Abstract
Escherichia coli is known to be an important uropathogenic agent. Several models were developed for investigating the uropathogensis of E. coli, including the recent biomimetic porcine urothelial in vitro model. The aim of this study was to assess the cytokine response of the cells of the biomimetic porcine urothelial model to different E. coli strains. The production of nine different cytokines in response to E. coli infection was evaluated using the commercial pre-configured immunoassay multiplex Cytokine & Chemokine 9-Plex Porcine ProcartaPlex™ Panel 1 kit. Our results showed that cells of the biomimetic porcine urothelial model reacted to the presence of all the employed different E. coli strains, albeit with some differences in levels and types of cytokines produced. Increased production of IL-10, IL-8, TNF-α, IL-1β, IL-4 and IL-12p40 was observed. Statistical analysis (Fisher’s exact test) revealed a correlation between the high fold change in the immune response and the presence of the cnf1 gene that encodes the cytotoxic necrotizing factor. Our results shed light on the cytokine response of normal urothelial cells to different E. coli strains and have the potential to fuel the search for understanding the mechanisms behind the different cytokine responses to different E. coli strains.
1. Introduction
Escherichia coli (E. coli) are a large and diverse group of bacteria. Most of the known strains are harmless [1], but some E. coli strains, which are capable of producing virulence factors, such as adhesins, iron acquisition systems, invasins, toxins and capsules, are considered pathogenic and cause infections of various severity [2]. Moreover, certain bacterial cell structures may induce cytokine production by host cells, which is important for an effective immune response.
Cytokines are mediators in many processes of innate and acquired immunity. They are linked to some initial processes of infection, for example, type 1 fimbriae- or P fimbriae-mediated adhesion can lead to IL-8 production in urinary tract cell models [3]. In addition, the presence of certain structures on bacterial cells, such as lipopolysaccharides, in general stimulates cytokine production in host cells [4]. Analysis of the presence and concentration of certain types of cytokines can provide additional information on how different E. coli strains affect certain immune processes, and thus indirectly reveal their (potential) pathogenicity [5].
In our previous study reported by Predojević et al. in 2022 [6], we established the biomimetic porcine in vitro model, which was constructed from normal, non-cancerous, urothelial cells originally derived from the porcine urinary bladder urothelium, as an alternative model system to study the pathogenicity of different human strains of E. coli. Details on the morphology, its molecular composition, and ultrastructural characteristics of the porcine urothelial model were also thoroughly described in our other studies [7,8,9,10,11,12,13]. The model is biomimetic and has a high-resolution capacity, as it also expresses the gene for Uroplakin Ia, which is a receptor for uropathogenic E. coli (UPEC) [14,15].
In our presented study, the urothelial in vitro model was infected with a diverse set of E. coli strains and analyzed for the production of cytokines as a response to infection. Our goal was to evaluate whether the normal porcine urothelial (NPU) cells of our in vitro model respond to infection with various human E. coli strains by secreting different cytokines into the cell-culture supernatant.
Finally, it should be stressed that since our in vitro model has a high physiological and genetic similarity to the human urothelium, there is a strong possibility that the results gathered on this model are also valid for human medicine.
2. Materials and Methods
2.1. In Vitro Biomimetic Model
In this research, we used the in vitro biomimetic model as described by Predojević et al. in 2022 [6] for the infection with E. coli strains.
2.2. Bacterial Strains
The strains used in this study are listed in Table 1. More details on the used strains are given in Supplementary Table S1. Bacterial strains, kept at −80 °C, were inoculated into 5 mL of Luria Broth (LB) medium (LLG Labware, Meckenheim, Germany) and grown overnight with aeration (180 rpm) at 37 °C. In the previous study by Predojević et al., 2022 [6], the used strains were already characterized for the presence of the following virulence-associated genes: cnf1, hlyA, usp, clbAQ, vat, fimH, papGII, papGIII, afa/draBC, sfaDE, iha, yfcV, fyuA, hbp, ireA, picU, iucD, iroN, kpsMTII, ompT, ompT-APEC, tcpC, traT, iss, neuB and ibeA.

Table 1.
Strains used in this research.
2.3. Infection Assay
The infection assay was performed as described by Predojević et al., 2022 [6], with the slight modification that after 3 h of incubation (37 °C, 0.5% CO2, without aeration), the supernatant from each well was collected, centrifuged (10 min, 4000× g), filtrated through 0.20 μm or 0.22 μm sterile filters and the bacteria-free supernatants were then stored at −80 °C for the analysis of cytokine production.
2.4. Cytokine Detection Assay
Using the commercial pre-configured immunoassay multiplex Cytokine & Chemokine 9-Plex Porcine ProcartaPlex™ Panel 1 kit (Invitrogen, Vienna, Austria), the concentrations of cytokines produced by the NPU cells were determined. Bacteria-free supernatants collected during the infection assays from infected models and models without bacteria (negative controls) were processed according to the manufacturer’s protocols (ProcartaPlex™ Multiplex Immunoassay USER GUIDE). The MagPix instrument (Luminex, Austin, TX, USA) was used to analyze the presence and amount of the following nine cytokines: IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12p40, IFN-α, IFN-γ and TNF-α. The fold change in cytokine production was calculated by dividing the obtained concentration of each analyte in the infection assay by the determined analyte concentration in the negative control. If the MagPix instrument reported for an analyte that its concentration was “below the limit of detection,” the values of the obtained limit of detection were used to calculate the fold change (Supplementary Table S2A,B).
2.5. Statistical Analysis
Analysis for statistical significance was performed using ANOVA, F-test and t-test in Microsoft Excel Analysis ToolPak. Statistical analysis of the possible correlations between the data of the cytokine production and presence of virulence-associated genes of E. coli strains was carried out using computer software for performing the Fischer’s exact test [19], followed by Bonferroni correction. For statistical analysis, three cytokine production groups were formed according to the intensity of total cytokine production by NPU cells in response to infection with bacterial strains, compared to the negative control (NC) and were as follows: (i) low fold change group I contained strains BJ65, DL80, BJ51, SE15, BJ95, BJ69, 536, BJ45, J96, MG1655, BJ23 and DL95 that caused low total cytokine production by NPU cells (from 1 to 1.99 fold change), (ii) the moderate fold change group II contained strains BJ97, DL75, DL1, BJ16, HS16, DL87 and DL53 that caused moderate total cytokine production by NPU cells (from 2 to 2.99 fold change), and (iii) the high fold change group III contained E. coli strains DL31, BJ50, BJ30, DL18 and DL102 that caused high total cytokine production by NPU cells (over 3 fold change). Statistical significance was taken into account for all p-values below or equal to 0.05.
3. Results and Discussion
3.1. Cytokine Response of Normal Urothelial Cells
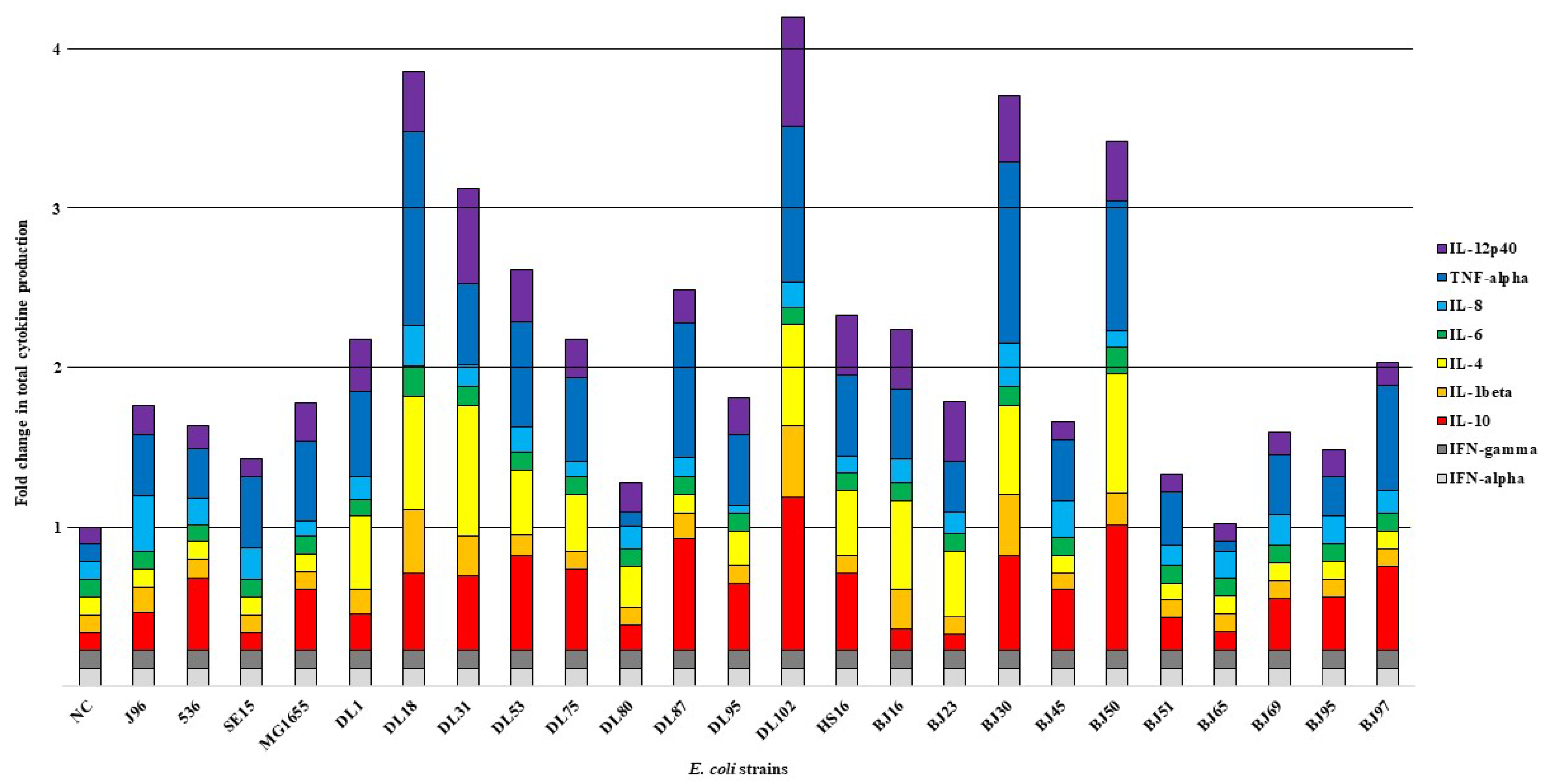
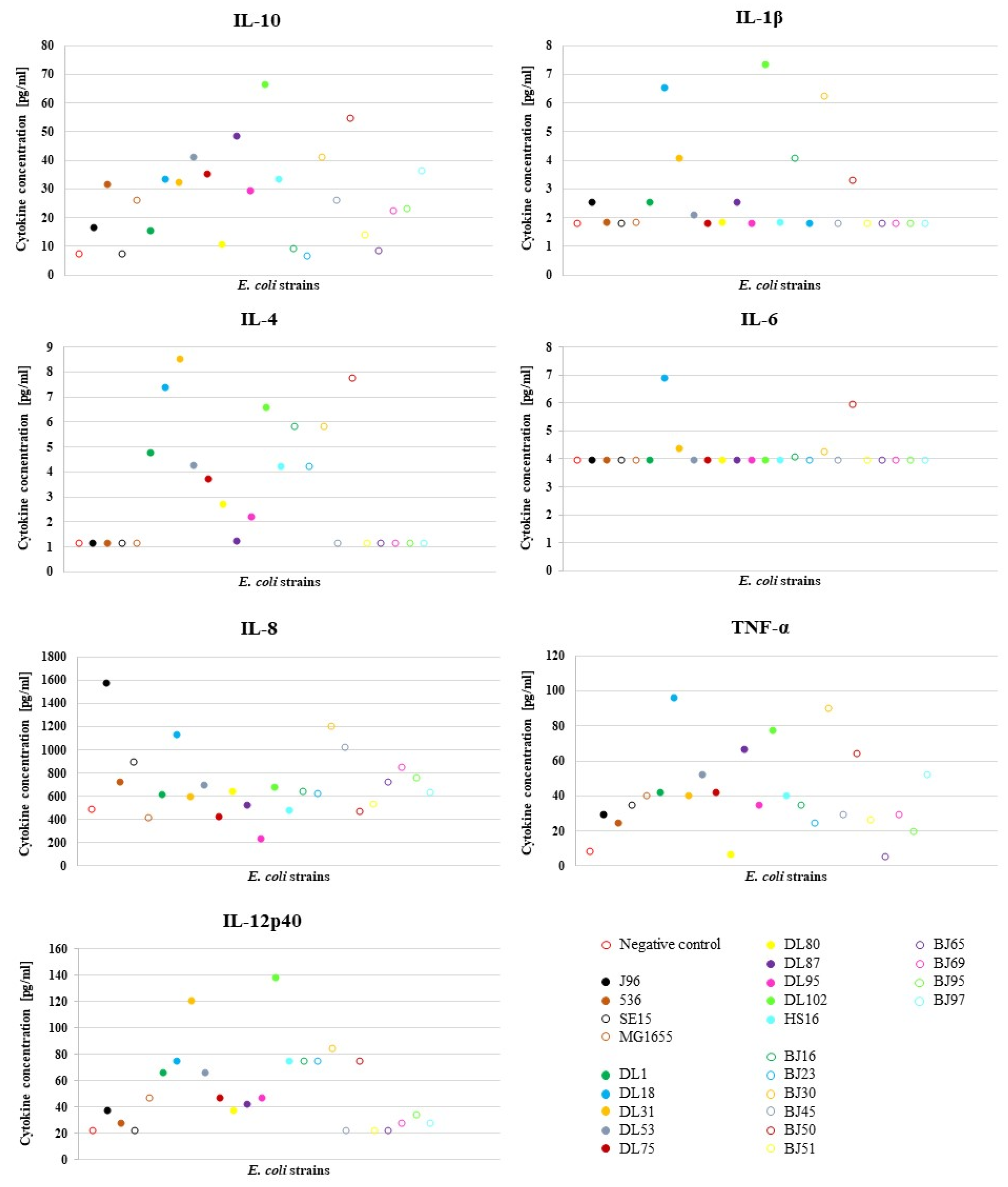
To characterize the effect of different E. coli strains on the NPU cells of the in vitro model upon infection, we analyzed NPU cell cytokine production using cytokine multiplex immunoassay. For the infection of the in vitro model, we used a diverse set of E. coli strains, which consisted of 24 E. coli strains (10 human UPEC strains designated as DL, isolated from urine and blood of patients with UTI; 10 human commensal strains designated as BJ, isolated from feces of healthy volunteers), as well as 4 well-described human E. coli strains (2 UPEC strains J96 and 536, commensal strain SE15 and laboratory strain MG1655). The results of the multiplex immunoassay for assessing the release of multiple cytokines into the cell culture supernatant after infection of the NPU cells and the obtained fold changes in the amounts of different cytokines relative to the negative control (NC) (in vitro model without bacteria) are presented in Figure 1. In Figure 2, the obtained average cytokine concentrations for each strain are plotted and Table 2 shows the calculated mean values ± SD for the cytokine concentrations in relation to the type of E. coli strain used (UPEC vs. commensal). The cytokine concentrations determined with the MagPix instrument and the calculated fold-changes can be found in the Supplementary Table S2A,B, respectively.

Figure 1.
Fold change in total cytokine production by NPU cells in response to infection with different E. coli strains relative to the value of cytokine production in the negative control (NC), (NC—negative control, not infected NPU cells). The experiment was performed in duplicates, fold change for each cytokine was calculated by dividing the average value of the concentration of each analyte measured by the instrument from both duplicates in the E. coli infection assay with the average value of both duplicates in the negative control.
Figure 2.
Average value of the cytokine concentrations produced by NPU cells in the E. coli infection assay. The experiment was performed in duplicates and the average value was calculated. Data for IFN-α and IFN-γ are not shown, as cytokine production of these two analytes was below the detection limit in all strains.

Table 2.
Mean ± SD concentrations of cytokines produced by NPU cells, depending on the E. coli type (UPEC vs. commensal).
As shown in Table 2, the NPU cells produced higher levels of cytokines in response to infection with UPEC strains, with the exception of IL-8, than in response to infection with the commensal strains, albeit the difference was not statistically significant. This is actually not surprising, since in our previous study [6], in which we used this model to assess the pathogenicity of strains, some DL strains, although isolated from the urine of UTI patients, had lower pathogenic potential, while some BJ strains, although isolated from the feces of healthy individuals, had higher pathogenic potential than would have been expected based on their isolation source. It is known that strains with a high virulence potential can be found in the intestinal microbiota of healthy individuals [20], while under certain circumstances (e.g., in immunocompromised patients), commensal E. coli lacking virulence-associated genes can cause disease [2].
In the case of both uropathogenic E. coli strains (J96 and 536) (Figure 1), higher levels of cytokine production were observed, compared to lower levels of cytokine production following infection with the non-pathogenic commensal strain (SE15). Compared to the control without bacteria (NC), strains J96 and 536 caused increased production of IL-10, IL-8, TNF-α and IL-12p40. In addition, the strain J96 caused also an increase in the IL-1β production. Infection of the model with two non-pathogenic strains caused elevated production of TNF-α, as well as IL-12p40 (SE15), IL-10 and IL-12p40 (MG1655). The obvious difference between the pathogenic and non-pathogenic strains was observed in the IL-1β release (a high increase in IL-1β in the case of J96) and the higher overall number of significantly increased levels of different cytokines. The highest increase in the amount of any cytokine relative to NC was in the case of TNF-α, after infection of NPU cells with strains BJ30 and DL18. Considerable high fold-change in the amounts of cytokines IL-10 and IL-4 was also observed.
Expression of IL-10 is highly activated during urinary infections due to its involvement in multiple biological pathways and importance in the early control of bacteria that cause urinary infections. Measurements of the concentration of IL-10 produced in individuals with cystitis showed an increase in IL-10 concentration compared to the IL-10 levels in individuals without infection, both in urine (over 40 pg/mL) and in plasma [21]. In our study, strain DL102 triggered IL-10 production even above 60 pg/mL. Pro-inflammatory cytokine IL-1β production is also known to be induced in epithelial cells of the urinary tract by UPEC [22]. In our model, strains DL18 and DL102 induced the greatest production of IL-1β, reaching concentrations above 6 pg/mL (Figure 2). An IL-1β production of above 6 pg/mL was also detected after infection with the strain BJ30, which also exhibited a similar level of pathogenicity as DL18 and DL102 in our previous study [6].
Elevated production of IL-4 by NPU cells was induced predominantly by UPEC strains. IL-4 is involved in modulating the inflammatory responses to infection, for example, it is involved in the pathogenesis of asthma [23], allergic reactions [24] and other type 2 immune responses [25]. The urothelium response to UPEC infection in vivo is also secretion of cytokine IL-6 [26]. It is known that some bacterial structures induce production of IL-6, for example P-fimbriae [27] or bacterial LPS [4], and UPEC can evade the host’s immune response by suppressing the secretion of IL-6, which presents a survival advantage of UPEC [28]. In concordance, almost all UPEC strains in our model (except from DL18) caused either no change or a slight change in the secretion of IL-6. IL-8 has a pivotal role in chemotactically attracting neutrophils to the site of infection and the initiation of inflammation [29]. The most prominent increase in production of IL-8, in our model, was caused by the J96 strain, which is a potent uropathogenic E. coli strain.
The host’s response to infection by UPEC relies on innate immune processes, for example, the activation of pathogen associated molecular pattern receptors (PAMP receptors) [30], which induces an inflammatory response, as well as the secretion of cytokines, among others TNF-α [31]. Compared to the reported values of 800 pg/mL in urine of some cystitis patients [32], in our model, the highest production of TNF-α was rather low (the highest production of this cytokine was induced by the DL18 strain (87.6 pg/mL). Interestingly, both potent UPEC strains J96 and 536 caused only a slight change in the secretion of this cytokine; the concentration was even lower than that detected with the MG1655. Recently, we showed that TNF-α-stimulated NPU cells exhibited marked inflammatory phenotypes with upregulated mRNA expression of the acute phase protein SAA3, complement component C3, chemokine CXCL10, several proinflammatory cytokines (IL1a, IL1b, IL8), and the cytokine receptor IFNGR1. In addition, protein levels of IL8 and IFNGR1 were also significantly higher in TNF-α-treated NPU cells compared with the controls, as determined by ELISA and Western blot, respectively [33]. Together with these results, we can further confirm the usefulness of our urothelial model, since it does not only represent normal bladder urothelium, but it can significantly respond to specific environmental conditions, as is the case in interstitial cystitis/bladder pain syndrome and also in infectious cystitis.
Strain DL102 induced the greatest production of IL-12p40, which, among others, drives the differentiation of the immune response towards different effector pathways, as for example, the promotion of INF-γ secretion by natural killer cells [34].
In our study, the production of cytokines INF-α and INF-γ was not detected, which was to be expected, as these two cytokines have not been previously associated with E. coli infections. They are involved in antiviral immune responses [35] and, in addition, INF-γ is also involved in immunological processes in infections caused by some bacteria (Mycobacterium tuberculosis, Salmonella, Listeria) and some parasites (Leishmania, Toxoplasma, Chlamydia) [36].
To sum up, each E. coli strain caused a distinct pattern of cytokine production by NPU cells. The majority of highly pathogenic DL and BJ strains provoked between a 3 and 4 fold change in total cytokine production (BJ30, DL18, DL102); strain DL53 provoked a 2–3 fold change. Interestingly, the two uropathogenic strains, J96 and 536, as well as the strain BJ23, which all possess many virulence-associated genes, induced much lower fold changes in total cytokine production, as would be expected.
The increase in concentrations of the individual cytokines secreted by the cells of our model, in response to infection with UPEC strains, is in concordance with the findings of other researchers. Thus, Sundac et al. in 2016 [32] reported elevated concentrations of multiple cytokines, in comparison to the negative control, in urine samples of patients with cystitis caused by UPEC, among others (IL-1β, IL-4, IL-10 and TNFα), as was also observed upon infection in our model with the high fold change group of E. coli strains. Another study that used urine samples from patients with recurring UTIs (rUTI) for the detection of multiple cytokines showed, among others, elevated concentrations of IL-1β and IL-10 [37], which is also in accordance with the response of our in vitro model to infection.
3.2. Statistical Analysis of Correlation between Strain’s Cytokine Production Group and Virulence-Associated Genes
The 24 tested E. coli strains were assigned to the following 3 different groups, according to the fold change in total cytokine production by NPU cells in response to infection with each strain: (i) the low fold change group I, containing strains BJ65, DL80, BJ51, SE15, BJ95, BJ69, 536, BJ45, J96, MG1655, BJ23 and DL95, which induced the low overall cytokine production of NPU cells in response to infection (from 1 to 1,99 fold change), (ii) moderate fold change group II, containing strains BJ97, DL75, DL1, BJ16, HS16, DL87 and DL53, which induced the moderate total cytokine production of NPU cells (from 2 to 2,99 fold change), and finally (iii) high fold change group III, containing the E. coli strains DL31, BJ50, BJ30, DL18 and DL102, which induced a high total cytokine production of NPU cells in response to infection with these strains (above 3 fold change). The Fisher’s exact test and Bonferroni correction were performed to reveal any statistical correlation between the assigned cytokine production groups and the presence of virulence-associated genes. As observed from Table 3 and Supplementary Table S3A,B, only one statistically significant correlation was observed, namely between the high fold change group III and cnf1 gene. Some UPEC produce the chromosomally encoded toxin cnf1 [38], which plays an important role in initiating infection, promoting inflammation [39] and generally increases the virulence of E. coli [40]. This infection often leads to inflammation [41], which in turn causes the production of pro-inflammatory cytokines [42], e.g., Il-6, IL-8, and TNF-α [43], which can also be observed from our results, as both UPEC strains DL18 and DL102 harboring the cnf1 gene caused the highest total cytokine production.

Table 3.
Distribution of virulence-associated genes among E. coli strain groups causing different intensities of NPU cell cytokine production.
Before we performed the Bonferroni correction, the tcpC and hlyA also correlated with the high fold change group III (p value before correction of 0.0474, Supplementary Table S3A). TcpC-producing E. coli use this host-defence-like protein to disrupt the innate immune response, among others (the IL-6 pathways), and cause inflammation and damage to the host’s tissues [44]. In our study, most of the strains did not cause considerable change in the production of IL-6, including many strains that harbored the tcpC gene (e.g., DL102). Alpha-hemolysin (HlyA) is cytotoxic to many types of cells and can cause tissue damage during urinary infections [45]. HlyA also promotes production of IL-6 and IL-8 in renal epithelial cells [46]. In our study, the majority of strains that encode HlyA induced production of IL-8.
To sum up, Fisher’s exact test revealed that three prominent virulence-associated genes, cnf1, hlyA and tcpC, correlated with the groups of strains that induced high total cytokine production by NPU cells in response to infection with these strains (above 3 fold change)—the high fold change group III. However, after we performed the Bonferroni correction, only the correlation of cnf1 with the high fold change group III remained statistically significant.
4. Conclusions
The results of this study show that the NPU cells in our urothelial model respond to infection with different E. coli strains by secreting different amounts of various cytokines. The statistical analysis showed that possession of the cnf1 gene correlated with higher cytokine production. In the current study, there were certain limitations, such as a small number of E. coli strains and the NPU cells used were only from one pig bladder (only one biological sample). However, the results obtained are interesting and an incentive for future studies using a larger number of bacterial strains and more biological samples of NPU cells. Moreover, the results obtained are an important basis for future studies on bacterial pathogenesis and response to infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12178567/s1. Table S1: E. coli strains used in this study; Table S2A: Raw data—average values of the amount of each cytokine obtained from the instrument MagPix; Table S2B: Fold change in the amount of each cytokine according to values in NC from Table Supplementary S2A; Table S3A: P (two-tail) values obtained with automated Fischer’s exact test computer software (presented with four decimals); Table S3B: Data obtained after Bonferroni correction applied to the data in the Supplementary Table S3A (presented with four decimals).
Author Contributions
Conceptualization, M.S.E. and M.E.K.; methodology, L.P., M.S.E., M.E.K. and D.Ž.B.; validation, L.P., M.S.E. and M.E.K.; investigation, L.P., D.K. and M.K.; writing—original draft preparation, L.P., M.S.E. and M.E.K.; writing—review and editing, L.P., M.S.E., M.E.K., D.K., D.Ž.B. and M.K.; visualization, L.P.; supervision, M.S.E. and M.E.K. All authors were involved in drafting the manuscript or critically revising it for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Slovenian Research Agency (Grant No. P1-0198, P3-0108, J7-2594 and MRIC UL IP-0510 Infrastructure program).
Institutional Review Board Statement
The animal study protocol was approved by the Administration for Food Safety, Veterinary Sector and Plant Protection (U34453-15/2013/2, 4 July 2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Data are freely available, under a license allowing re-use by any third party for any lawful purpose.
Acknowledgments
The authors are thankful to Sanja Čabraja, Sabina Železnik, Zdravko Podlesek and Miša Pavletič for their technical assistance and to Nejc Draganjec for analyzing the data with his computer program, an automated Fisher exact test. The authors are also thankful to Eva Moreno for providing the J96 and 536 strain, to Eric Oswald for the SE15 strain, and to Christophe Beloin for the MG1655 strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.W.; Lai, H.T.; Tsai, T.C.; Wu, Y.C.; Yang, Y.T.; Chen, K.Y.; Chen, C.M.; Li, Y.S.; Chen, C.N. Difference in the regulation of IL-8 expression induced by uropathogenic E. coli between two kinds of urinary tract epithelial cells. J. Biomed. Sci. 2009, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.D.; Mulvey, M.A.; Vincent, C.D.; Lorenz, R.G.; Hultgren, S.J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 2001, 166, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Sullivan, M.J.; Duell, B.L.; Goh, K.G.K.; Katupitiya, L.; Gosling, D.; Chamoun, M.N.; Kakkanat, A.; Chattopadhyay, D.; Crowley, M.; et al. Rapid bladder interleukin-10 synthesis in response to uropathogenic Escherichia coli is part of a defense strategy triggered by the major bacterial flagellar filament FliC and contingent on TLR5. mSphere 2019, 4, e00545-19. [Google Scholar] [CrossRef]
- Predojević, L.; Keše, D.; Žgur Bertok, D.; Železnik Ramuta, T.; Veranič, P.; Erdani Kreft, M.; Starčič Erjavec, M. A biomimetic porcine urothelial model for assessing Escherichia coli pathogenicity. Microorganisms 2022, 10, 783. [Google Scholar] [CrossRef]
- Višnjar, T.; Kocbek, P.; Kreft, M.E. Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem. Cell Biol. 2012, 137, 177–186. [Google Scholar] [CrossRef]
- Višnjar, T.; Kreft, M.E. The complete functional recovery of chitosan-treated biomimetic hyperplastic and normoplastic urothelial models. Histochem. Cell Biol. 2015, 143, 95–107. [Google Scholar] [CrossRef]
- Višnjar, T.; Jerman, U.D.; Veranič, P.; Kreft, M.E. Chitosan hydrochloride has no detrimental effect on bladder urothelial cancer cells. Toxicol. Vitr. 2017, 44, 403–413. [Google Scholar] [CrossRef]
- Resnik, N.; Erman, A.; Veranič, P.; Kreft, M.E. Triple labelling of actin filaments, intermediate filaments and microtubules for broad application in cell biology: Uncovering the cytoskeletal composition in tunneling nanotubes. Histochem. Cell Biol. 2019, 152, 311–317. [Google Scholar] [CrossRef]
- Tratnjek, L.; Romih, R.; Kreft, M.E. Differentiation-dependent rearrangements of actin filaments and microtubules hinder apical endocytosis in urothelial cells. Histochem. Cell Biol. 2017, 148, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Lojk, J.; Bregar, V.B.; Strojan, K.; Hudoklin, S.; Veranič, P.; Pavlin, M.; Kreft, M.E. Increased endocytosis of magnetic nanoparticles into cancerous urothelial cells versus normal urothelial cells. Histochem. Cell Biol. 2018, 149, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.; Tratnjek, L.; Janev, A.; Seme, K.; Starčič Erjavec, M.; Kreft, M.E. The antibacterial activity of human amniotic membrane against multidrug-resistant bacteria associated with urinary tract infections: New insights from normal and cancerous urothelial models. Biomedicines 2021, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef]
- Višnjar, T.; Chesi, G.; Iacobacci, S.; Polishchuk, E.; Resnik, N.; Robenek, H.; Kreft, M.; Romih, R.; Polishchuk, R.; Kreft, M.E. Uroplakin traffic through the Golgi apparatus induces its fragmentation: New insights from novel in vitro models. Sci. Rep. 2017, 7, 12842. [Google Scholar] [CrossRef]
- Rijavec, M.; Starčič Erjavec, M.; Ambrožič Avguštin, J.; Reissbrodt, R.; Fruth, A.; Križan-Hergouth, V.; Žgur-Bertok, D. High prevalence of multidrug resistance and random distribution of mobile genetic elements among uropathogenic Escherichia coli (UPEC) of the four major phylogenetic groups. Curr. Microbiol. 2006, 53, 158–162. [Google Scholar] [CrossRef]
- Rijavec, M.; Müller-Premru, M.; Zakotnik, B.; Žgur-Bertok, D. Virulence factors and biofilm production among Escherichia coli strains causing bacteraemia of urinary tract origin. J. Med. Microbiol. 2008, 57, 1329–1334. [Google Scholar] [CrossRef]
- Starčič Erjavec, M.; Jesenko, B.; Petkovšek, Ž.; Žgur-Bertok, D. Prevalence and associations of tcpC, a gene encoding a Toll/interleukin-1 receptor domain-containing protein, among Escherichia coli urinary tract infection, skin and soft tissue infection, and commensal isolates. J. Clin. Microbiol. 2010, 48, 966–968. [Google Scholar] [CrossRef]
- Hrovat, K.; Draganjec, N.; Starčič Erjavec, M. A tool for the Fisher’s exact test. In Proceedings of the Genetics, 4th Colloquium of Genetics, Piran, Slovenia, 19 September 2014. [Google Scholar]
- Starčič Erjavec, M.; Žgur-Bertok, D. Virulence potential for extraintestinal infections among commensal Escherichia coli isolated from healthy humans--the Trojan horse within our gut. FEMS Microbiol. Lett. 2015, 362, fnu061. [Google Scholar] [CrossRef]
- Duell, B.L.; Carey, A.J.; Tan, C.K.; Cui, X.; Webb, R.I.; Totsika, M.; Schembri, M.A.; Derrington, P.; Irving-Rodgers, H.; Brooks, A.J.; et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 2012, 188, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Demirel, I.; Persson, A.; Brauner, A.; Särndahl, E.; Kruse, R.; Persson, K. Activation of the NLRP3 inflammasome pathway by uropathogenic Escherichia coli is virulence factor-dependent and influences colonization of bladder epithelial cells. Front. Cell. Infect. Microbiol. 2018, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Choi, P.; Reiser, H. IL-4: Role in disease and regulation of production. Clin. Exp. Immunol. 1998, 113, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.J.; Barlow, J.L.; McKenzie, A.N. Insights into the initiation of type 2 immune responses. Immunology 2011, 134, 378–385. [Google Scholar] [CrossRef]
- Svanborg, C.; Godaly, G.; Hedlund, M. Cytokine responses during mucosal infections: Role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 1999, 2, 99–105. [Google Scholar] [CrossRef]
- Wullt, B.; Bergsten, G.; Connell, H.; Röllano, P.; Gebratsedik, N.; Hang, L.; Svanborg, C. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell. Microbiol. 2001, 3, 255–264. [Google Scholar] [CrossRef]
- Hunstad, D.A.; Justice, S.S.; Hung, C.S.; Lauer, S.R.; Hultgren, S.J. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 2005, 73, 3999–4006. [Google Scholar] [CrossRef]
- Rao, W.H.; Evans, G.S.; Finn, A. The significance of interleukin 8 in urine. Arch. Dis. Child. 2001, 85, 256–262. [Google Scholar] [CrossRef]
- Hagberg, L.; Hull, R.; Hull, S.; McGhee, J.R.; Michalek, S.M.; Svanborg Edén, C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 1984, 46, 839–844. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; Becknell, B.; Watson, J.; Hains, D.S. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 2014, 29, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Sundac, L.; Dando, S.J.; Sullivan, M.J.; Derrington, P.; Gerrard, J.; Ulett, G.C. Protein-based profiling of the immune response to uropathogenic Escherichia coli in adult patients immediately following hospital admission for acute cystitis. Pathog. Dis. 2016, 74, ftw062. [Google Scholar] [CrossRef] [PubMed]
- Kuret, T.; Peskar, D.; Kreft, M.E.; Erman, A.; Veranič, P. Comprehensive transcriptome profiling of urothelial cells following TNFα stimulation in an in vitro interstitial cystitis/bladder pain syndrome model. Front. Immunol. 2022, 13, 960667. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Murira, A.; Lamarre, A. Type-I interferon responses: From friend to foe in the battle against chronic viral infection. Front. Immunol. 2016, 7, 609. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concept. 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Drage, L.K.L.; Robson, W.; Mowbray, C.; Ali, A.; Perry, J.D.; Walton, K.E.; Harding, C.; Pickard, R.; Hall, J.; Aldridge, P.D. Elevated urine IL-10 concentrations associate with Escherichia coli persistence in older patients susceptible to recurrent urinary tract infections. Immun. Ageing 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, A.; Falbo, V.; Ruggeri, F.M.; Baldassarri, L.; Bisicchia, R.; Ippolito, G.; Romoli, E.; Donelli, G. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J. Clin. Microbiol. 1987, 25, 146–149. [Google Scholar] [CrossRef]
- Rippere-Lampe, K.E.; O’Brien, A.D.; Conran, R.; Lockman, H.A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf(1)) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 2001, 69, 3954–3964. [Google Scholar] [CrossRef]
- Andreu, A.; Stapleton, A.E.; Fennell, C.; Lockman, H.A.; Xercavins, M.; Fernandez, F.; Stamm, W.E. Urovirulence determinants in Escherichia coli strains causing prostatitis. J. Infect. Dis. 1997, 176, 464–469. [Google Scholar] [CrossRef]
- Rappuoli, R. Pushing the limits of cellular microbiology: Microarrays to study bacteria-host cell intimate contacts. Proc. Natl. Acad. Sci. USA 2000, 97, 13467–13469. [Google Scholar] [CrossRef] [Green Version]
- Knaus, U.G. Rho GTPase signaling in inflammation and transformation. Immunol. Res. 2000, 21, 103–109. [Google Scholar] [CrossRef]
- Falzano, L.; Quaranta, M.G.; Travaglione, S.; Filippini, P.; Fabbri, A.; Viora, M.; Donelli, G.; Fiorentini, C. Cytotoxic necrotizing factor 1 enhances reactive oxygen species-dependent transcription and secretion of proinflammatory cytokines in human uroepithelial cells. Infect. Immun. 2003, 71, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Zhang, J.; Fischer, H.; Huang, W.; Lutay, N.; Cirl, C.; Lum, J.; Miethke, T.; Svanborg, C. Inhibition of TIR domain signaling by TcpC: MyD88-dependent and independent effects on Escherichia coli virulence. PLoS Pathog. 2010, 6, e1001120. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Mulvey, M.A. The RTX pore-forming toxin α-hemolysin of uropathogenic Escherichia coli: Progress and perspectives. Futur. Microbiol. 2013, 8, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, P.; Laestadius, A.; Jahnukainen, T.; Söderblom, T.; Bäckhed, F.; Celsi, G.; Brismar, H.; Normark, S.; Aperia, A.; Richter-Dahlfors, A. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 2000, 405, 694–697. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).