Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of P. ostreatus Mushrooms
2.2. Synthesis of Mushroom ZnO NPs (Zinc Oxide Nanoparticles)
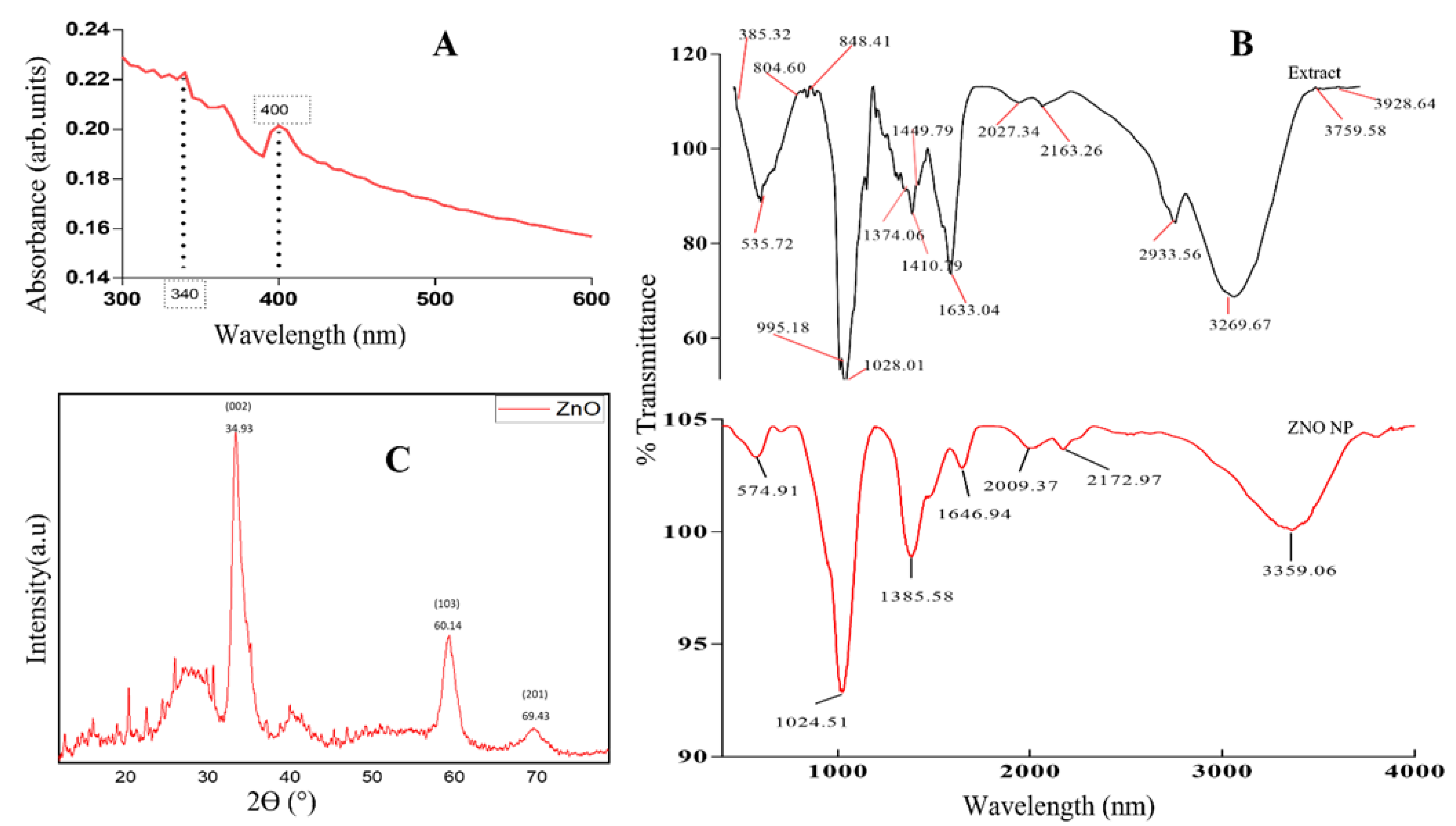
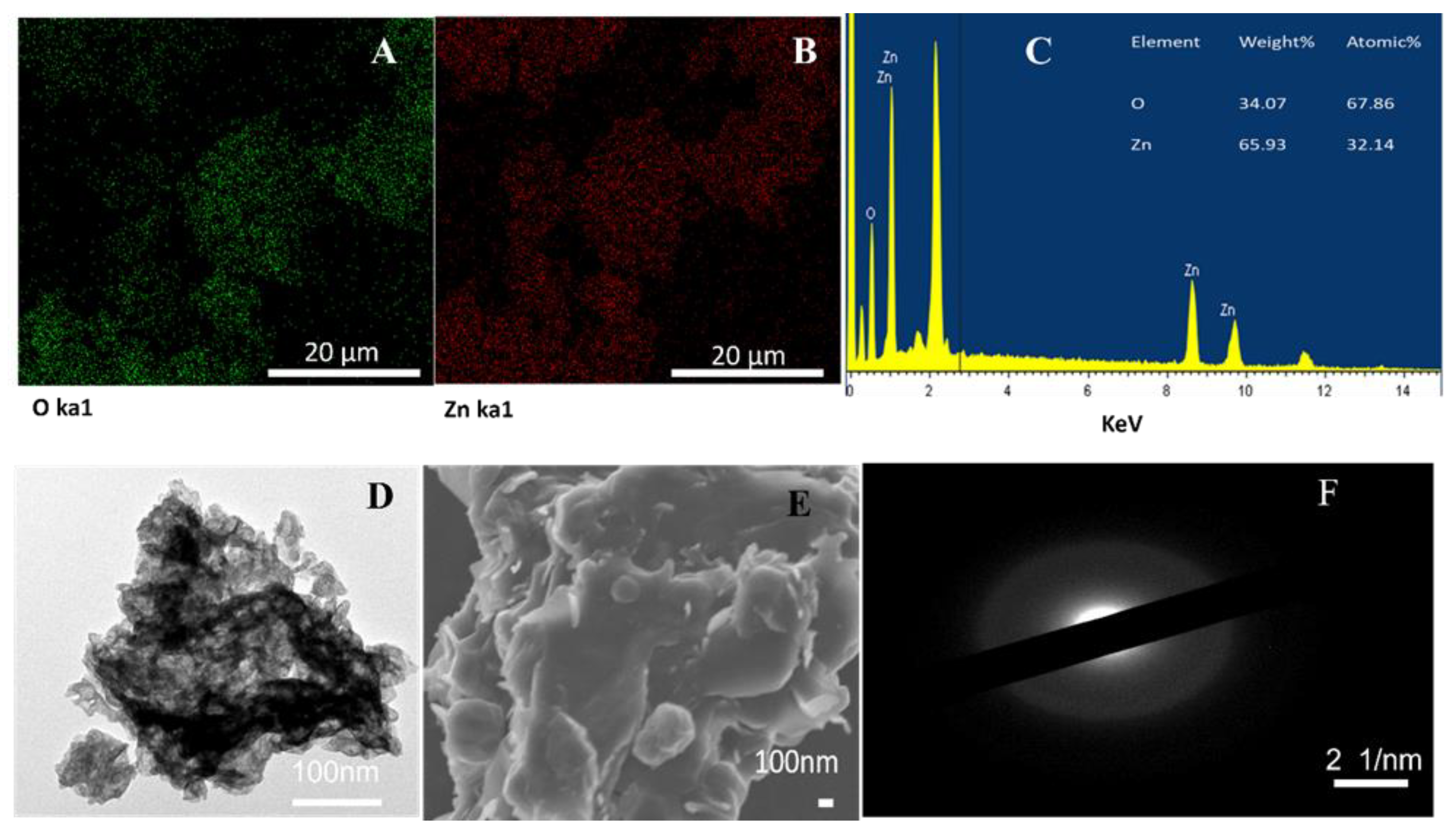
2.3. Characterization of ZnO NPs
2.4. Antimicrobial Activity Assay
2.5. DNA Cleavage Studies
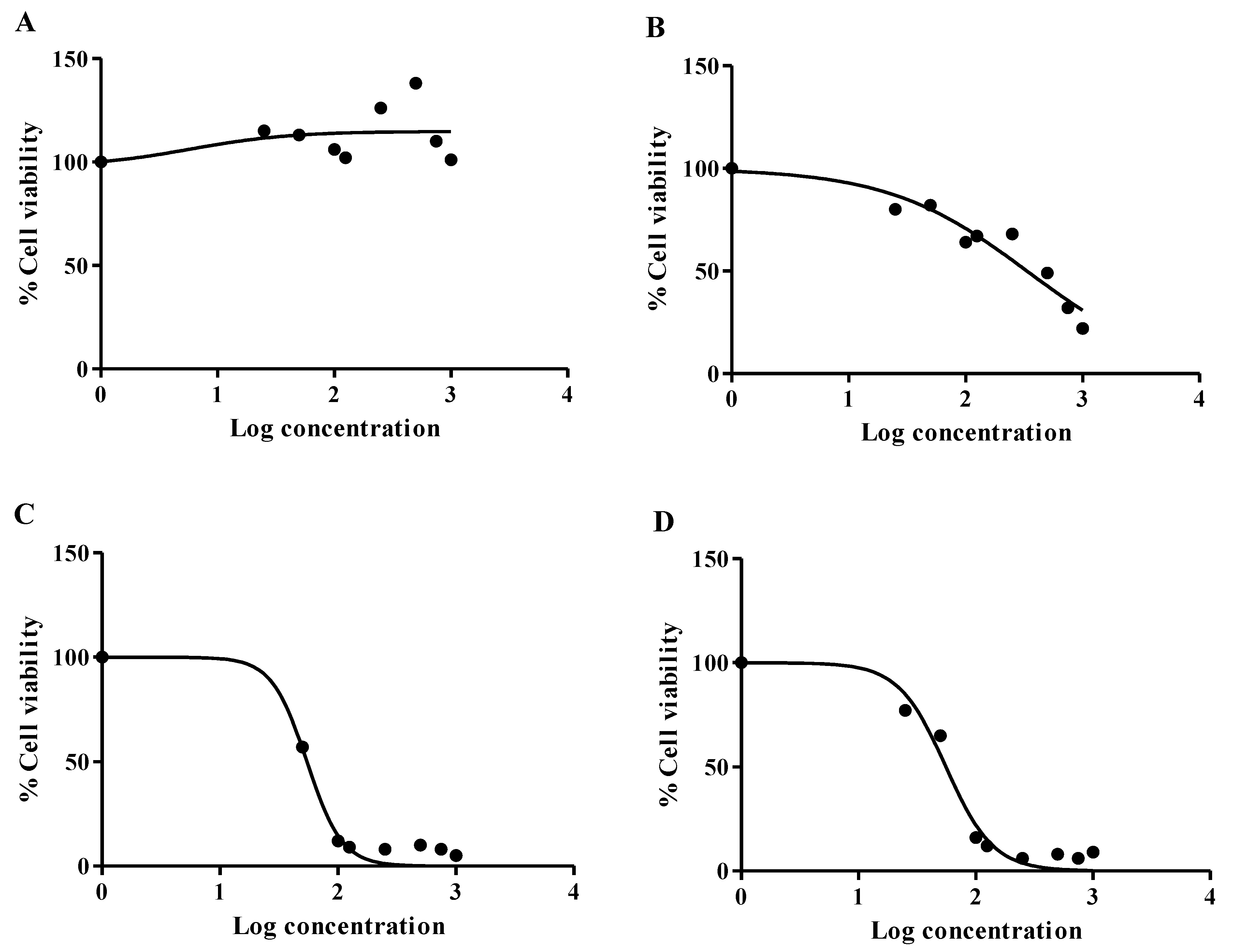
2.6. Cytotoxicity Assay
2.7. Statistical Analysis of Results
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ye, F.; Zhao, Y.; El-Sayed, R.; Muhammed, M.; Hassan, M. Advances in nanotechnology for cancer biomarkers. Nano Today 2018, 18, 103–123. [Google Scholar] [CrossRef]
- Ovais, M.; Raza, A.; Naz, S.; Islam, N.U.; Khalil, A.T.; Ali, S.; Khan, M.A.; Shinwari, Z.K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; Debella, M.; Dibella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N. Biomedical Applications of Nanoparticles Synthesized from Mushrooms. In Green Nanoparticles. Nanotechnology in the Life Sciences; Patra, J., Fraceto, L., Das, G., Campos, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 289–303. [Google Scholar] [CrossRef]
- Mthana, M.S.; Mthiyane, D.M.N.; Onwudiwe, D.C.; Singh, M. Biosynthesis of ZnO Nanoparticles Using Capsicum chinense Fruit Extract and Their In Vitro Cytotoxicity and Antioxidant Assay. Appl. Sci. 2022, 12, 4451. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sharma, G.; Nam, J.S.; Sharma, A.R.; Lee, S.S. Antimicrobial potential of silver nanoparticles synthesized using medicinal herb coptidis rhizome. Molecules 2018, 23, 2268. [Google Scholar] [CrossRef]
- Muzammil, S.; Hayat, S.; Fakhar-e-Alam, M.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A.; et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci. Elite 2018, 10, 352–374. [Google Scholar] [CrossRef] [Green Version]
- Mickymaray, S. One-step synthesis of silver nanoparticles using Saudi arabian desert seasonal plant Sisymbrium irio and antibacterial activity against multidrug-resistant bacterial strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Bhonchal Bhardwaj, S. Enterococci: An Important Nosocomial Pathogen. In Pathogenic Bacteria; IntechOpen: London, UK, 2020; ISBN 978-1-78985-988-1. [Google Scholar] [CrossRef]
- Labovská, S. Pseudomonas aeruginosa as a Cause of Nosocomial Infections. In Pseudomonas aeruginosa—Biofilm Formation, Infections and Treatments; IntechOpen: London, UK, 2021; ISBN 978-1-83968-648-1. [Google Scholar] [CrossRef]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: Implications of a mutually antagonistic relationship on virulence and inflammation in the CF airway. Front. Microbiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Lee, K.; Lee, K.M.; Kim, D.; Yoona, S.S. Molecular determinants of the thickened matrix in a dual-species Pseudomonas aeruginosa and Enterococcus faecalis biofilm. Appl. Environ. Microbiol. 2017, 83, e01182-17. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Guarcello, R.; Venturella, G.; Palazzolo, E.; Francesca, N.; Moschetti, G.; Settanni, L.; Saporita, P.; Gargano, M.L. Microbiological, chemical and sensory aspects of bread supplemented with different percentages of the culinary mushroom Pleurotus eryngii in powder form. Int. J. Food Sci. Technol. 2019, 54, 1197–1205. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Gonçalves, O.; Pereira, R.; Gonçalves, F.; Mendo, S.; Coimbra, M.A.; Rocha, S.M. Evaluation of the mutagenicity of sesquit-erpenic compounds and their influence on the susceptibility towards antibiotics of two clinically relevant bacterial strains. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 18–25. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, G. Biosynthesis of Nanoparticles Using Mushrooms. In Biology of Macrofungi; Springer Nature: Cham, Switzerland, 2018; pp. 351–360. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Khaliullin, S.M.; Zhuravlev, V.D.; Ermakova, L.V.; Buldakova, L.Y.; Yanchenko, M.Y.; Porotnikova, N.M. Solution Combustion Synthesis of ZnO Using Binary Fuel (Glycine + Citric Acid). Int. J. Self Propagating High Temp. Synth. 2019, 28, 226–232. [Google Scholar] [CrossRef]
- Morin, J.; Fujimoto, K.; Preston, A.; Guillen, D.P. Synthesis Methods for Nanoparticle Morphology Control in Energy Applications. In The Minerals, Metals and Materials Series; Springer Nature: Cham, Switzerland, 2022; pp. 21–31. [Google Scholar] [CrossRef]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Banerjee, K.; Ravishankar Rai, V. A Review on Mycosynthesis, Mechanism, and Characterization of Silver and Gold Nanoparticles. Bionanoscience 2018, 8, 17–31. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Cloete, J.; Basson, A.K.; Zharare, G.E. Performance of Pleurotus ostreatus mushroom grown on maize stalk residues supplemented with various levels of maize flour and wheat bran. Food Sci. Technol. 2016, 36, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Mkhize, S.S.; Simelane, M.B.C.; Gasa, N.L.; Pooe, O.J. Evaluating the antioxidant and heavy metal content of Pleurotus ostreatus mushrooms cultivated using sugar cane agro-waste. Pharmacogn. J. 2021, 13, 844–852. [Google Scholar] [CrossRef]
- Muhammad, F.R.; Nurgaha, E.S.; Fahim, M.T. Synthesis of ZnO nanoparticles by precipitation method with their antibacterial effect. Indones. J. Chem. 2016, 16, 117–123. [Google Scholar] [CrossRef]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) C.A.Sm—Medicinal plants used in South Africa. BMC Complement. Altern. Med. 2018, 18, 315. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Naghiha, R.; Kheirizadeh, M.; Sadatfaraji, H.; Mirzaei, A.; Alvand, Z.M. Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: Synthesis, characterization, and biological properties. Mater. Sci. Eng. C 2017, 78, 1109–1118. [Google Scholar] [CrossRef]
- Kongsema, M.; Tadakittisarn, S.; Chumnanpuen, P. Riceberry rice bran protein hydrolyzed fractions 2 induced apoptosis, senescence and G1/S cell cycle 3 arrest in human colon cancer cell lines 4 5 Vichugorn Wattayagorn. Appl. Sci. 2022, 12, 6917. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Nasser Alabdan, A.; Alharbi, S.; Ali Mohammed Alateeq, B.; Abdulrahman Al Mohsen, A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological Applications of Synthesized ZnO Nanoparticles Using Pleurotus djamor Against Mosquito Larvicidal, Histopathology, Antibacterial, Antioxidant and Anticancer Effect. J. Clust. Sci. 2021, 32, 1635–1647. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Daumann, S.; Andrzejewski, D.; Di Marcantonio, M.; Hagemann, U.; Wepfer, S.; Vollkommer, F.; Bacher, G.; Epple, M.; Nannen, E. Water-free synthesis of ZnO quantum dots for application as an electron injection layer in light-emitting electrochemical cells. J. Mater. Chem. C 2017, 5, 2344–2351. [Google Scholar] [CrossRef]
- Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Abilaji, S.; Prakash, C.; Rajasekar, A.; Nanthini, A.U.R.; Valli, G. Myco-Synthesis of Zinc Oxide Nanoparticles as Potent Anti-corrosion of Copper in Cooling Towers. J. Clust. Sci. 2019, 30, 1583–1590. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Synthesis of zinc oxide using Agaricus bisporus and its in-vitro biological activities. J. Environ. Chem. Eng. 2020, 8, 104192. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Sun, S. Preparation and characterization of ZnO nanoparticles supported on amorphous SiO2. Nanomaterials 2017, 7, 217. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An in-vitro investigation of swelling controlled delivery of insulin from egg albumin nanocarriers. Iran. J. Pharm. Res. 2016, 15, 695–711. [Google Scholar] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-mediated zinc oxide nanoparticles: Advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics 2021, 13, 1662. [Google Scholar] [CrossRef]
- Espitia, P.J.; Soares, N.D.; Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1022–S1031. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Anitha, R.; Ramesh, K.V.; Ravishankar, T.N.; Sudheer Kumar, K.H.; Ramakrishnappa, T. Cytotoxicity, antibacterial and an-tifungal activities of ZnO nanoparticles prepared by the Artocarpus gomezianus fruit mediated facile green combustion method. J. Sci. Adv. Mater. Devices 2018, 3, 440–451. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Zhang, L.; Zhang, C.; Peng, H.; Lan, F.; Peng, S.; Liu, C.; Guo, J. Zinc oxide nanoparticles induce mitochondrial biogenesis impairment and cardiac dysfunction in human ipsc-derived cardiomyocytes. Int. J. Nanomed. 2020, 15, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Bae, H.C.; Kim, J.; Jeong, S.H.; Yang, S.I.; Son, S.W. Zinc oxide nanoparticles induce HIF-1α protein stabilization through increased reactive oxygen species generation from electron transfer chain complex III of mitochondria. J. Dermatol. Sci. 2018, 91, 104–107. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- De Lima, R.; Feitosa, L.; Pereira, A.D.; De Moura, M.R.; Aouada, F.A.; Mattoso, L.H.; Fraceto, L.F. Evaluation of the genotoxicity of chitosan nanoparticles for use in food packaging films. J. Food Sci. 2010, 75, N89–N96. [Google Scholar] [CrossRef]
- Rafeeq, C.M.; Paul, E.; Vidya Saagar, E.; Manzur Ali, P.P. Mycosynthesis of zinc oxide nanoparticles using Pleurotus floridanus and optimization of process parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]


| Microorganisms | Mushroom ZnO NPs | Mushroom Extract | * Control Drug |
|---|---|---|---|
| P. aeruginosa | 0.04 | 0.04 | 0.003 |
| E. faecalis | 0.08 | 1.25 | 0.006 |
| C. albicans | 0.31 | 0.63 | 0.003 |
| K. pneumoniae | 0.16 | 0.63 | 0.008 |
| B. cereus | 0.63 | 1.25 | 0.004 |
| P. vulgaris | 0.31 | 0.16 | 0.001 |
| S. aureus | 0.63 | 0.63 | 0.004 |
| E. coli | 0.16 | 0.63 | 0.001 |
| M. catarrhalis | 1.25 | 1.25 | 0.003 |
| M. hominis | 0.16 | 0.63 | 0.004 |
| Sample | Cell Lines | IC25 (mg/mL) | IC50 (mg/mL) |
|---|---|---|---|
| Mushroom extract | Hek293 | 0.074 (74 µg/mL) | 0.335 (335 µg/mL) |
| HepG2 | - | - | |
| Mushroom ZnO NPs | Hek293 | 0.034 (34 µg/mL) | 0.056 (56 µg/mL) |
| HepG2 | 0.037 (37 µg/mL) | 0.055 (55 µg/mL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhize, S.S.; Pooe, O.J.; Khoza, S.; Mongalo, I.N.; Khan, R.; Simelane, M.B.C. Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom. Appl. Sci. 2022, 12, 8563. https://doi.org/10.3390/app12178563
Mkhize SS, Pooe OJ, Khoza S, Mongalo IN, Khan R, Simelane MBC. Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom. Applied Sciences. 2022; 12(17):8563. https://doi.org/10.3390/app12178563
Chicago/Turabian StyleMkhize, Senzosenkosi Surprise, Ofentse Jacob Pooe, Sandile Khoza, Ishmael Nkoana Mongalo, Rene Khan, and Mthokozisi Blessing Cedric Simelane. 2022. "Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom" Applied Sciences 12, no. 17: 8563. https://doi.org/10.3390/app12178563





