Microstructural Characteristics, Modeling of Mechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Ordinary Portland Cement
2.1.2. Glass Waste
2.1.3. Fine and Coarse Aggregates
2.1.4. Superplasticizer
2.2. Experimental Design and Methods
Mix Design
2.3. Sample Preparation
2.4. Evaluation Methods
2.4.1. Setting Time and Workability
2.4.2. Compressive Strength
2.4.3. Density of the Sample
2.4.4. Thermal Treatment of Concrete
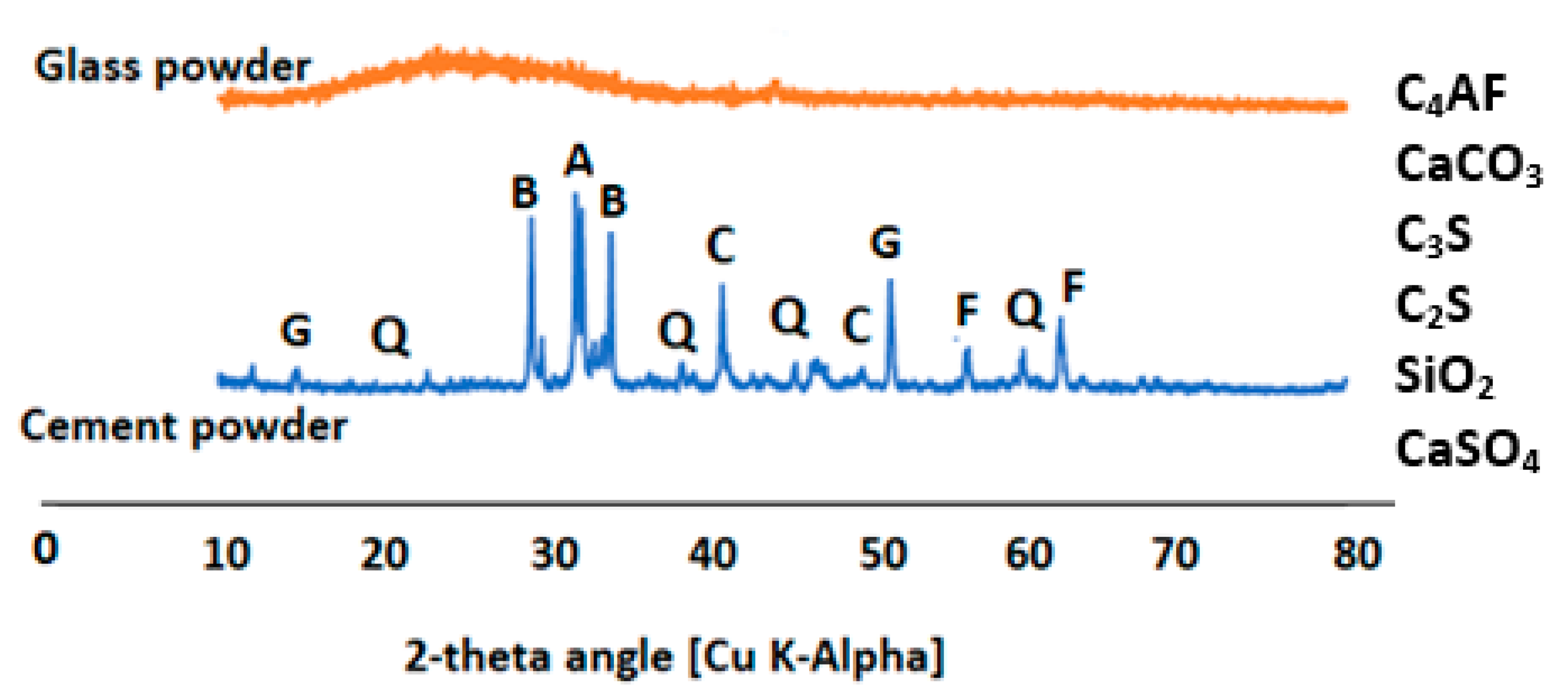
2.4.5. Microstructural Characterization of the Specimens
3. Discussion of Results
3.1. Effect of Glass on the Setting Time of Mortar
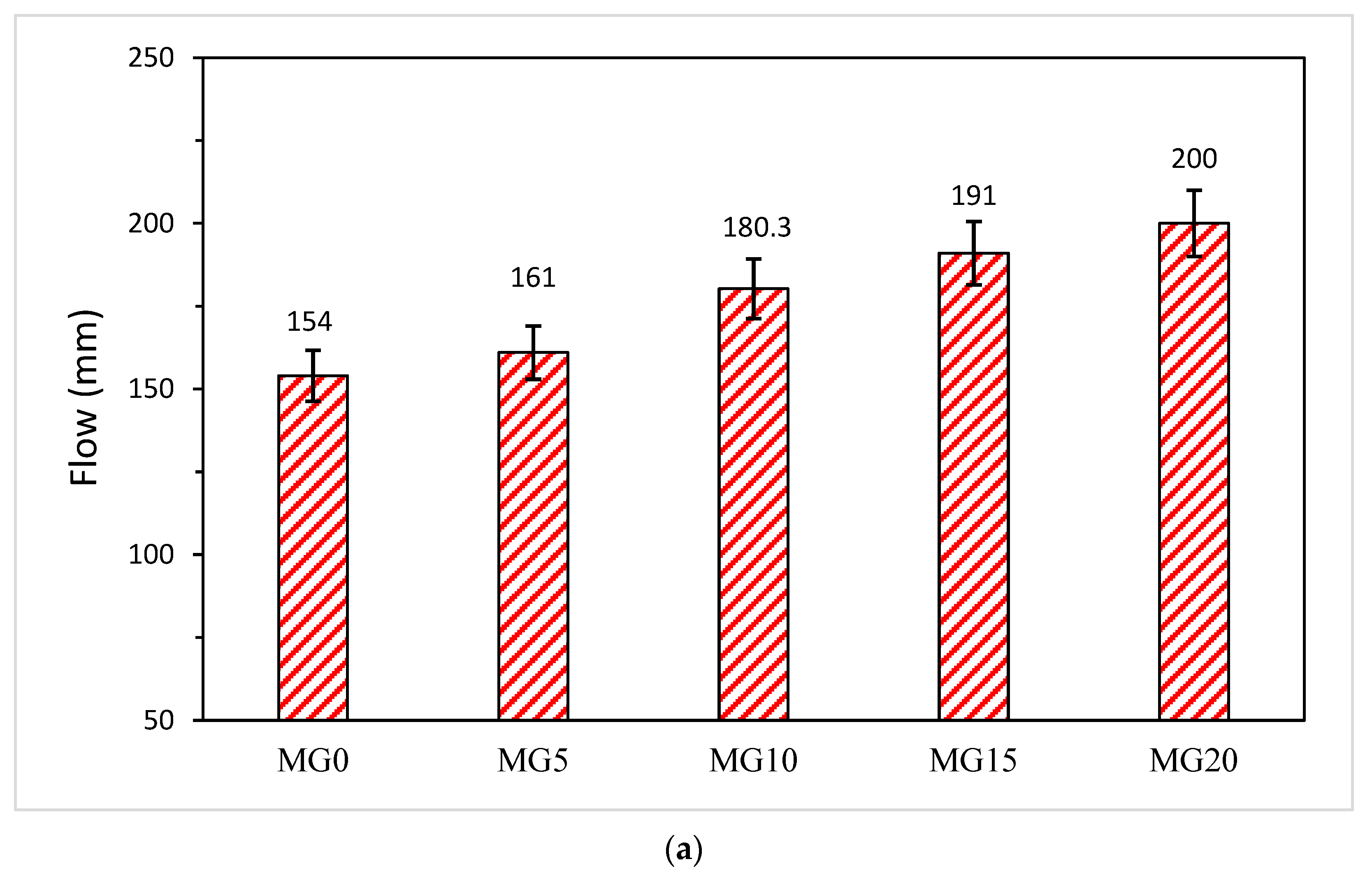
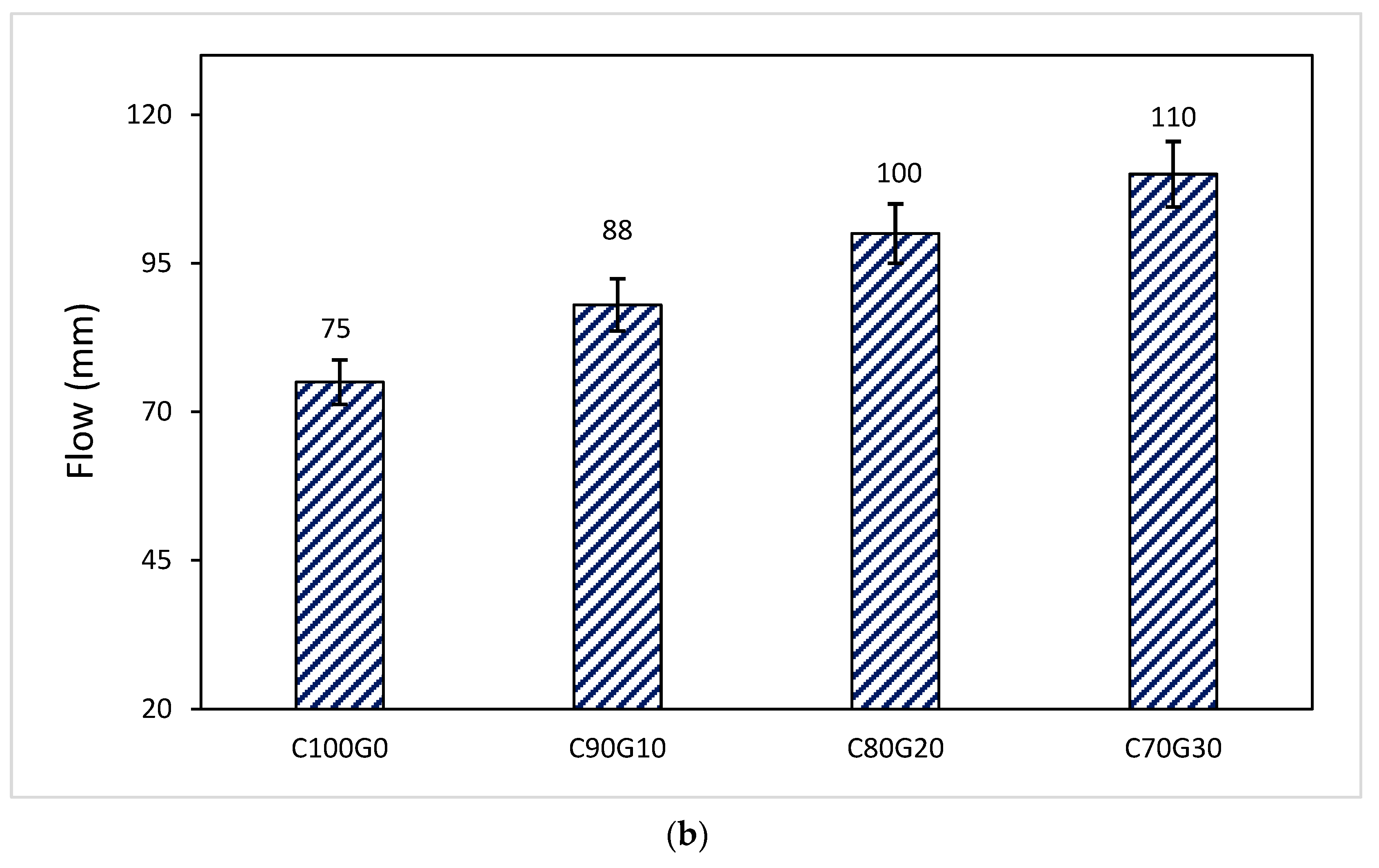
3.2. Effect of Glass on Workability of Mortar and Concrete
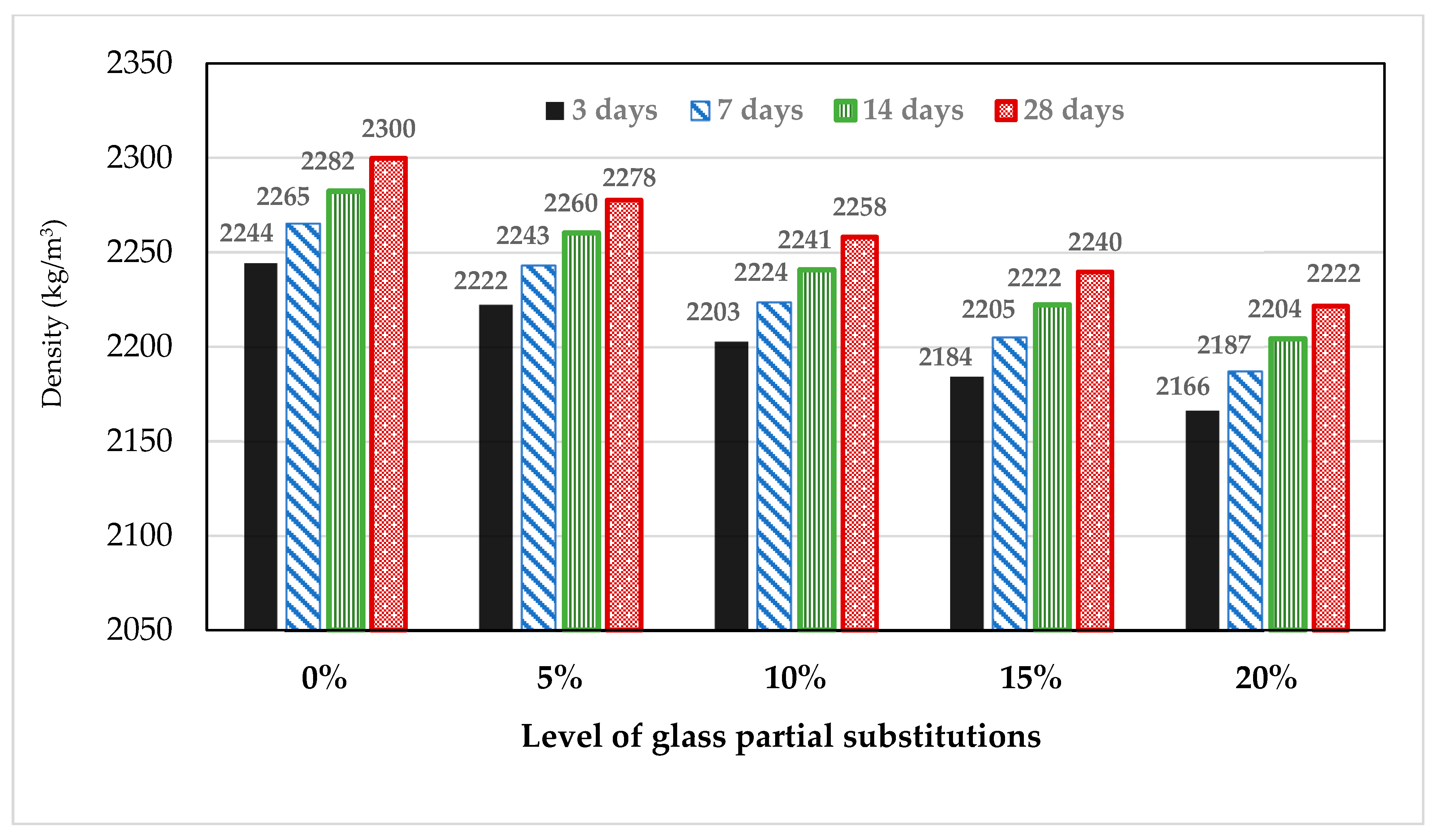
3.3. Effect of Glass on the Density of Mortar
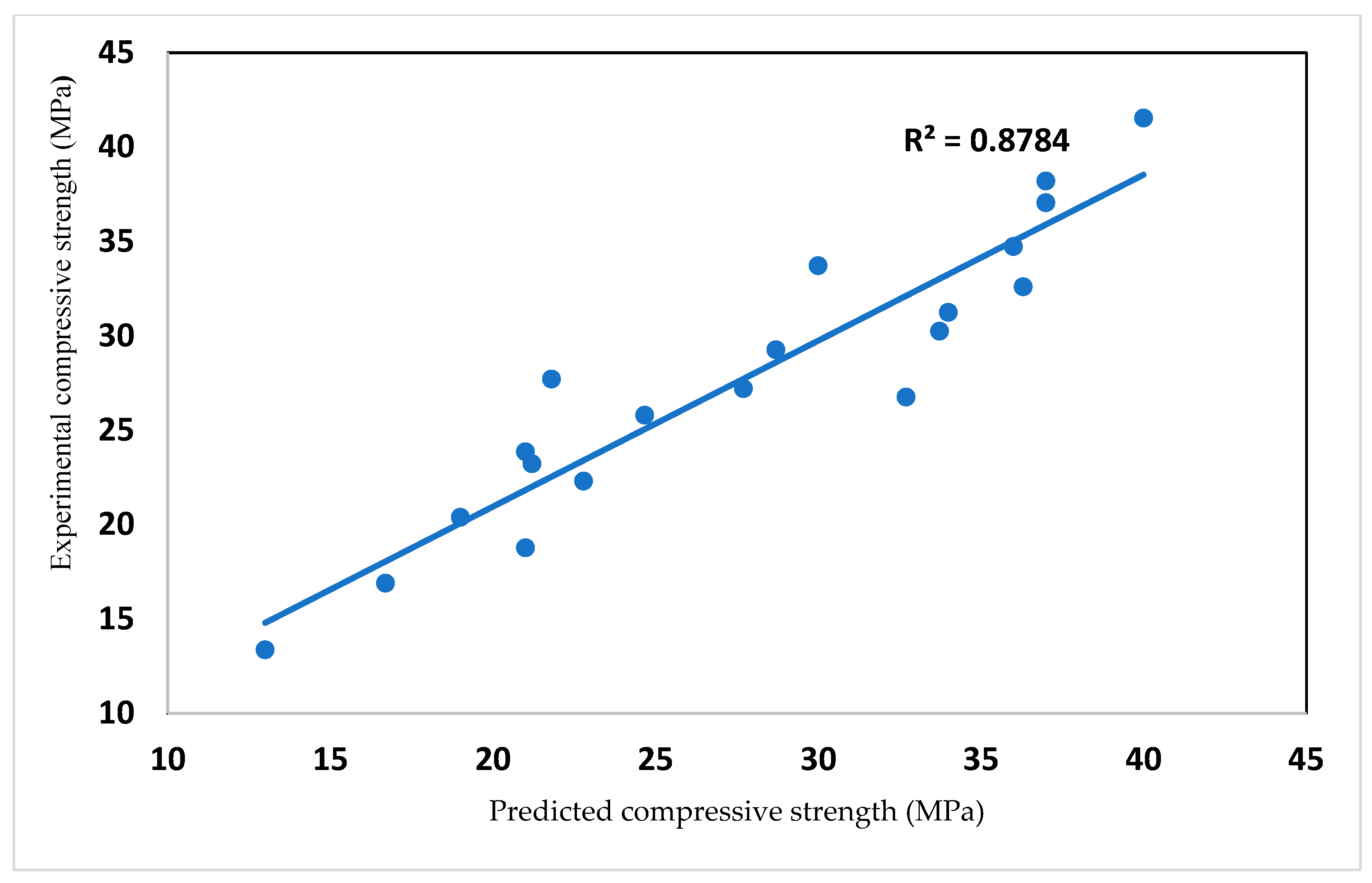
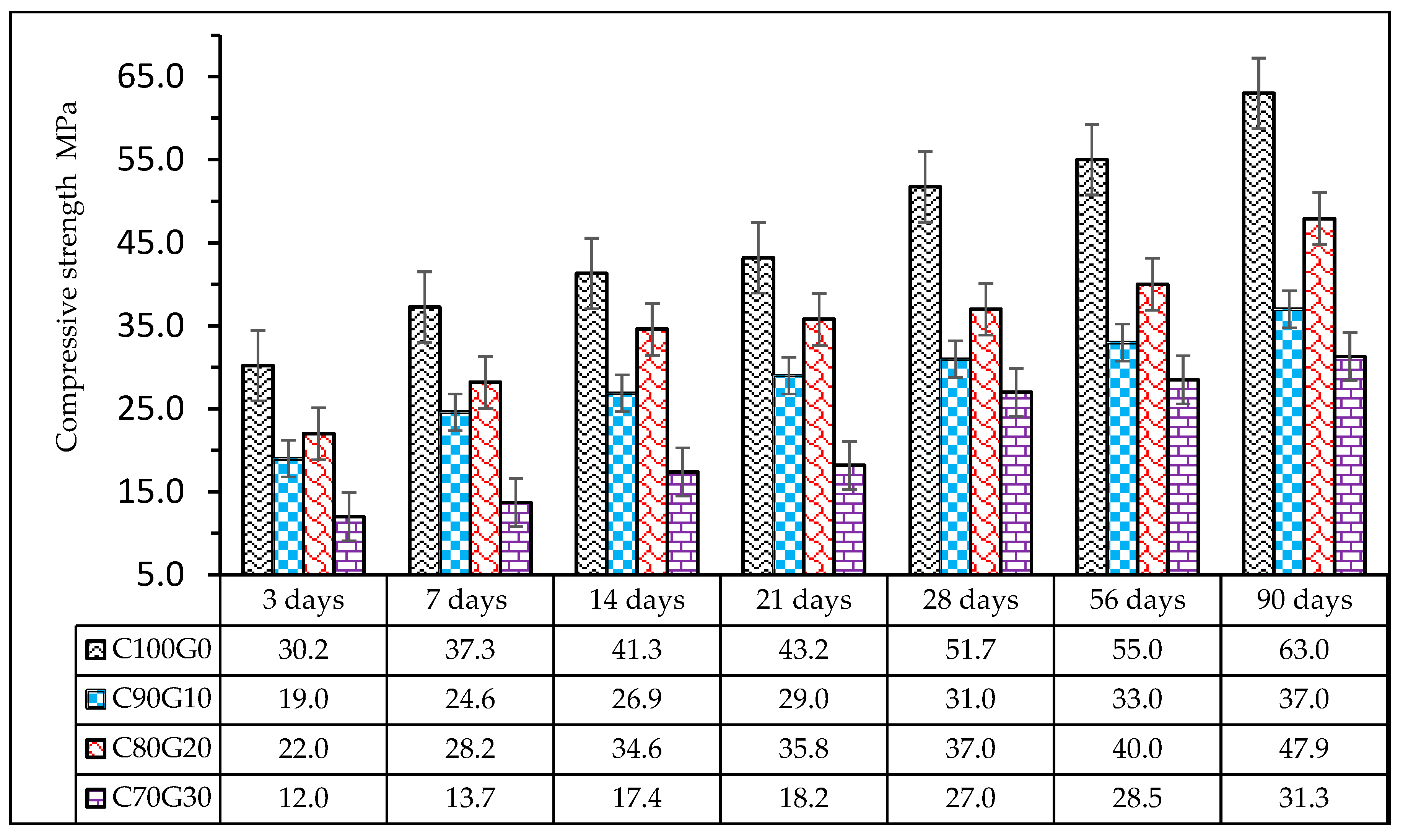
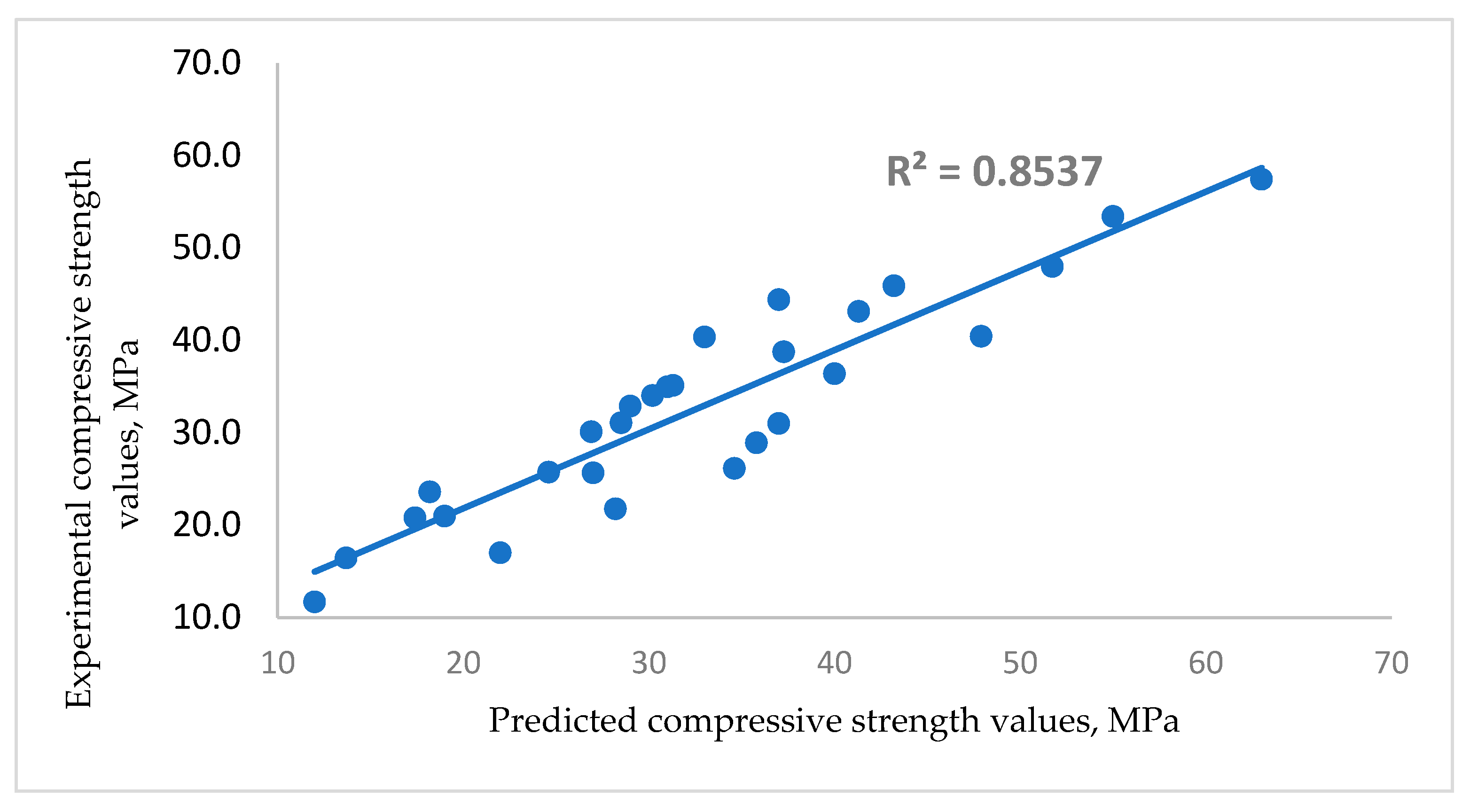
3.4. Effect of Glass on Compressive Strength of Mortar and Concrete
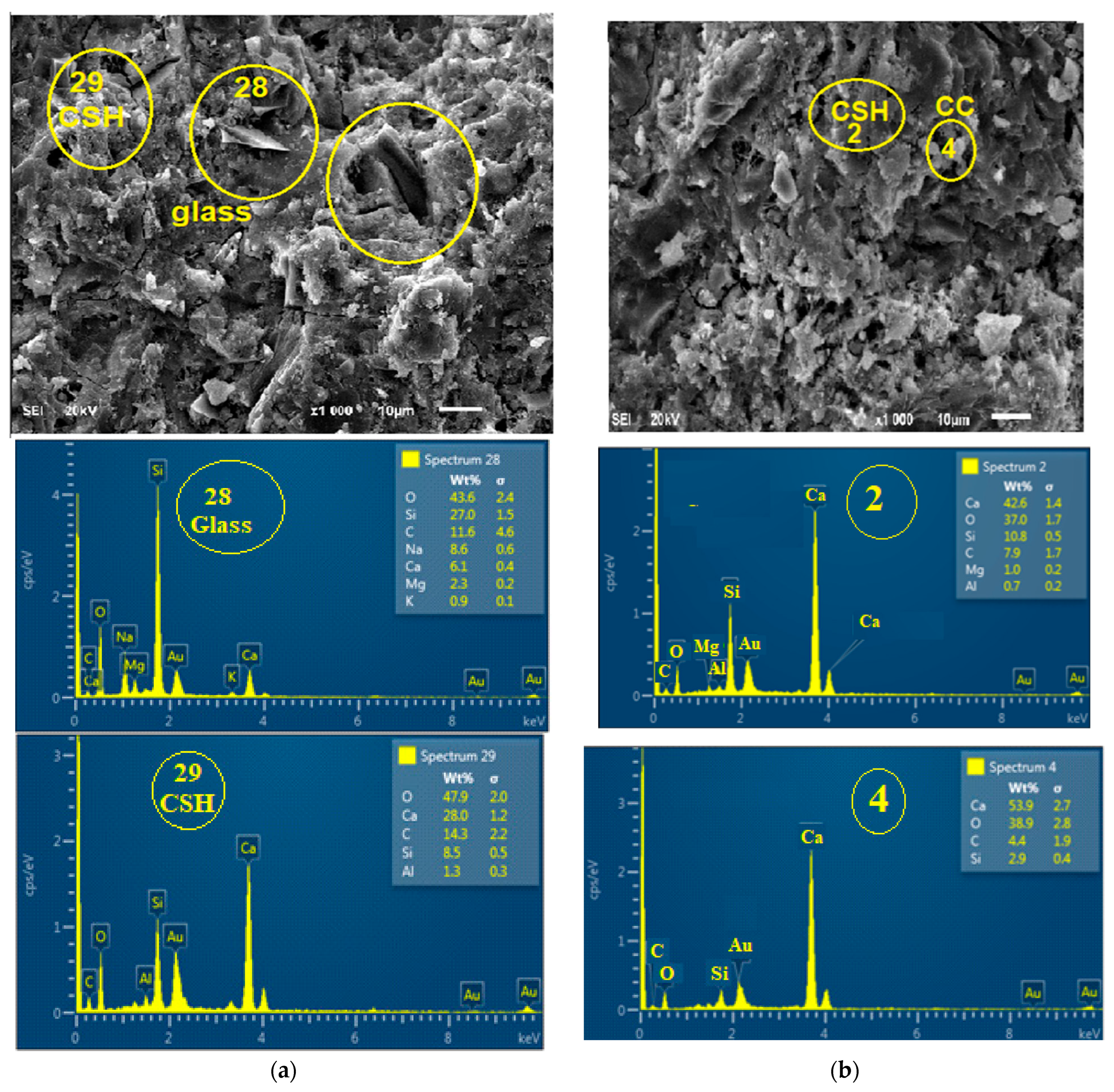
3.5. Effect of Glass on the Morphology of the Binder
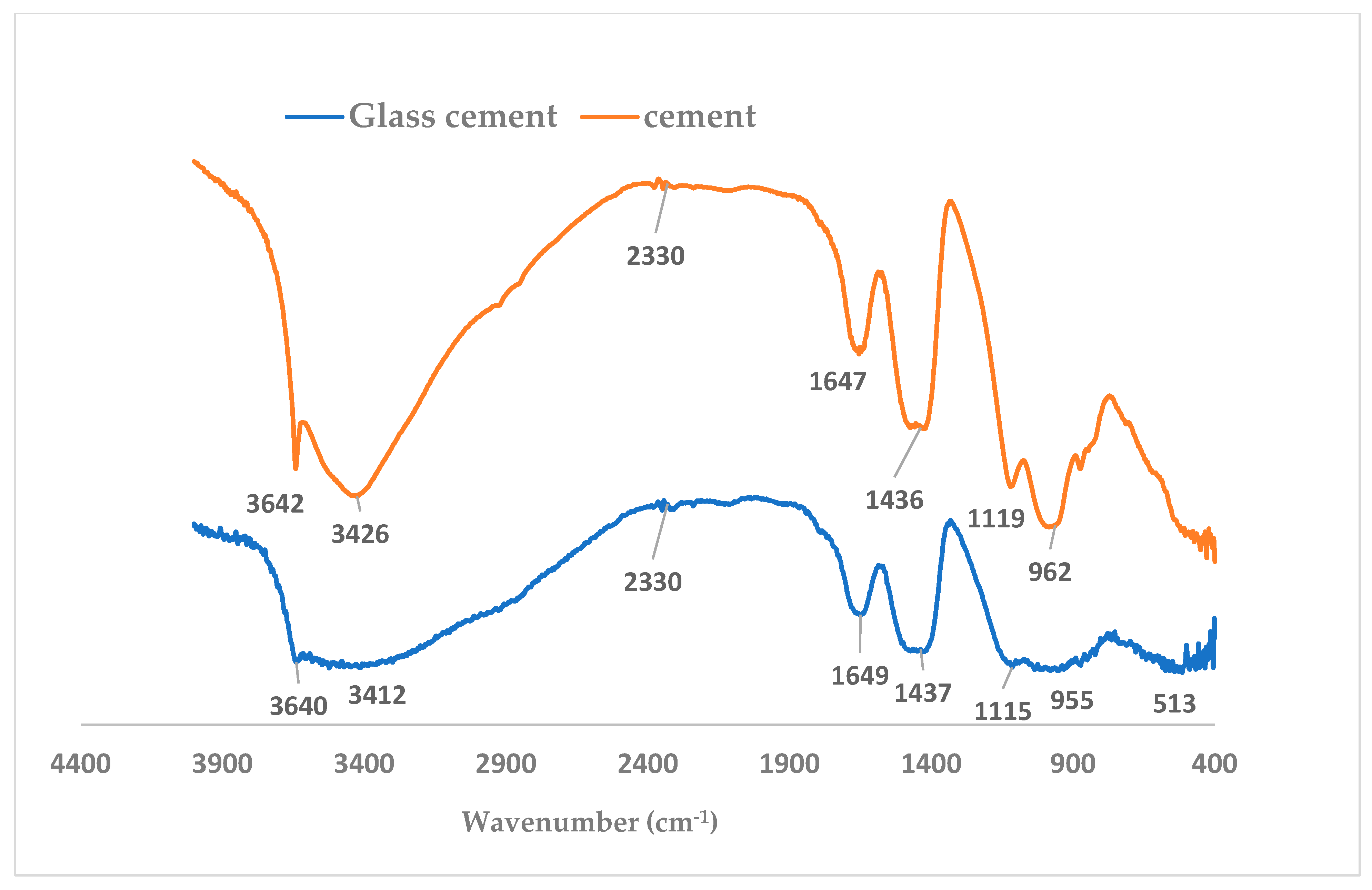
3.6. Effect of Glass on Hydroxyl Ion, C=O, Water Molecule Vibrations and Silica Reorganization of the Binder
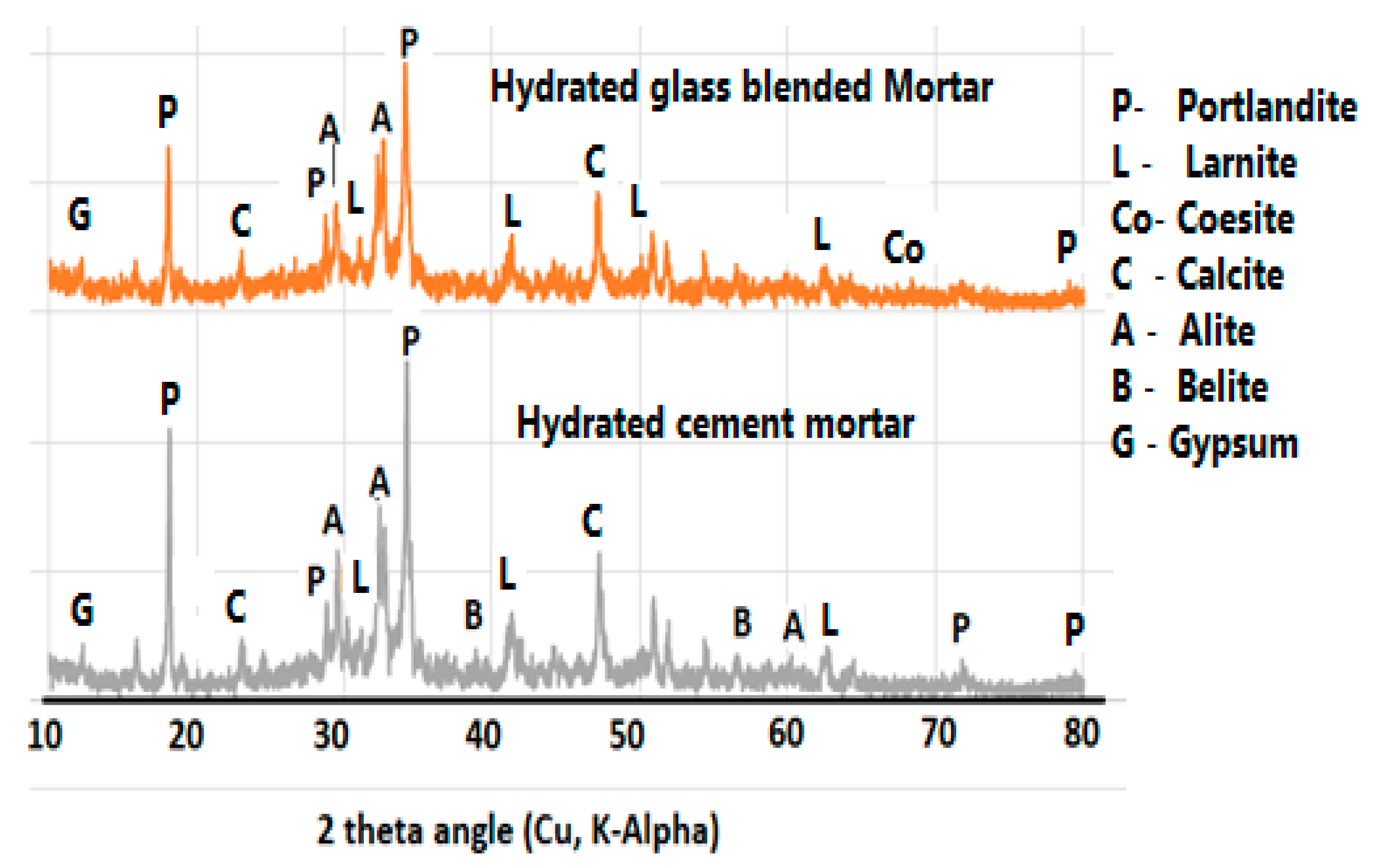
3.7. Effect of Glass on the Formation of Mineral Phases in the Product
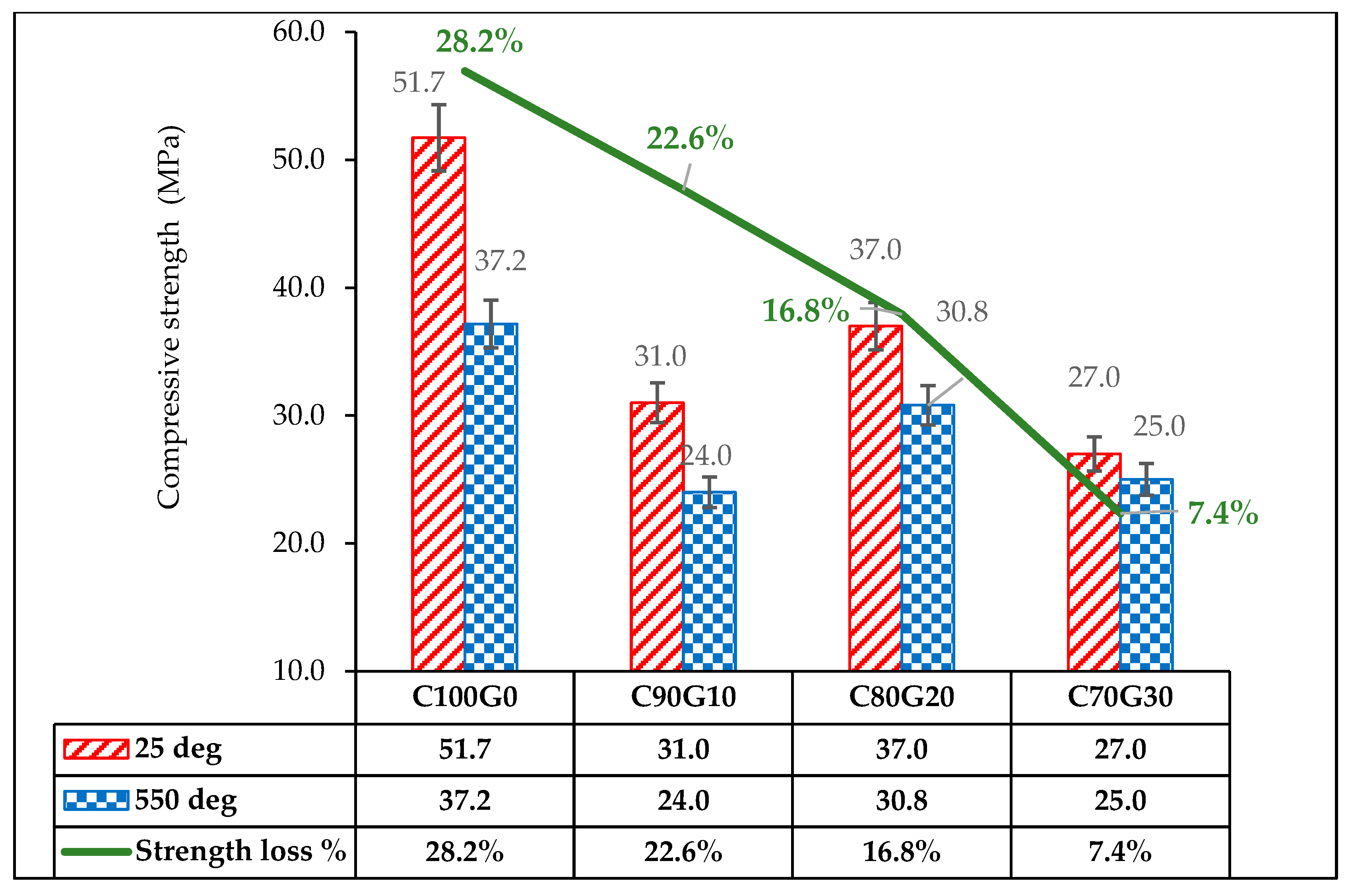
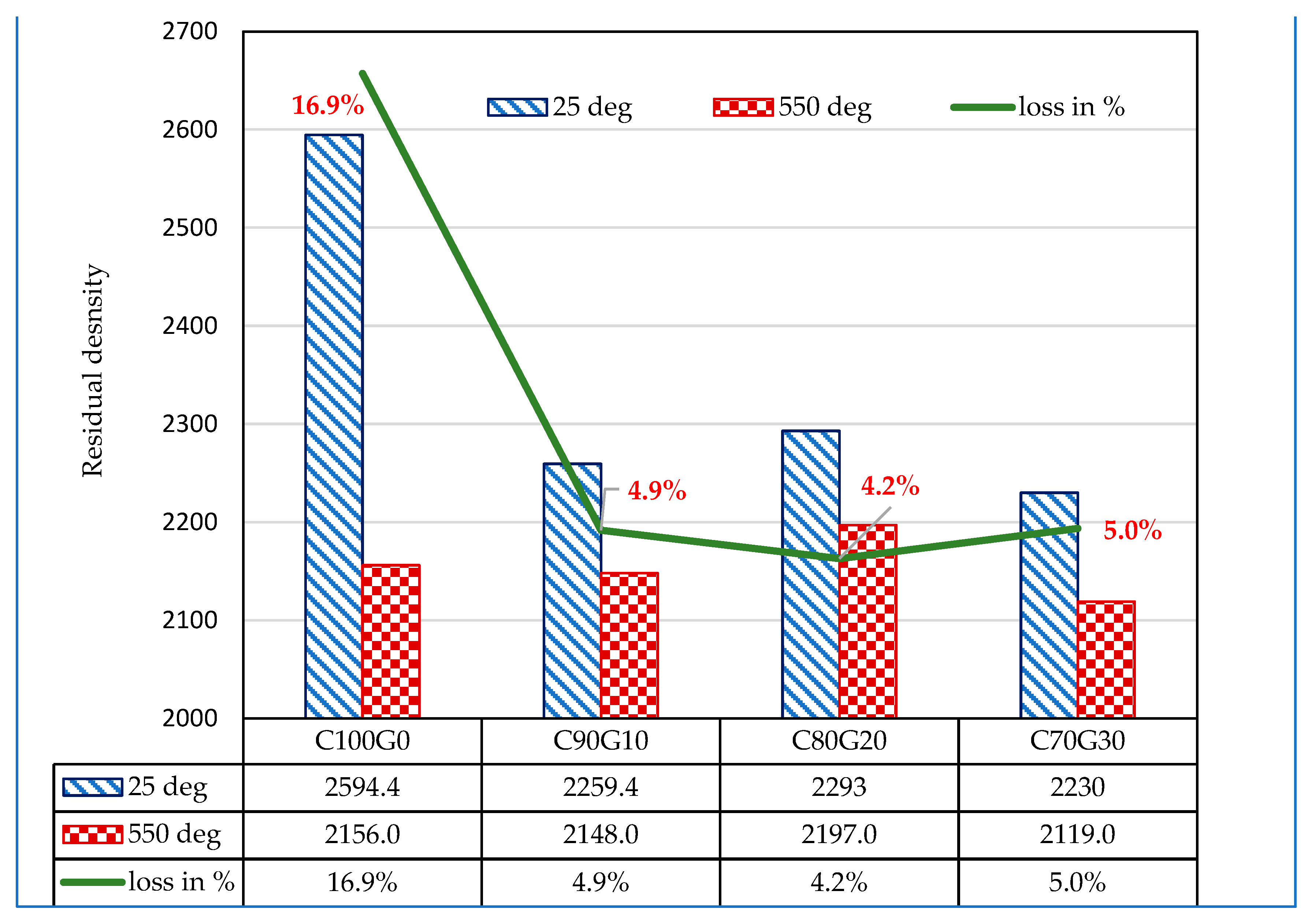
3.8. Effect of Glass on 28 Day Concrete Exposed to Elevated Temperatures (550 °C)
4. Conclusions
- (i)
- Glass reduced the setting time and enhanced the workability of both glass blended mortar and concrete.
- (ii)
- Presence of glass caused an interfacial transition zone within the binder matrix, and this led to weak microstructural stability that prevented early strength development.
- (iii)
- Cement content, glass content and age constituted key elements in the models predicting the strength and density of mortar/concrete.
- (iv)
- Presence of glass affected the vibrational frequencies of hydroxyl (-OH), C=O, water molecules, Si-O and S-O, as observed in the Fourier infrared spectroscopy results.
- (v)
- The maximum 28-day compressive strengths in mortar and concrete were 33 MPa and 37 MPa, prepared at water/binder ratios of 0.4 and 0.42 with the optimum OPC partially substituted glass content of 10 wt.% and 20 wt.%, respectively. In addition, the maximum 90-day strength achieved in concrete was 47 MPa.
- (vi)
- Generally, OPC (C100G0) and glass blended concrete (C80G20) subjected to thermal exposure of 550 °C had strength reductions of 28.2% and 16.8%, while the loss of density was 16.9% and 4.2%, respectively.
- (vii)
- Glass blended concrete could be easily used in fire-resistant structures such as kitchens and incinerators to achieve better durability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Qiao, H.; Li, A.; Li, G. Performance of waste glass powder as a pozzolanic material in blended cement mortar. Constr. Build. Mater. 2022, 324, 126531. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (EPA). Facts and Figures about Materials, Waste and Recycling. Glass: Material-Specific Data. 2021. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/glass-material-specific-data (accessed on 5 August 2022).
- Tamanna, N.; Tuladhar, R. Sustainable use of recycled glass powder as cement replacement in concrete. Open Waste Manag. J. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Cao, J.; Lu, J.; Jiang, L.; Wang, Z. Sinterability, microstructure and compressive strength of porous glass-ceramics from metallurgical silicon slag and waste glass. Ceram. Int. 2016, 42, 10079–10084. [Google Scholar] [CrossRef]
- Lu, J.; Duan, Z.; Poon, C.S. Combined use of waste glass powder and cullet in architectural mortar. Cem. Concr. Compos. 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Šimonová, H.; Zahálková, J.; Rovnaníková, P.; Bayer, P.; Schmid, P. Mechanical fracture parameters of cement-based mortars with waste glass powder. Procedia Eng. 2017, 190, 86–91. [Google Scholar] [CrossRef]
- Lu, J.; Zhan, B.; Duan, Z.; Sun, C. Using glass powder to improve the durability of architectural mortar prepared with glass aggregates. Mater. Des. 2017, 135, 102–111. [Google Scholar] [CrossRef]
- Shoaei, P.; Ameri, F.; Reza Musaeei, H.; Ghasemi, T.; Cheah, C.B. Glass powder as a partial precursor in Portland cement and alkali-activated slag mortar: A comprehensive comparative study. Constr. Build. Mater. 2020, 251, 118991. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Use of waste glass as sand in mortar: Part II-Alkali-silica reaction and mitigation methods. Cem. Concr. Compos. 2013, 35, 118–126. [Google Scholar] [CrossRef]
- Tan, K.H.; Du, H. Use of waste glass as sand in mortar: Part I-Fresh, mechanical and durability properties. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Lu, J.X.; Duan, Z.H.; Poon, C.S. Fresh properties of cement pastes or mortars incorporating waste glass powder and cullet. Constr. Build. Mater. 2017, 131, 793–799. [Google Scholar] [CrossRef]
- Sadati, S.; Khayat, K.H. Rheological and hardened properties of mortar incorporating high-volume ground glass fiber. Constr. Build. Mater. 2017, 152, 978–989. [Google Scholar] [CrossRef]
- Alhumoud, J.M.; Al-Mutairi, N.Z.; Terro, M.J. Recycling crushed glass in concrete mixes. Int. J. Environ. Waste Manag. 2008, 2, 111–124. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Use of recycled CRT funnel glass as fine aggregate in dry-mixed concrete paving blocks. J. Clean. Prod. 2014, 68, 209–215. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef]
- Harbec, D.; Tagnit-Hamou, A.; Gitzhofer, F. Waste-glass fume synthesized using plasma spheroidization technology: Reactivity in cement pastes and mortars. Constr. Build. Mater. 2016, 107, 272–286. [Google Scholar]
- Kamali, M.; Ghahremaninezhad, A. An investigation into the hydration and microstructure of cement pastes modified with glass powders. Constr. Build. Mater. 2016, 112, 915–924. [Google Scholar] [CrossRef]
- Lu, J.X.; Poon, C.S. Use of waste glass in alkali activated cement mortar. Constr. Build. Mater. 2018, 160, 399–407. [Google Scholar] [CrossRef]
- Zheng, K. Pozzolanic reaction of glass powder and its role in controlling alkali-silica reaction. Cem. Concr. Compos. 2016, 67, 30–38. [Google Scholar] [CrossRef]
- Sikora, P.; Horszczaruk, E.; Skoczylas, K.; Rucinska, T. Thermal properties of cement mortars containing waste glass aggregate and nanosilica. Procedia Eng. 2017, 196, 159–166. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S.; Kou, S.C. Feasibility of using recycled glass in architectural cement mortars. Cem. Concr. Compos. 2011, 33, 848–854. [Google Scholar] [CrossRef]
- Ling, H.Z.C.S.P.C.L. Utilizing recycled cathode ray tube funnel glass sand as river sand replacement in the high-density concrete. J. Clean. Prod. 2013, 51, 184–190. [Google Scholar] [CrossRef]
- Gorospe, K.; Booya, E.; Ghaednia, H.; Das, S. Strength, Durability, and Thermal Properties of Glass Aggregate Mortars. J. Mater. Civ. Eng. 2019, 31, 04019231. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Murphy, T.; Wuhrer, R. High temperature performance of mortars containing fine glass powders. J. Clean. Prod. 2017, 162, 16–26. [Google Scholar]
- Ibrahim, K.I.M. Recycled waste glass powder as a partial replacement of cement in concrete containing silica fume and fly ash. Case Stud. Constr. Mater. 2021, 15, e00630. [Google Scholar] [CrossRef]
- Mehta, A.; Ashish, D.K. Silica fume and waste glass in cement concrete production: A review. J. Build. Eng. 2020, 29, 100888. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Al-Sodani, K.A.A.; Alateah, A.H.; Al-Tholaia, M.M.H.; Adewumi, A.A.; Bakare, A.O.; Usman, A.K.; Momohjimoh, I. Performances of the synergy of silica fume and waste glass powder in ternary blended concrete. Appl. Sci. 2022, 12, 1–16. [Google Scholar]
- ASTM C150-07; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM C494/C494M-08; Standard Specification for Chemical Admixtures for Concrete. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM C191-21; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1437-20; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C109/C109M-20; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2020.
- BS EN 12390-1: 2000; Testing Hardened Concrete-Part 1: Shape, Dimensions and Other Requirements for Specimens and Molds. British Standards Institution: London, UK, 2000.
- Aili, A.; Maruyama, I. Review of several experimental methods for characterization of micro- and nano-scale pores in cement-based material. Int. J. Concr. Struct. Mater. 2020, 14, 1–18. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Sarker, P.K. Effect of waste glass fine aggregate on the strength, durability and high temperature resistance of alkali-activated fly ash and GGBFS blended mortar. Constr. Build. Mater. 2020, 263, 120177. [Google Scholar] [CrossRef]
- Bekiaris, G.; Bruun, S.; Peltre, C.; Houot, S.; Jensen, L.S. FTIR–PAS: A powerful tool for characterising the chemical composition and predicting the labile C fraction of various organic waste products. Waste Manag. 2015, 39, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Takeuchi, M.; Otsuka, M.; Ben-Nissan, B.; Grossin, D. Effect of carbon dioxide on self-setting apatite cement formation from tetracalcium phosphate and dicalcium phosphate dihydrate; ATR-IR and chemoinformatic analysis. Colloid Polym. Sci. 2015, 239, 2781–2788. [Google Scholar] [CrossRef]
- Christensen, A.N.; Jensen, T.R.; Hanson, J.C. Formation of ettringite, Ca6Al2(SO4)3(OH)12·26H2O, AFt, and monosulfate, Ca4Al2O6(SO4)·14H2O, AFm-14, in hydrothermal and hydration of Portland cement and of calcium aluminum oxide—calcium sulfate dihydrate mixtures studied by in situ synchrotron X-ray powder diffraction. J. Solid State Chem. 2004, 177, 1944–1951. [Google Scholar] [CrossRef]
- Carsana, M.; Frassoni, M.; Bertolini, L. Comparison of ground waste glass with other supplementary cementitious materials. Cem. Concr. Compos. 2014, 45, 39–45. [Google Scholar] [CrossRef]
- Richhariya, G.; Dora, D.T.K.; Parmar, K.R.; Pant, K.K.; Singhal, N.; Lal, K.; Kundu, P.P. Development of self-healing cement slurry through the incorporation of dual-encapsulated polyacrylamide for the prevention of water ingress in oil well. Materials 2020, 13, 2921. [Google Scholar] [CrossRef]
- Feng, P.; Bullard, J.W. A multiscale microstructure model of cement paste sulfate attack by crystallization pressure a multiscale microstructure model of cement paste sulfate a ack by crystallization pressure. Model. Simul. Mater. Sci. Eng. 2017, 25, 065013. [Google Scholar] [CrossRef]






| Oxides | Cement | Glass |
|---|---|---|
| SiO2 | 20.17 | 68.1 |
| Al2O3 | 5.58 | 0.9 |
| Fe2O3 | 2.86 | 0.6 |
| CaO | 63.51 | 14.5 |
| MgO | 3.15 | 1.8 |
| Na2O | 0.12 | 12.2 |
| K2O | 0.57 | 0.8 |
| SO3 | 2.56 | 0.4 |
| SiO2 + Al2O3 + Fe2O3 | 26.89 | 69.6 |
| Specific gravity (water) | 3.14 | 2.48 |
| Specific surface area (m2/kg) | 329.5 | 223.0 |
| LOI (%) | 2.80 | 0.80 |
| Property | Values |
|---|---|
| Fineness modulus | 2.61 |
| Specific gravity | 2.57 |
| Sample ID | Water (kg/m3) | Sand (kg/m3) | Cement * (kg/m3) | Glass Waste * (kg/m3) | Superplasticizers (kg/m3) | Sand Absorption (%) | w/c Ratio | Sand/Binder Ratio |
|---|---|---|---|---|---|---|---|---|
| MG0 | 140 | 962.5 | 350 | 0 | 4.95 | 1.75 | 0.4 | 2.75 |
| MG5 | 140 | 962.5 | 332.5 | 17.5 | 4.95 | 1.75 | 0.4 | 2.75 |
| MG10 | 140 | 962.5 | 297.5 | 35 | 4.95 | 1.75 | 0.4 | 2.75 |
| MG15 | 140 | 962.5 | 243.5 | 54 | 4.95 | 1.75 | 0.4 | 2.75 |
| MG20 | 140 | 962.5 | 173.5 | 70 | 4.95 | 1.75 | 0.4 | 2.75 |
| Mixes | Cement * (kg/m3) | Glass * (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | Water (kg/m3) | SP (kg/m3) | w/Binder Ratio | Fine/Total Aggregates |
|---|---|---|---|---|---|---|---|---|
| C100G0 | 350 | 0 | 767 | 1128 | 147 | 1.75 | 0.42 | 0.40 |
| C90G10 | 315 | 35 | 735 | 1120 | 147 | 1.75 | 0.42 | 0.40 |
| C80G20 | 280 | 70 | 738 | 1120 | 147 | 1.75 | 0.42 | 0.40 |
| C70G30 | 245 | 105 | 737 | 1120 | 147 | 1.75 | 0.42 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, M.O.; Al-Sodani, K.A.A.; Adewumi, A.A.; Alateah, A.H.; Al-Tholaia, M.M.H.; Shamsah, S.M.I.; Qazi, U.Y.; Alanazi, G.D.S. Microstructural Characteristics, Modeling of Mechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete. Appl. Sci. 2022, 12, 8600. https://doi.org/10.3390/app12178600
Yusuf MO, Al-Sodani KAA, Adewumi AA, Alateah AH, Al-Tholaia MMH, Shamsah SMI, Qazi UY, Alanazi GDS. Microstructural Characteristics, Modeling of Mechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete. Applied Sciences. 2022; 12(17):8600. https://doi.org/10.3390/app12178600
Chicago/Turabian StyleYusuf, Moruf Olalekan, Khaled A. Alawi Al-Sodani, Adeshina A. Adewumi, Ali H. Alateah, Mohammed M. H. Al-Tholaia, Sami M. Ibn Shamsah, Umair Yaqub Qazi, and Ghazi Dibas S. Alanazi. 2022. "Microstructural Characteristics, Modeling of Mechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete" Applied Sciences 12, no. 17: 8600. https://doi.org/10.3390/app12178600
APA StyleYusuf, M. O., Al-Sodani, K. A. A., Adewumi, A. A., Alateah, A. H., Al-Tholaia, M. M. H., Shamsah, S. M. I., Qazi, U. Y., & Alanazi, G. D. S. (2022). Microstructural Characteristics, Modeling of Mechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete. Applied Sciences, 12(17), 8600. https://doi.org/10.3390/app12178600










