- Puiso, J.; Laukaitis, G.; Leskela, M.; Lindroos, S. Growth of PbS thin films on silicon substrate by SILAR technique. Thin Solid Films 2002, 403–404, 457–461. [Google Scholar] [CrossRef]
- Gulen, Y. Characteristics of Ba-Doped PbS Thin Films Prepared by the SILAR Method. Acta Phys. Polonica A 2014, 126, 763–767. [Google Scholar] [CrossRef]
- Guneri, E.; Gode, F.; Cevik, S. Influence of grain size on structural and optic properties of PbS thin films produced by SILAR method. Thin Solid Films 2015, 589, 578–583. [Google Scholar] [CrossRef]
- Preetha, K.; Murali, K.; Ragina, A.; Deepa, K.; Remadevi, T. Effect of cationic precursor pH on optical and transport properties of SILAR deposited nano crystalline PbS thin films. Curr. Appl. Phys. 2012, 12, 53–59. [Google Scholar] [CrossRef]
- Vishal, V.; Devan, R.; Pawar, S.; Namdev, S.; Payil, V.; Rao, K.; Ma, Y.; Ae, J. Chemically synthesized PbS nanoparticulate thin films for a rapid NO2 gas sensor. Mater. Sci. 2016, 34, 204–211. [Google Scholar] [CrossRef]
- Sartale, S.; Lokhande, C. Studies on large area (∼50 cm2) MoS2 thin films deposited using successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2001, 71, 94–97. [Google Scholar] [CrossRef]
- Deshpande, M.; Krisha, C.; Kiran, P.; Rajput, P.; Bhoi, R.; Chaki, S. Study of Sb2S3 thin films deposited by SILAR method. Mater. Res. Express 2018, 5, 056410. [Google Scholar] [CrossRef]
- Sankapal, R.; Mane, R.; Lokhande, D. Preparation and characterization of Sb2S3 thin films using a successive ionic layer adsorption and reaction (SILAR) method. J. Mater. Sci. Lett. 1999, 18, 1453–1455. [Google Scholar] [CrossRef]
- Ying, W.; Chen, J.; Jiang, L.; Liu, F.; Li, J.; Lai, Y. Characterization of Bi2S3 thin films synthesized by an improved successive ionic layer adsorption and reaction (SILAR) method. Mater. Lett. 2017, 209, 479–482. [Google Scholar]
- Bouachri, M.; Farri, H.; Taibi, M.; Beraich, M.; Fahoume, M. Influence of cycle numbers on optical parameters of nanostructured Bi2S3 thin films using SILAR method for solar cells light harvesting. Materialia 2021, 20, 101242. [Google Scholar] [CrossRef]
- Dipalee, D.; Shaeed, S.; Farha, S.; Birajdar, R.; Anil, G.; Sharma, R. Enhancement of photosensitivity by annealing in Bi2S3 thin films grown using SILAR method. Compos. B Eng. 2013, 46, 1–6. [Google Scholar]
- Shrikant, S.; Jyotsna, A.; Babasaheb, R. SILAR deposited Bi2S3 thin film towards electrochemical supercapacitor. Phys. E. 2017, 87, 209–212. [Google Scholar]
- Damisa, J.; Emegha, J. Growth and Optical Analysis of Cobalt Tin Sulphide Thin Films using SILAR Technique. Niger. Res. J. Eng. Environ. Sci. 2021, 6, 642–648. [Google Scholar]
- Gosavi, M.; Chaudhari, B.; Gosavi, S.; Deshpande, N. Chemical synthesis and characterization of CdSe thin films deposited by SILAR technique for optoelectronic applications. J. Sci. Adv. Mater. Dev. 2016, 1, 476–481. [Google Scholar]
- Pathan, M.; Sankapal, R.; Desai, J.; Lokhande, D. Preparation and characterization of nanocrystalline CdSe thin films deposited by SILAR method. Mater. Chem. Phys. 2002, 78, 11–14. [Google Scholar] [CrossRef]
- Kale, B.; Sartale, D.; Lokhande, D.; Chougule, B. Growth and characterization of nanocrystalline CdSe thin films deposited by the successive ionic layer adsorption and reaction method. Semicond. Sci. Technol. 2004, 19, 980–986. [Google Scholar] [CrossRef]
- Sankapal, R.; Lokhande, C. Photoelectrochemical characterization of Bi2Se3 thin films deposited by SILAR technique. Mater. Chem. Phys. 2002, 73, 151–155. [Google Scholar] [CrossRef]
- Lokhande, D.; Mane, R.; Sankapal, R. Preparation and characterization of Bi2Se3 thin films deposited by successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2000, 63, 230–234. [Google Scholar]
- Sankapal, R.; Pathan, M.; Lokhande, D. Growth of multilayer Bi2Se3-Sb2Se3 thin films by SILAR technique. Indian J. Eng. Mater. Sci. 2001, 8, 223–227. [Google Scholar]
- Kale, B.; Lokhande, D. Room temperature deposition of ZnSe thin films by successive ionic layer adsorption and reaction (SILAR) method. Mater. Res. Bull. 2004, 39, 1829–1839. [Google Scholar] [CrossRef]
- Geethanjali, M.; Depa, K.; Remadevi, T. Effect of number of cycles on SILAR deposited ZnSe thin films. AIP Conf. Proc. 2021. [Google Scholar] [CrossRef]
- Guzeldir, B.; Saglam, M.; Ates, A. Preparation and characterization of CdSe, ZnSe and CuSe thin films deposited by the successive ionic layer adsorption and reaction method. J. Optoelectron. Adv. Mater. 2012, 14, 224–229. [Google Scholar]
- Ho, S.M. An investigation of SILAR grown cobalt selenide thin films. Asian J. Basic Sci. Res. 2022, 4, 1–9. [Google Scholar]
- Ho, S.M. Properties Study of SILAR Deposited Cobalt Selenide Thin Films. Int. J. Res. Rev. 2021, 8, 119–124. [Google Scholar]
- Soonmin, H.; Shiong, N. Effect of pH on the Synthesis of Cobalt Selenide Films by SILAR Method. Oriental J. Chem. 2021, 37, 791–796. [Google Scholar]
- Soonmin, H. Characterization of Cobalt Selenide Films using FESEM and EDX. Int. J. Thin Films Sci. Technol. 2022, 11, 1. [Google Scholar]
- Egwunyenga, J.; Onuabuchi, V.; Okoli, L.; Nwankwo, E. Effect of SILAR Cycles on the Thickness, Structural, Optical Properties of Cobalt Selenide Thin Films. Int. Res. J. Multidiscip. Tech. 2021, 3, 1–9. [Google Scholar]
- Ridvan, D.; Aktas, S.; Betul, G.; Saglam, M. The properties of the ZnInSe thin film grown by SILAR method. In Proceedings of the Materials Science & Technology 2013, Montreal, QC, Canada, 27–31 October 2013. [Google Scholar]
- Berruet, M.; Shreiner, H.; Cere, S.; Vazqez, M. Deposition and characterization of CuInSe2 films for solar cells using an optimized chemical route. J. Alloys Compd. 2011, 509, 3019–3024. [Google Scholar] [CrossRef]
- Yang, J.; Jin, Z.; Liu, T.; Yong, S. An investigation into effect of cationic precursor solutions on formation of CuInSe2 thin films by SILAR method. Sol. Energy Mater. Sol. Cells 2008, 92, 621–627. [Google Scholar] [CrossRef]
- Ivanauskas, A.; Ancutiene, I.; Ivanauskas, R. The deposition of CuInSe2 layer on glass substrate by SILAR method. Chalcogen. Lett. 2016, 13, 373–380. [Google Scholar]
- Aykut, A.; Yunus, A.; Muhamme, Y. Conversion of SILAR deposited Cu3Se2 thin films to Cu2-xSe by annealing. Mater. Lett. 2016, 166, 9–11. [Google Scholar]
- Algimantas, I.; Remigijius, I.; Ancutiene, I. Effect of In-Incorporation and Annealing on CuxSe Thin Films. Materials 2021, 14, 3810. [Google Scholar] [CrossRef]
- Betul, G.; Saglam, M. Using different chemical methods for deposition of copper selenide thin films and comparison of their characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 111–119. [Google Scholar]
- Tapio, K.; Seppo, L.; Jarkko, I.; Markka, L. Growth of lead selenide thin films by the successive ionic layer adsorption and reaction (SILAR) technique. J. Mater. Chem. 1996, 6, 983–986. [Google Scholar] [CrossRef]
- Remadevi, T.; Preetha, K. Effect of hydrazine hydrate concentration on structural, surface morphological and optoelectronic properties of SILAR deposited PbSe thin films. Mater. Sci. Semicond. Process. 2015, 39, 178–187. [Google Scholar]
- Khot, S.; Nikam, P.; Malavekar, D.; Patil, D.; Lokhande, C.; Ubale, S. SILAR synthesized dysprosium selenide (Dy2Se3) thin films for hybrid electrochemical capacitors. Synth. Met. 2022, 287, 117075. [Google Scholar] [CrossRef]
- Bagwade, P.; Malavekar, D.; Ubale, S.; Bulakhe, R.; Ghogare, T.; Patil, U. Characterization of Dy2S3 thin films deposited by successive ionic layer adsorption and reaction (SILAR) method. Solid State Sci. 2021, 38, 48–52. [Google Scholar] [CrossRef]
- Ertap, H.; Yuksek, M.; Mevlut, K. Structural and Optical Properties of Indium Selenide (InSe) Thin Films Deposited on Glass and GaSe Single Crystal Substrates by SILAR Method. Cumhuriyet Sci. J. 2019, 40, 602–611. [Google Scholar] [CrossRef]
- Sankapal, B.; Ganesan, V.; Lokhande, D. Studies on deposition of antimony triselenide thin films by chemical method: SILAR. Indian J. Pure Appl. Phys. 2000, 38, 606–610. [Google Scholar]
- Chodankar, V.; Patil, S.; Lokhande, V.; Lokhande, D. Chemically prepared La2Se3 nanocubes thin film for supercapacitor application. J. Colloid Interface Sci. 2016, 469, 318–324. [Google Scholar]
- Dhokne, J.; Ubale, A.; Sangawar, S.; Kulkarni, K. Characterization of nanocrystalline cadmium telluride thin films grown by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci. 2006, 29, 165–168. [Google Scholar]
- Kulkarni, K.; Ubale, U. Studies on size dependent properties of cadmium telluride thin films deposited by using successive ionic layer adsorption and reaction method. Indian J. Pure Appl. Phys. 2006, 44, 254–259. [Google Scholar]
- Swapna, S.; Salunke, D.; Patil, R.; Gosavi, R. Structural and Optoelectronic Properties of Nanocrystalline CdTe Thin Films Synthesized by Using SILAR Technique. J. Nano Electron. Phys. 2017, 9, 05028-1. [Google Scholar] [CrossRef]
- Patil, S.; Lokhande, C.; Lee, W.; Kim, H.; Lokhande, D. Chemical synthesis and supercapacitive properties of lanthanum telluride thin film. J. Colloid Interface Sci. 2017, 490, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Mane, S.; Pathan, H.; Shaikh, A.; Han, S. Preparation and characterization of ZnTe thin films by SILAR method. Appl. Surf. Sci. 2007, 253, 4335–4337. [Google Scholar] [CrossRef]
- Jignesh, R. ZnTe Films Deposited Using SILAR Method: Can Be Used as Optical Window; LAP Lambert Academic Publishing: Chisinau, Moldova, 2014. [Google Scholar]
- Amalnerkar, S.; Pathan, M.; Seth, T.; Lokhande, D. Preparation and characterization of copper telluride thin films by modified chemical bath deposition (M-CBD) method. Appl. Surf. Sci. 2003, 218, 291–297. [Google Scholar]
- Kumbhar, S.; Lokhande, A.; Gaikwad, S.; Lokhande, C. Synthesis of samarium telluride thin films by successive ionic layer adsorption and reaction (SILAR) method for supercapacitor application. Mater. Sci. Semicond. Process. 2016, 46, 29–34. [Google Scholar] [CrossRef]
- Raidou, A.; Sall, T.; Aggour, M.; Benmalek, F.; Fahoume, M. Characterization of ZnO Thin Films Grown by SILAR Method. Open Access Libr. J. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Sreedev, P.; Rakhesh, V.; Roshima, S.; Shankar, B. Preparation of Zinc Oxide Thin films by SILAR method and its Optical analysis. J. Phys. Conf. Ser. 2019, 1172, 012024. [Google Scholar] [CrossRef]
- Bijoy, C.; Syed, F.; Abdul, M.; Shanta, M.; Guo, Q.; Tooru, T.; Atiqur, R. Influence of the Substrate, Process Conditions, and Postannealing Temperature on the Properties of ZnO Thin Films Grown by the Successive Ionic Layer Adsorption and Reaction Method. ACS Omega 2021, 6, 2665–2674. [Google Scholar]
- Nirmala, M.; Kavitha, B.; Banu, A. Optical analysis of ZnO thin films by SILAR method. J. Environ. Nanotechnol. 2018, 6, 36–41. [Google Scholar]
- Yergaliuly, G.; Baktiyar, S.; Sandugash, K.; Baenov, Z.; Almagul, M. Effect of thickness and reaction media on properties of ZnO thin films by SILAR. Sci. Rep. 2022, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.; Cruz, M.; Pineda, N.; Rivas, A.; Lopez, M.; Tostado, F. Growth of lily flower-like ZnO structures by Successive Ionic Layer Adsorption and Reaction method. In Microscopy and Imaging Science: Practical Approaches to Applied Research and Education; Mendez, A., Ed.; Formatex Research Center: Badajoz, Spain, 2017. [Google Scholar]
- Desai, D.; Joo, O.; Jung, K.; Min, K.; Pathan, H. Preparation and characterization of titanium dioxidethin films by SILAR method. Mater. Chem. Phys. 2006, 97, 5–9. [Google Scholar]
- Oommen, R.; Usha, R.; Saranya, R.; Sudha, A. Characterization of TiO2 thin films deposited by SILAR method. Adv. Mater. Res. 2013, 678, 75–79. [Google Scholar] [CrossRef]
- Garcia, N.; Segura, B.; Parra, E.; Lopez, A. Synthesis of TiO2 thin films by the SILAR method and study of the influence of annealing on its structural, morphological and optical properties. Ingeniare. Rev. Chil. Ing. 2015, 23, 622–629. [Google Scholar] [CrossRef]
- More, M.; Joo, S.; Lokhande, D.; Gunjaar, L. Fabrication of hydrophobic surface of titanium dioxide films by successive ionic layer adsorption and reaction (SILAR) method. Appl. Surf. Sci. 2009, 255, 6067–6072. [Google Scholar] [CrossRef]
- Agnes, N.; Chime, K.; Asogwa, L.; Nwanya, C.; Ezema, F.; Jen, T. A study on titanium dioxide nanoparticles synthesized from titanium isopropoxide under SILAR-induced gel method: Transition from anatase to rutile structure. Inorg. Chem. Commun. 2019, 112, 107705. [Google Scholar] [CrossRef]
- Patil, U.; Gurav, K.; Joo, O.; Lokhande, D. Synthesis of photosensitive nanograined TiO2 thin films by SILAR method. J. Alloys Compd. 2009, 478, 711–715. [Google Scholar] [CrossRef]
- Beevi, M.; Anusuya, M.; Saravanan, V. Characterization of CdO Thin Films Prepared By SILAR Deposition Technique. Int. J. Chem. Eng. Appl. 2010, 1, 151–154. [Google Scholar] [CrossRef]
- Nwanya, A.; Cosmas, C.; Osuji, U.; Ezugwu, S.; Malik, M.; Ezema, F. Transformation of cadmium hydroxide to cadmium oxide thin films synthesized by SILAR deposition process: Role of varying deposition cycles. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 20, 49–54. [Google Scholar] [CrossRef]
- Salunkhe, R.; Dhawale, D.; Gujar, P.; Lokhande, D. Structural, electrical and optical studies of SILAR deposited cadmium oxide thin films: Annealing effect. Mater. Res. Bull. 2009, 44, 364–368. [Google Scholar] [CrossRef]
- Rajini, M.; Marimuthu, K.; Kasinathan, K. An Investigation of SILAR Grown CdO Thin Films. Iran. J. Chem. Chem. Eng. 2019, 38, 11–17. [Google Scholar]
- Kulal, P.; Dubal, P.; Fulari, V.; Lokhande, D. Chemical synthesis of Fe2O3 thin films for supercapacitor application. J. Alloys Compd. 2011, 509, 2567–2571. [Google Scholar] [CrossRef]
- Sheik, S.; Mythili, N.; Mohamed, M.; Ravi, G.; Saravanakumar, K. Studies on the simplified SILAR deposited magnetite (Fe3O4) thin films annealed at different temperatures. J. Mater. Sci. Mater. Electron. 2016, 27, 3420–3426. [Google Scholar] [CrossRef]
- Fareed, S.; Ravi, G.; Murugan, R.; Mythili, N.; Mohamed, M. Properties of SILAR deposited magnetite (Fe3O4) thin films: Effect of bath temperatures. J. Mater. Sci. Mater. Electron. 2017, 28, 9450–9455. [Google Scholar] [CrossRef]
- Ozge, E. Effect of cycle numbers on the structural, linear and nonlinear optical properties in Fe2O3 thin films deposited by SILAR method. Curr. Appl. Phys. 2022, 34, 7–18. [Google Scholar]
- Sreekrishnan, R.; Karthika, S.; Roshima, S.; Rakhesh, V. Structural and electrical properties of tin oxide films deposited by SILAR and spin coating techniques. AIP Conf. Proc. 2019, 2162, 020137. [Google Scholar] [CrossRef]
- Shipra, R.; Kumar, S.; Kumar, S.; Kar, P.; Roy, P. Deposition of Tin Oxide Thin Films by Successive Ionic Layer Adsorption Reaction Method and Its Characterization. J. Nanosci. Nanotechnol. 2018, 18, 2569–2575. [Google Scholar]
- Yunus, A.; Ates, A.; Ali, M. Characteristics of SnO2 thin films prepared by SILAR. Solid State Sci. 2012, 14, 1282–1288. [Google Scholar]
- Joohee, J.; Yim, H.; Cho, Y.; Kang, D.; Choi, J. Optical and Electronic Properties of SnO2 Thin Films Fabricated Using the SILAR Method. J. Sens. Sci. Technol. 2015, 24, 364–367. [Google Scholar]
- Yunus, A.; Tuba, C. Fabrication and characterization of NiO thin films prepared by SILAR method. J. Alloys Compd. 2015, 625, 144–148. [Google Scholar]
- Tuba, C. Influence of annealing on properties of SILAR deposited nickel oxide films. Vacuum 2019, 167, 189–194. [Google Scholar]
- Mahima, D.; Roy, A.; Ayan, M.; Mitra, P.; Das, S. Influence of dipping cycle on SILAR synthesized NiO thin film for improved electrochemical performance. Electrochim. Acta 2018, 273, 105–114. [Google Scholar]
- Sandesh, U.; Navale, T.; Jadhav, V.; Swapnil, B.; Mu, N.; Patil, B.; Florian, J. Solution-processed nickel oxide films and their liquefied petroleum gas sensing activity. J. Alloys Compd. 2017, 695, 2008–2015. [Google Scholar]
- Agnes, C.; Nwanya, C.; Nwankwo, U.; Ekwealor, C.; Ezema, F.; Malik, M.; Bucher, R.; Rose, U. Structural, optical and electrochemical properties of SILAR-deposited zirconium-doped cadmium oxide thin films. Mater. Res. Express 2019, 6, 096439. [Google Scholar] [CrossRef]
- Tuba, T. Synthesis of copper-doped nickel oxide thin films: Structural and optical studies. Chem. Phys. Lett. 2020, 738, 136884. [Google Scholar] [CrossRef]
- Sales, A.; Natarajan, G.; Joycee, S. Investigation of the properties of tungsten doped ZnO thin films synthesized by SILAR method. Mater. Res. Innov. 2021, 26, 263–269. [Google Scholar] [CrossRef]
- Zahirullah, S.; Joseph, P.; Fermi, P. Structural and optical properties of Cu-doped ZnO nanorods by silar method. Mater. Technol. 2017, 32, 755–763. [Google Scholar] [CrossRef]
- Devi, K.; Selvan, G.; Raj, P.; Ganesh, V.; Karunakaran, M. Enhanced room temperature ammonia gas sensing properties of strontium doped ZnO thin films by cost-effective SILAR method. Mater. Sci. Semicond. Process. 2020, 119, 105117. [Google Scholar] [CrossRef]
- Amalraj, S.; Joycee, S.; Rajah, J. Influence of Ni dopant on surface morphology of nanostructured ZnO thin films grown by SILAR method. Mater. Res. Innov. 2019, 24, 341–348. [Google Scholar] [CrossRef]
- Taskopru, T.; Zor, M.; Sahin, B.; Bayansal, F. Structural and optical properties of Co-doped NiO films prepared by SILAR method. Philos. Mag. 2014, 95, 32–40. [Google Scholar] [CrossRef]
- Ozkan, B.; Erdal, I.; Harun, G.; Onder, S. The role of cobalt doping on the optical and structural properties of Mn3O4 nanostructured thin films obtained by SILAR technique. Superlattices Microstruct. 2019, 128, 212–220. [Google Scholar]
- Guder, H.; Gulen, Y.; Bayansal, F.; Sahin, B. Fabrication and characterization of Mn-doped CuO thin films by the SILAR method. Ceram. Int. 2013, 39, 6475–6480. [Google Scholar]
- Navathe, G.; Jadhav, P.; Shinde, V. Manganese oxide thin films deposited by SILAR method for supercapacitor application. AIP Conf. Proc. 2013, 1536, 679. [Google Scholar] [CrossRef]
- Mangesh, D.; Amol, S.; Bhapkar, A.; Ganesh, D.; Sartale, D. An investigation of chemical and electrochemical conversion of SILAR grown Mn3O4 into MnO2 thin films. J. Environ. Manag. 2021, 299, 113564. [Google Scholar] [CrossRef]
- Nkele, A.; Chime, U.; Blessing, E.; Nwanya, A.; Malik, M.; Paul, M.; Rose, U. Enhanced electrochemical property of SILAR-deposited Mn3O4 thin films decorated on graphene. J. Mater. Res. Technol. 2020, 9, 9049–9058. [Google Scholar] [CrossRef]
- Malavekar, D.; Mane, V.; Ubale, S.; Lokhande, D.; Lokhande, V. Manganese dioxide thin films deposited by chemical bath and successive ionic layer adsorption and reaction deposition methods and their supercapacitive performance. Inorg. Chem. Commun. 2020, 119, 105117. [Google Scholar] [CrossRef]
- Waikar, M.; Shaikh, A.; Sonkawade, R. Effect of different precursors on electrochemical properties of manganese oxide thin films prepared by SILAR method. Synth. Met. 2019, 247, 1–9. [Google Scholar]
- Immanuel, P.; Mohanraj, K.; Senguttuvan, G. Enhanced Activity of Chemically Synthesized Nanorod Mn3O4 Thin Films for High Performance Supercapacitors. Int. J. Thin Films Sci. Technol. 2020, 9, 57–67. [Google Scholar]
- Nanasaheb, M.; Ajay, D.; Vijay, S.; Tanka, R.; Kim, J.; Lokhande, D. Wet chemical synthesis of WO3 thin films for supercapacitor application. Korean J. Chem. Eng. 2015, 32, 974–979. [Google Scholar]
- Jayeoba, B.; Olowofila, I. Successive Ionic Layer and Absorption Reaction (SILAR): A synthesis technique of optimising the properties of zinc oxide/silver (Zno-Ag) nanocomposite thin films. In Proceedings of the 4th National Development conference of the School Pure and Applied Sciences, Ilaro, Nigeria, 2–5 December 2019; The Federal Polytechnic: Nekede, Nigeria, 2019. [Google Scholar]
- Kathalingam, A.; Ali, A.; Yang, W.; Kim, H.; Valanarasu, S. Photosensing effect of indium-doped ZnO thin films and its heterostructure with silicon. J. Asian Ceram. Soc. 2022, 10, 108–119. [Google Scholar] [CrossRef]
- Akaltun, Y.; Aslan, M.; Yetim, T.; Celik, A.; Cayir, T. The effect of wettability on corrosion resistance of oxide films produced by SILAR method on magnesium, aluminum and copper substrates. Surf. Coat. Technol. 2016, 292, 121–131. [Google Scholar] [CrossRef]
- Edison, D.; Nirmala, W.; Arun, K.; Ganesh, V.; Mohd, S.; Valanarasu, S. Structural, optical and nonlinear optical studies of AZO thin film prepared by SILAR method for electro-optic applications. Phys. B Condens. Matter 2017, 523, 31–38. [Google Scholar] [CrossRef]
- Shrikant, S.; Bisen, O.; Sankpal, R. Synthesis of interconnected needle-like Bi2O3 using successive ionic layer adsorption and reaction towards supercapacitor application. Ionics 2017, 23, 1831–1837. [Google Scholar] [CrossRef]
- Ozaslan, D.; Erken, O.; Gunes, M.; Gumus, C. The effect of annealing temperature on the physical properties of Cu2O thin film deposited by SILAR method. Phys. B Condens. Matter 2019, 580, 411922. [Google Scholar] [CrossRef]
- Lohar, G.; Patil, A.; Fulari, J. Structural, morphological, optical and photoelectrochemical cell properties of copper oxide using modified SILAR method. J. Mater. Sci. Mater. Electron. 2016, 27, 9550–9557. [Google Scholar]
- Alauddin, M.; Syed, F.; Islam, N.; Tooru, T.; Guo, Q.; Jamal, U.; Majed, M.; Chang, H. Facile synthesis of Cu2O nanorods in the presence of NaCl by successive ionic layer adsorption and reaction method and its characterizations. Royal Soc. Open Sci. 2022, 9, 211899. [Google Scholar] [CrossRef]
- Rafea, M.; Rousshdy, N. Determination of the optical band gap for amorphous and nanocrystalline copper oxide thin films prepared by SILAR technique. J. Phys. D Appl. Phys. 2009, 42, 015413. [Google Scholar] [CrossRef]
- Lee, W.; Wang, J. Structural, Optical, and Electrical Properties of Copper Oxide Films Grown by the SILAR Method with Post-Annealing. Coatings 2021, 11, 864. [Google Scholar] [CrossRef]
- Farid, S.; Hossain, A.; Tanvir, I.; Rumana, A.; Abdul, M.; Atiqur, R. Structural, optical, electrical, and photoelectrochemical properties of cuprous oxide thin films grown by modified SILAR method. Mater. Sci. Semicond. Process. 2019, 95, 68–75. [Google Scholar] [CrossRef]
- Kandalkar, S.; Lokhande, D.; Gunjakar, L. Preparation of cobalt oxide thin films and its use in supercapacitor application. Appl. Surf. Sci. 2008, 254, 5540–5544. [Google Scholar] [CrossRef]
- Raut, S.; Sankapal, R. First report on synthesis of ZnFe2O4 thin film using successive ionic layer adsorption and reaction: Approach towards solid-state symmetric supercapacitor device. Electrochim Acta 2016, 198, 203–211. [Google Scholar] [CrossRef]

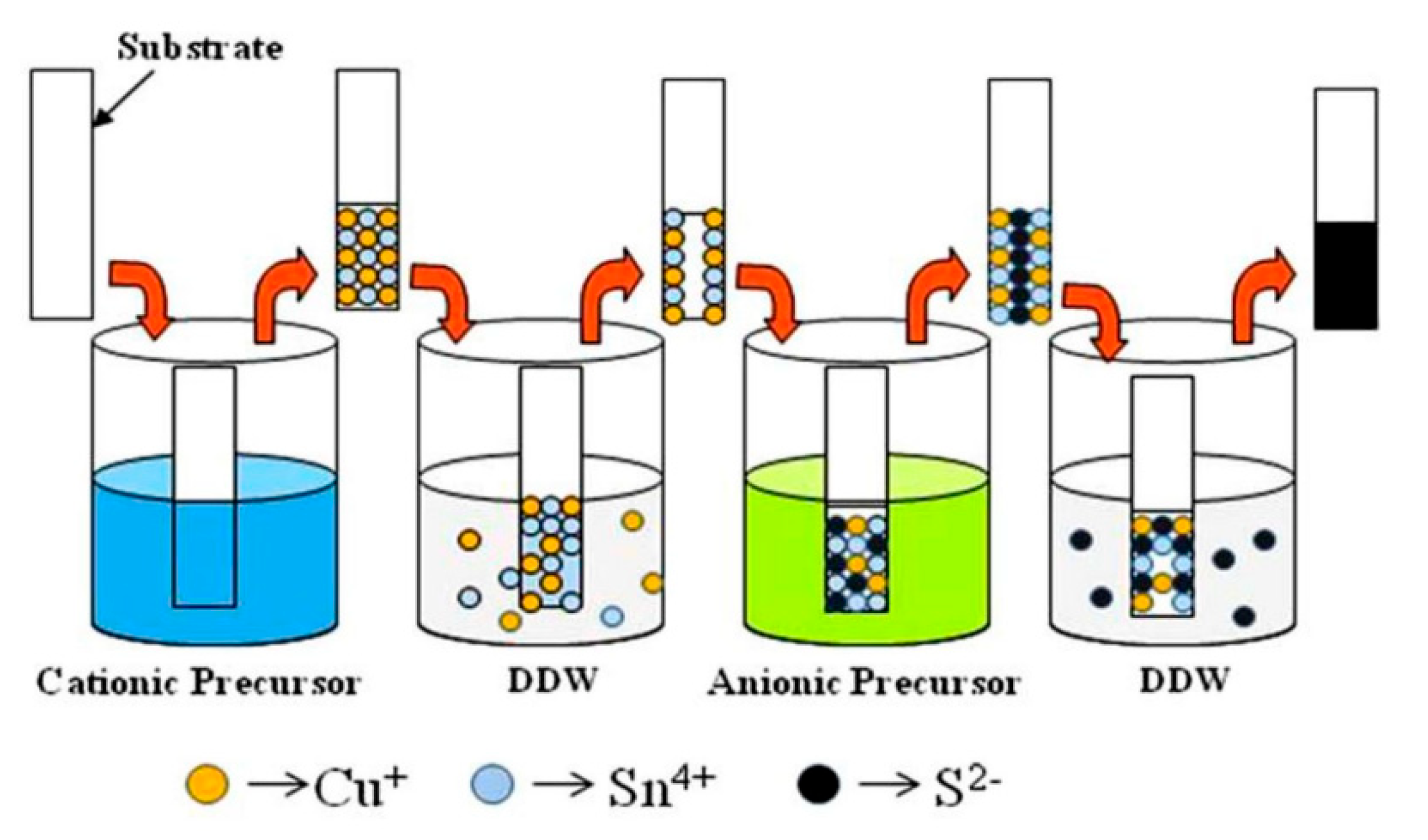
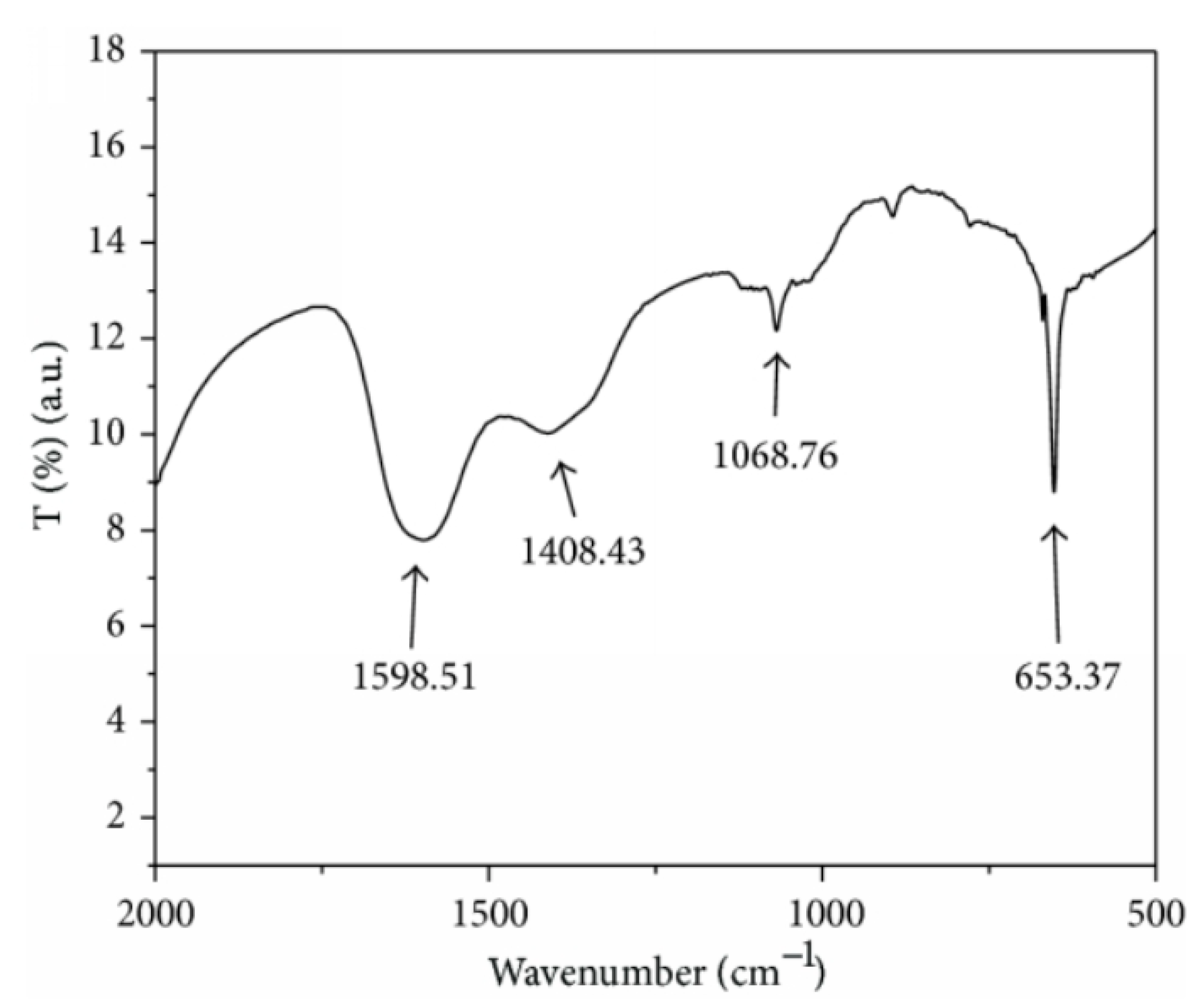
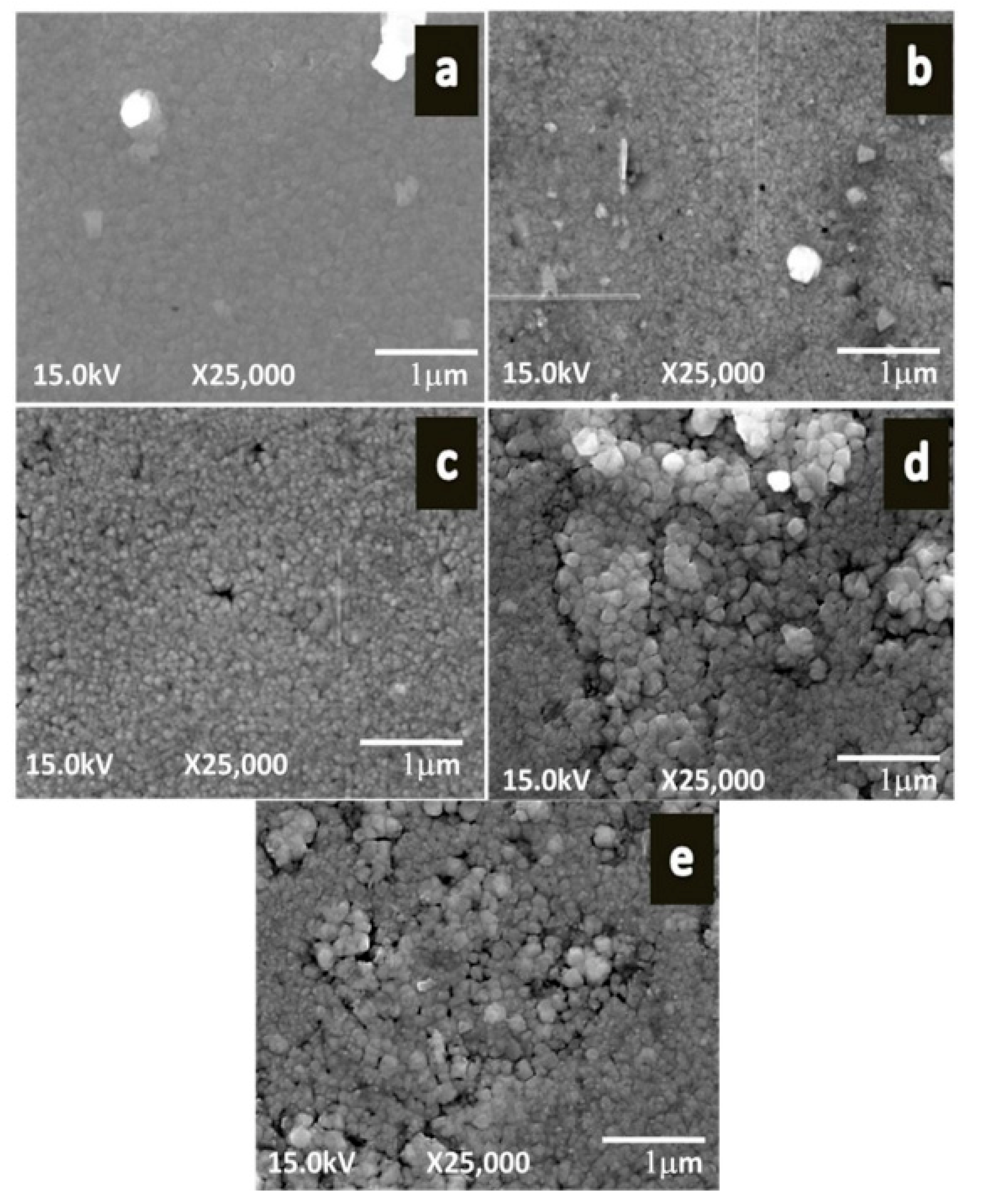
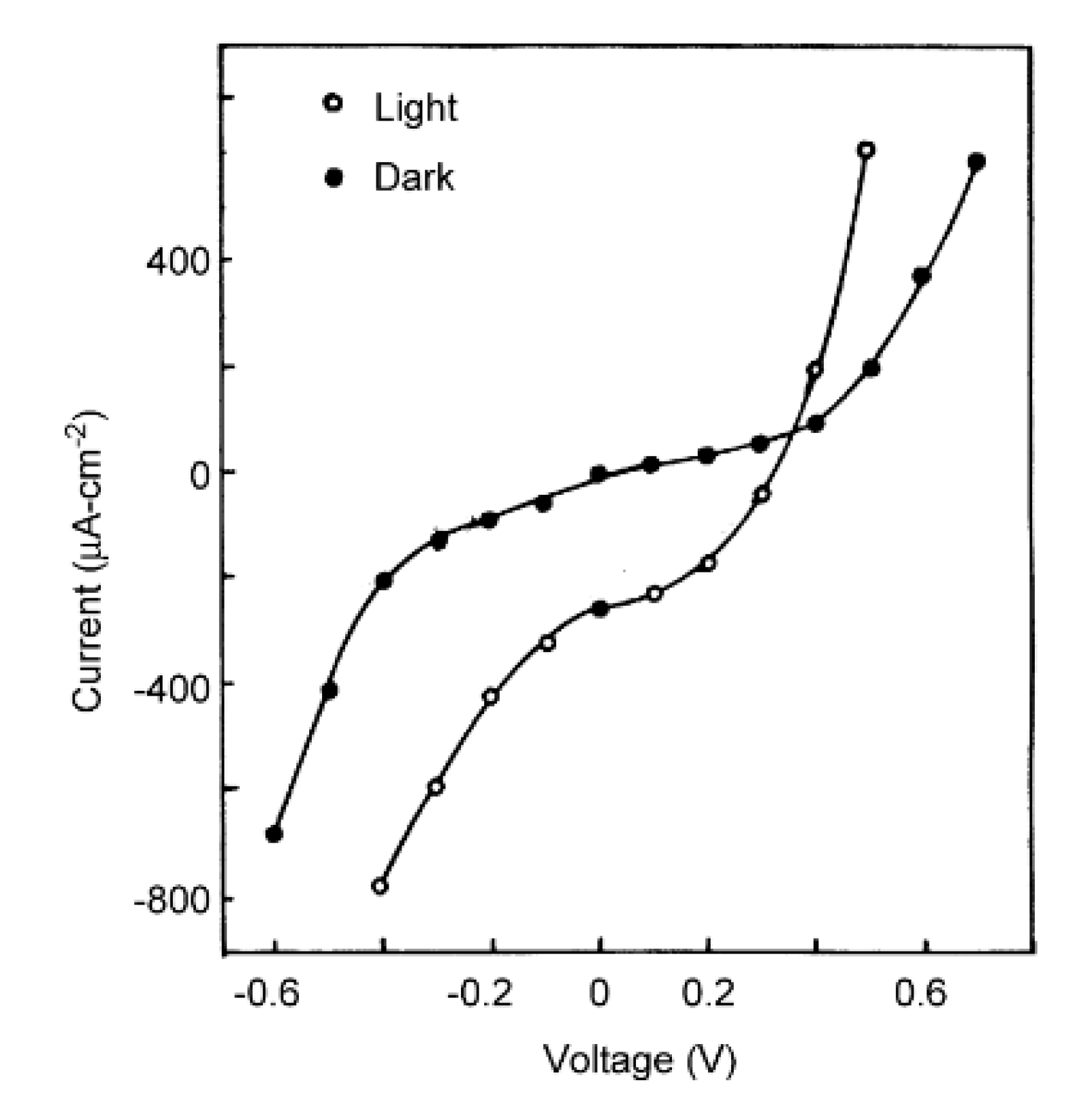
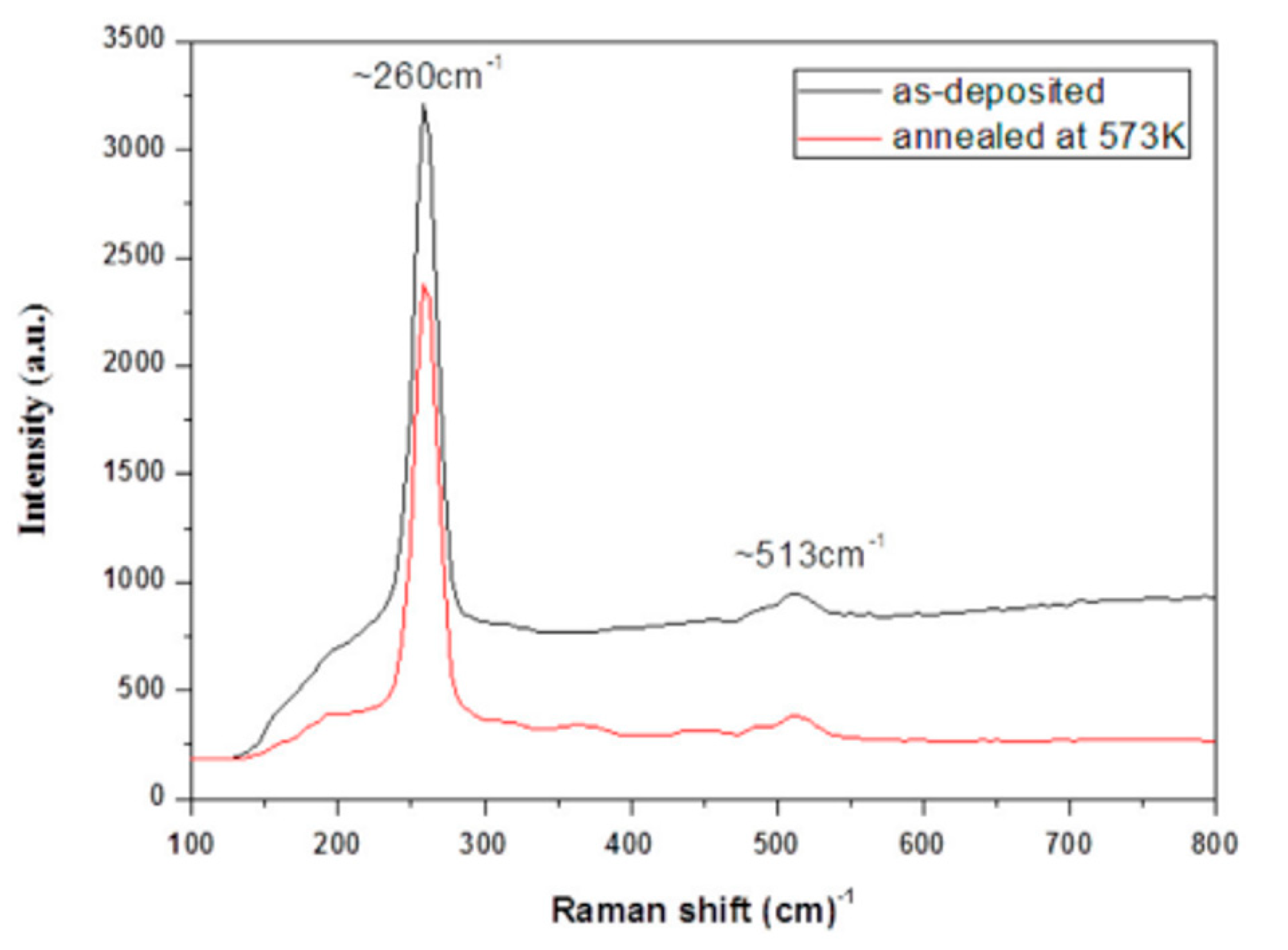
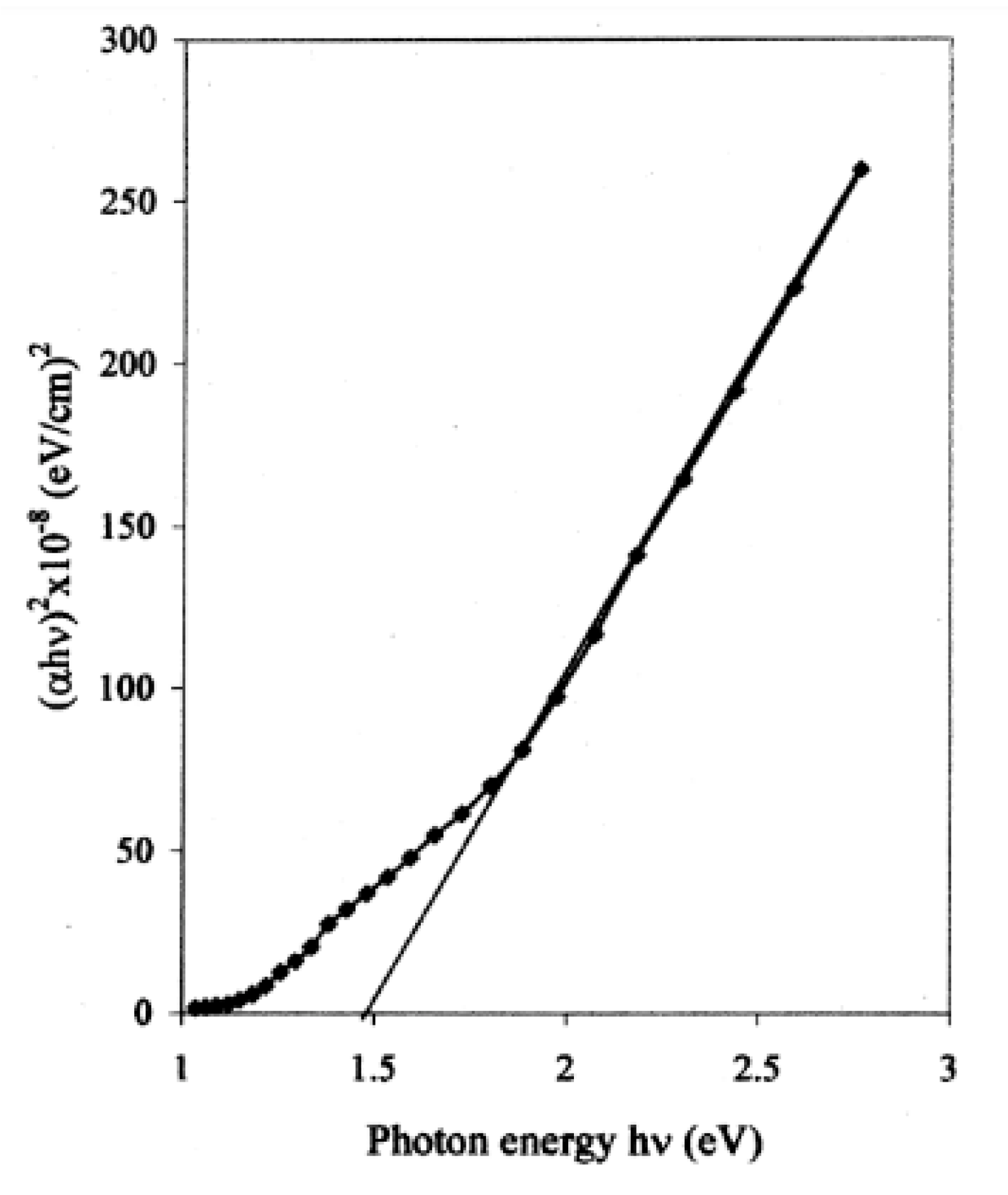
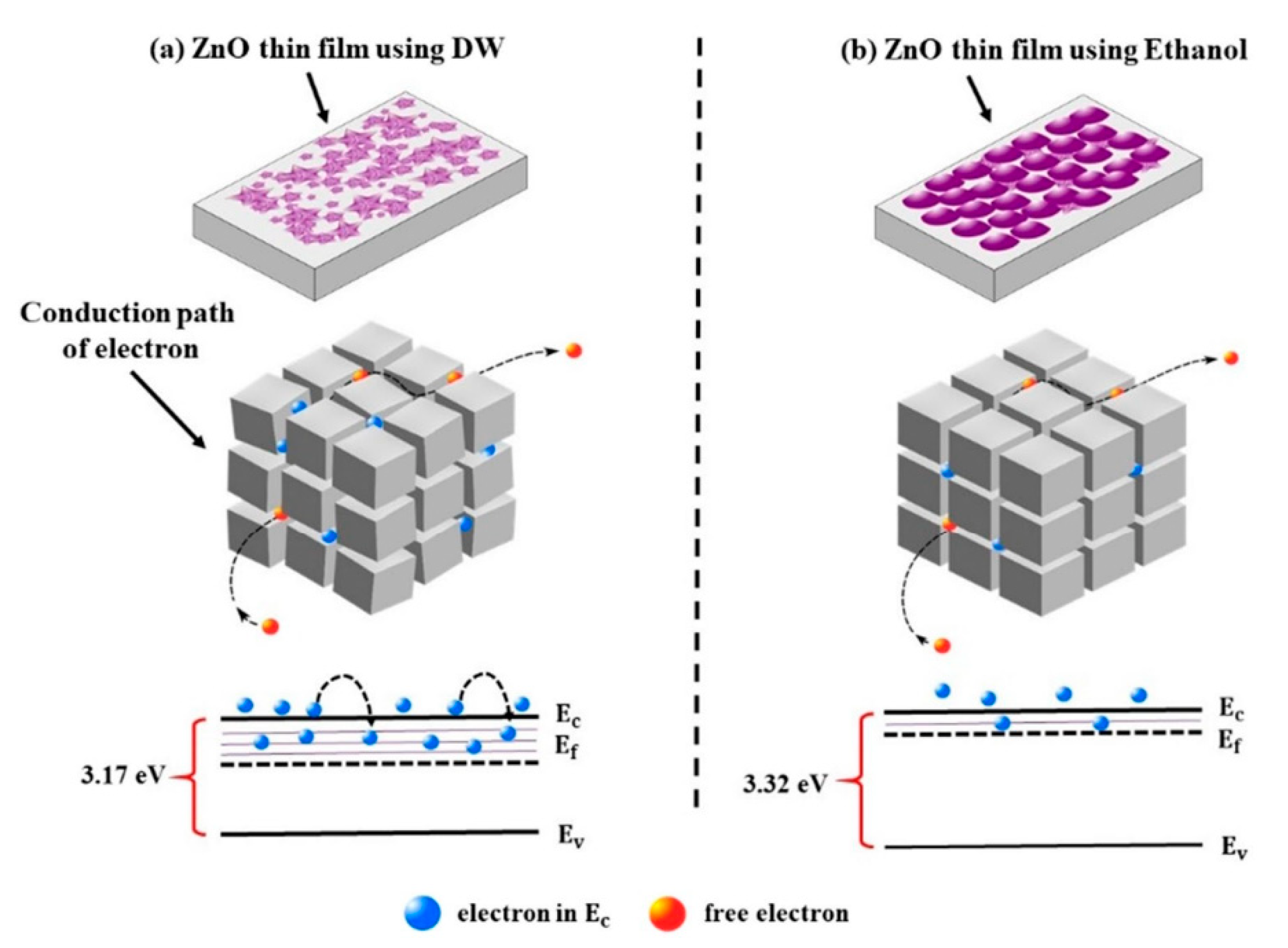
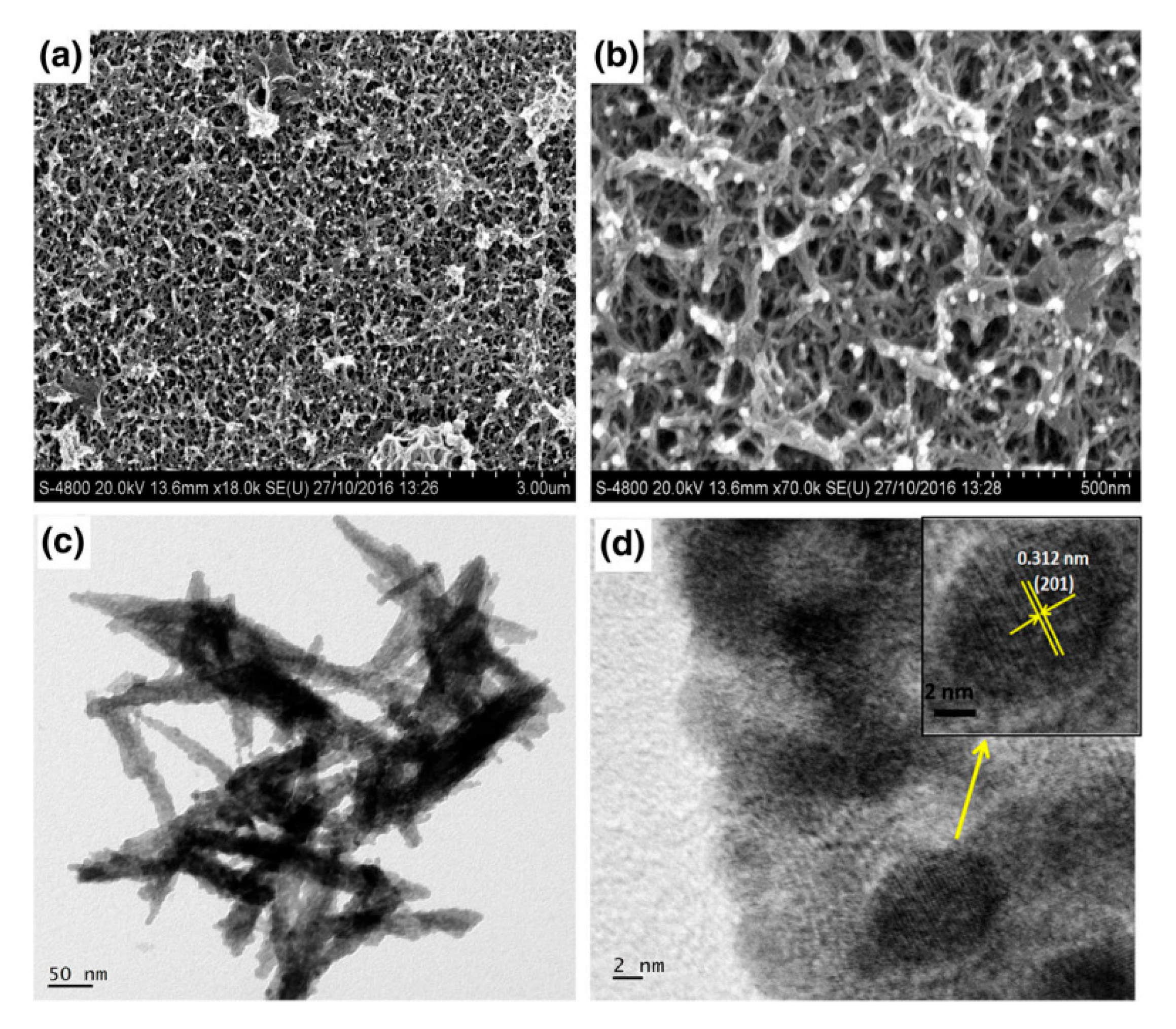
| Deposition Method | Benefits | Disadvantages |
|---|---|---|
| Spray pyrolysis | Low-cost deposition method, high growth rate | Very complicated process; low yield |
| Chemical bath deposition | Cheaper and simple method; deposition process requires substrate and solution in the container | Wastage of solution after the deposition process |
| Electron beam evaporation | Multiple thin films can be produced | The non-uniform evaporating rate could be seen due to filament degradation |
| Electrodeposition method | Cheaper deposition method; high deposition rate | Not suitable for large-scale production |
| Ion beam deposition | Uniform morphology of the obtained films | Deposition rate very low; expensive |
| Atomic layer epitaxy | Can synthesize high-quality films | High energy waste rate observed during the deposition process |
| Chemical vapor deposition | Can synthesis thick films under a high deposition rate | High temperature is high-temperature deposition during the deposition |
| Molecular beam epitaxy | High purity and epitaxial materials could be produced | Very expensive |
| Magnetron sputtering | High adhesion and uniform morphology of the obtained films | Very expensive; low deposition rate |
| Pulsed laser deposition | Dense and porous morphology observed in the obtained samples | Very expensive |
| Spin coating | Very thin, fine, and uniform morphology of the obtained sample via this method | High-speed spinning becomes very difficult when the size of the substrate increases |
| Thermal evaporation | High deposition rate during the deposition process | Very poor coverage due to low vacuum |
| Similarity Results: | ||
|---|---|---|
| ||
| Differences: | ||
| Properties | Thermal evaporated films | SILAR-deposited films |
| Color of the thin films | Violet in color. | Bluish white in color. |
| Structure of the films | Strong peak corresponding to the (111) plane. | Strong peak corresponding to (111), (220), and (311) planes. |
| Stress | Stress increased when the film thickness increased. This technique needs high thermal energy and the vapor atoms have high thermal energy. The film thickness increased during the experiment and was exposed to already-synthesized layers (to the heat radiated from the evaporation boat). | Stress reduced when the film thickness increased. This deposition method requires low temperatures. When the film thickness increased during the experiment, subsequent layers were produced on the films (already synthesized on the glass substrate). |
| Crystallinity | Better crystallinity could be observed due to the higher surface mobility of the impinging atoms. | Poor crystallinity. |
| Crystallite size | Larger crystallite size could be observed due to the higher adatom mobility; 19, 29, and 32 nm were found when the film thickness was 330, 440, and 550 nm, respectively. | Smaller crystallite size could be observed; 4.1, 6.2, and 12.4 nm were found when the film thickness was 330, 440, and 550 nm, respectively. |
| Absorption value | Low absorption in the ultraviolet region. | High absorption in the ultraviolet region due to the granular structure. |
| Absorption coefficient | Low absorption coefficient value. | Can absorb UV light more efficiently and produce high optoelectronic conversion efficiency because of the high absorption coefficient value. |
| Carrier mobility | A higher carrier mobility could be observed (23 to 37.5 cm2V−1⋅S−1) when the film thickness was 330–550 nm. | A low carrier mobility could be observed (6.5 to 18.5 cm2V−1⋅S−1) when the film thickness was 330–550 nm. |
| Carrier concentration | A higher carrier concentration could be observed (26 × 1014 to 73 × 1014 cm−3) when the film thickness was 330–550 nm. | A low carrier concentration could be observed (0.8 × 1014 to 10 × 1014 cm−3) when the film thickness was 330–550 nm. |
| Resistivity | Low resistivity could be observed (4.3 to 10.8 ohm-cm) when the film thickness was 330–550 nm. | High resistivity could be observed (37.6 to 62.5 ohm-cm) when the film thickness was 330–550 nm. |
| Thin Films | Crystal Structure | Band Gap Energy | Carrier Concentration (cm−3) | Carrier Mobility (cm2/V/S) | Resistivity (Ω·cm) |
|---|---|---|---|---|---|
| As-deposited films | Cu2O | 1.9 | 2.9 × 1015 | 1.8 | 1.22 × 103 |
| 200 °C, 60 min annealed | Cu2O | 1.87 | 1 × 1014 | 51.1 | 1.27 × 103 |
| 300 °C, 60 min annealed | Cu2O and CuO | 1.4 | 1.8 × 1012 | 93.7 | 3.72 × 104 |
| 300 °C, 120 min annealed | CuO | 1.36 | 8 × 1014 | 10.7 | 7.94 × 102 |
| 300 °C, 240 min annealed | CuO | 1.34 | 7.4 × 1016 | 2.3 | 1.02 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soonmin, H. Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films. Appl. Sci. 2022, 12, 8184. https://doi.org/10.3390/app12168184
Soonmin H. Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films. Applied Sciences. 2022; 12(16):8184. https://doi.org/10.3390/app12168184
Chicago/Turabian StyleSoonmin, Ho. 2022. "Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films" Applied Sciences 12, no. 16: 8184. https://doi.org/10.3390/app12168184
APA StyleSoonmin, H. (2022). Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films. Applied Sciences, 12(16), 8184. https://doi.org/10.3390/app12168184






