Abstract
Vinegars are natural products manufactured by two-step fermentation. One of the most famous is balsamic vinegar, especially that produced in Modena, Italy. Its unique production process positively distinguishes it from other vinegars. There are basically three types of balsamic vinegar: common balsamic vinegar, Balsamic vinegar of Modena, and Traditional balsamic vinegar of Modena. The chemical analysis of these vinegars is mainly carried out by using gas or liquid chromatography, often coupled to mass spectrometric detection. Although gas chromatography is generally used for the determination of the overall profile of volatile organic compounds, furfurals, phenolic compounds, and organic acids, high-performance liquid chromatography is typically applied for the determination of amino acids, sugars, and polyphenols. The two complementary techniques, the combination of which is useful for the detailed characterization of balsamic vinegars, are reviewed and discussed in this article.
1. Introduction
Balsamic vinegar produced in Modena and Reggio Emilia, Italy, is one of the most widely known vinegars on the European continent. The name “balsamic” comes from the word balm (medicinal ointment) because its miraculous medicinal abilities were assumed, for example, in the fight against the plague. The first mention of this vinegar dates to the 11th century, when it was given to the Emperor of the Holy Roman Empire, Henry III. In the following centuries, it was a gift to other important personalities [1]. At the end of the 16th century, the Dukes of Este used this vinegar as a gift for the nobles. The Napoleonic Wars, during which Duke Ercola III’s supplies were stolen, may have been responsible for spreading balsamic vinegar into the world. Another act, spreading balsamic vinegar throughout the world, occurred in 1862, when an Italian lawyer, Francesco Agezzotti, revealed in a letter the secret of making balsamic vinegar to his friend, from whom the recipe spread next [2]. To preserve its tradition and origin, balsamic vinegar received a protected designation of origin in the year 2000, which allows products called “traditional balsamic vinegar of Modena” (TBVM) or “traditional balsamic vinegar of Reggio Emilia” (TBVRE) and “balsamic vinegar from Modena” (BVM) to be produced only in these two Italian provinces [3]. Vinegars, known as “balsamic vinegars” (BV), do not bear any protected designation of origin and can be produced anywhere in the world [4].
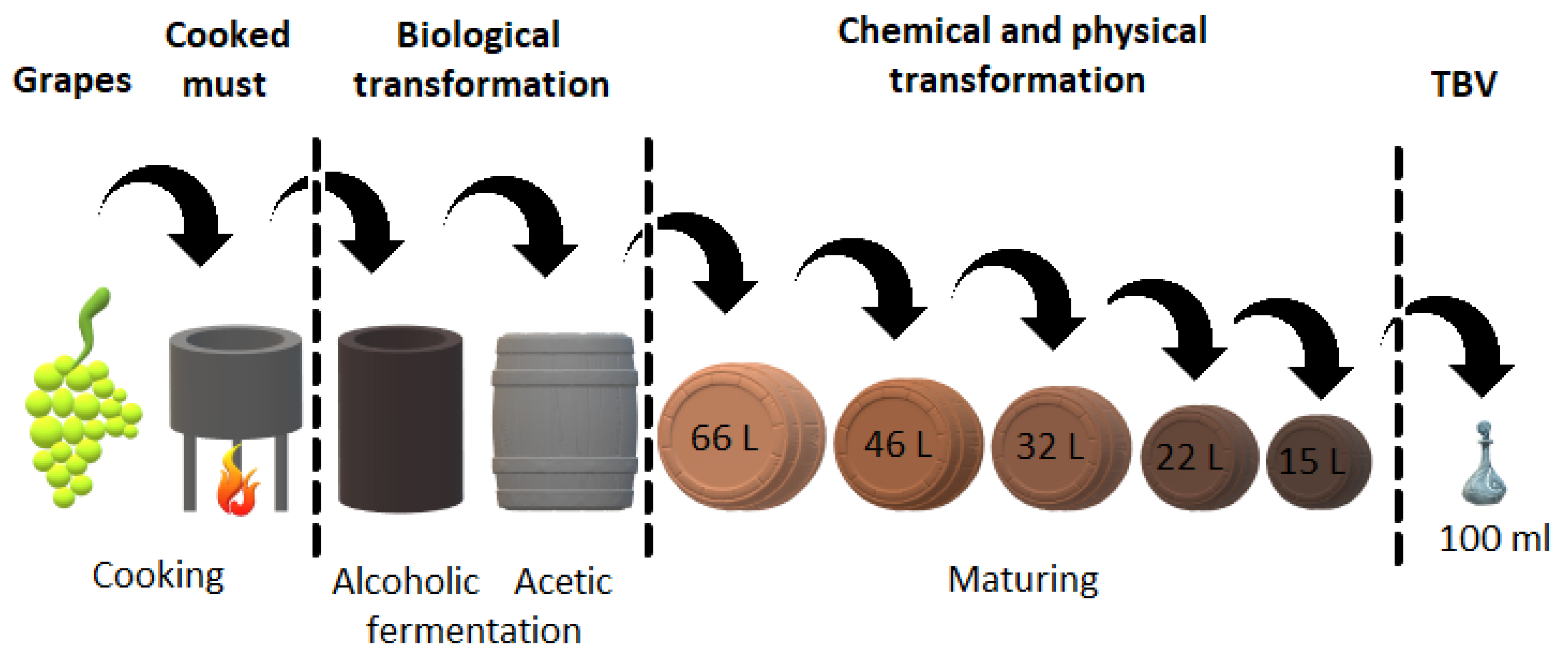
The TBVM and TBVRE production process (Figure 1) is completely unique and includes the concentration of grape must (the most used varieties are Trebbiano Modenese, and Lambrusco) on an open fire (for at least 30 min and at a temperature higher than 80 °C) followed by the first fermentation, in which the sugars of the grape must are oxidized to ethanol by means of yeast. Then, ethanol is bacterially oxidized to acetic acid in the second fermentation, mainly by the genera Acetobacter and Gluconacetobacter species [5]. This usually occurs in a series of five wooden barrels, where the final product passes in sequence through all barrels. The material for barrel production is, for example, mulberry, cherry, chestnut, oak, or juniper wood. Each of these barrels gives the vinegar a unique property. Traditional balsamic vinegars mature in barrels for at least 12 years (TBVM—affinato) or 25 years (TBVM—extravecchio). During this time, a replenishment process occurs, which means maintaining a constant level of intermediate in the barrels by transferring the liquid from one barrel to another. Liquid losses during evaporation are compensated by adding fresh must to the first barrel. Vinegar ages in a factory with uncontrolled temperature changes, while high summer temperatures support microbial activity and low winter temperatures support the evolution of taste. Grapes and all steps in the production process, including the aging of vinegar, must occur in the Italian provinces of Modena and Reggio Emilia. No other additives may be added during the maturation process and no techniques may be used to speed up the production process. The result is a dark brown liquid with a high density, sweet and sour, fruity, and complex taste [6,7,8,9].
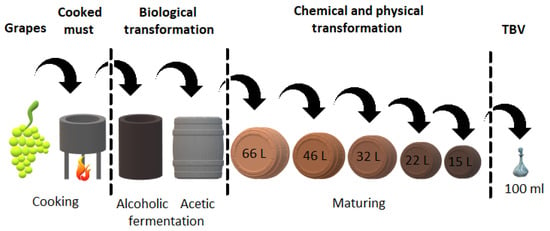
Figure 1.
Scheme of the production of TBVM.
There are many differences between BVM and TBVM, including production, organoleptic properties, and economic aspects of the production. The production of BVM is simpler and involves mixing cooked grape must with wine vinegar, and with the possibility of adding a small amount of dye or caramel, which can give dark color and a sweeter taste to the product. As for the production time of BVM, the producers have a free hand here and it only depends on them how long they will let the vinegar mature. While BVM shows a variable degree of acidity and tastes more fruity and sweeter than regular wine vinegars, TBVM is characterized by low acidity and unique aroma, which is given by aging in wooden barrels. Another difference is in the volume of the bottle available in a store. Bottles of TBVM usually have a volume of 250 mL or higher. Bottles of TBVM are sold in smaller 100 mL bottles. Due to production processes and maturing time, the products also differ in price. The price of a BVM bottle with a volume of 250 mL starts at 2 euros. On the other hand, the TBVM bottle is available for an average of 200 euros [4].
Balsamic vinegars contain a wide range of compounds, some of which have not yet been completely characterized. Most of the current studies focused on the analysis of sugars, organic acids, phenolic compounds, and furfurals. An overview of the compounds contained in balsamic vinegar is presented in Figure 2 and Figure 3. The methods used for their analysis are summarized in Table 1 for analyses using gas chromatography (GC) and Table 2 for analyses by high-performance liquid chromatography (HPLC).
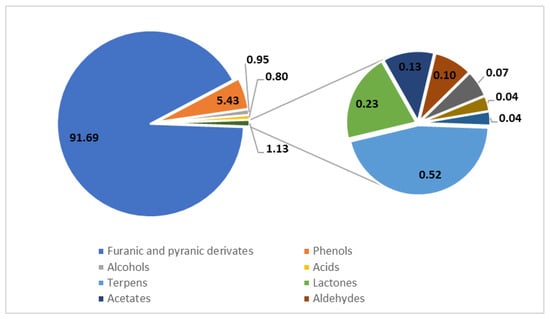
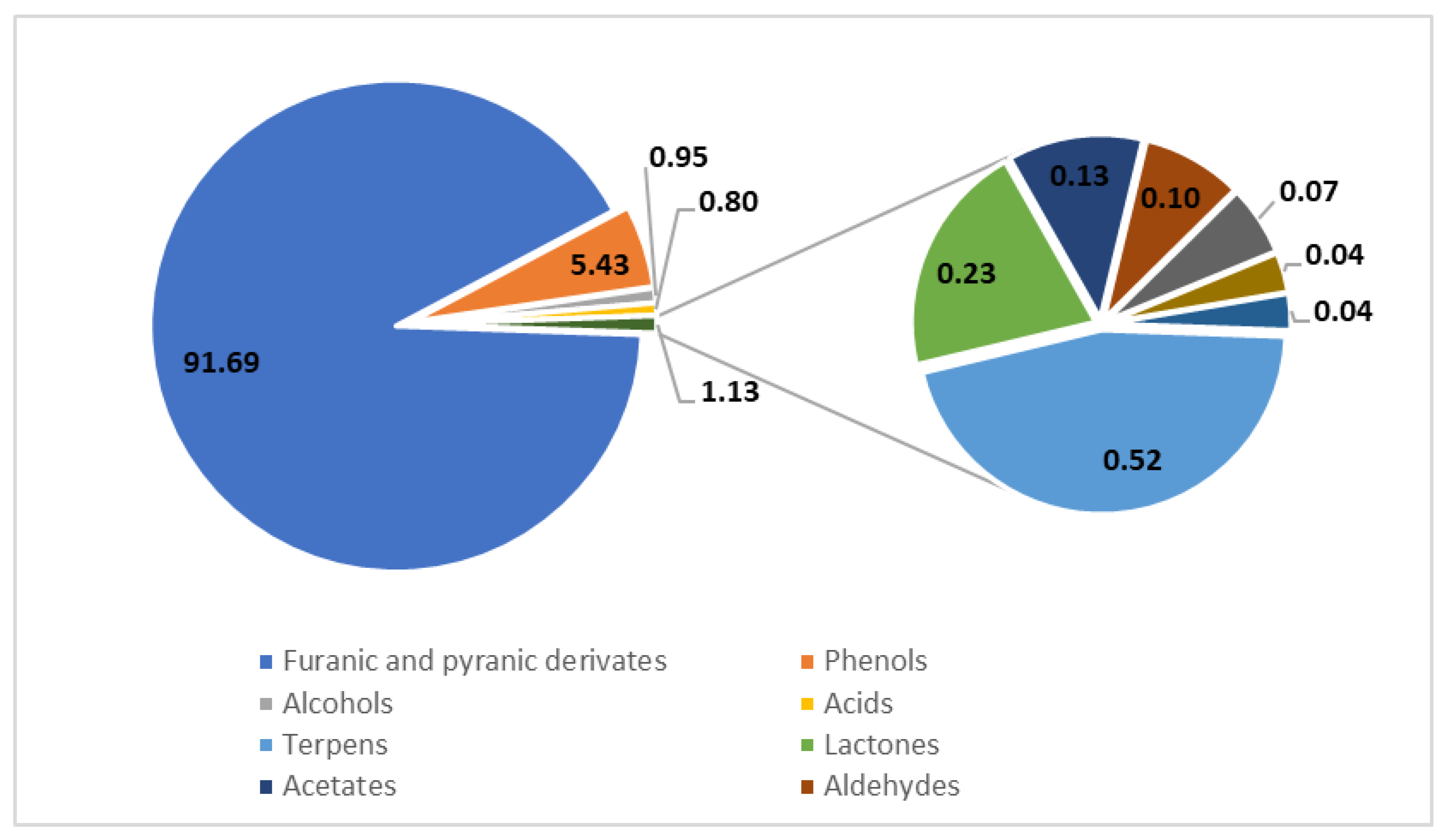
Figure 2.
Profile of volatile compounds in balsamic vinegars determined using GC with mass spectrometric detection after solid-phase extraction. The values are presented in % (w/w); based on data from [10].
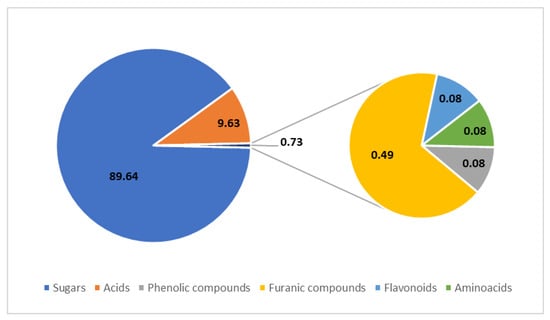
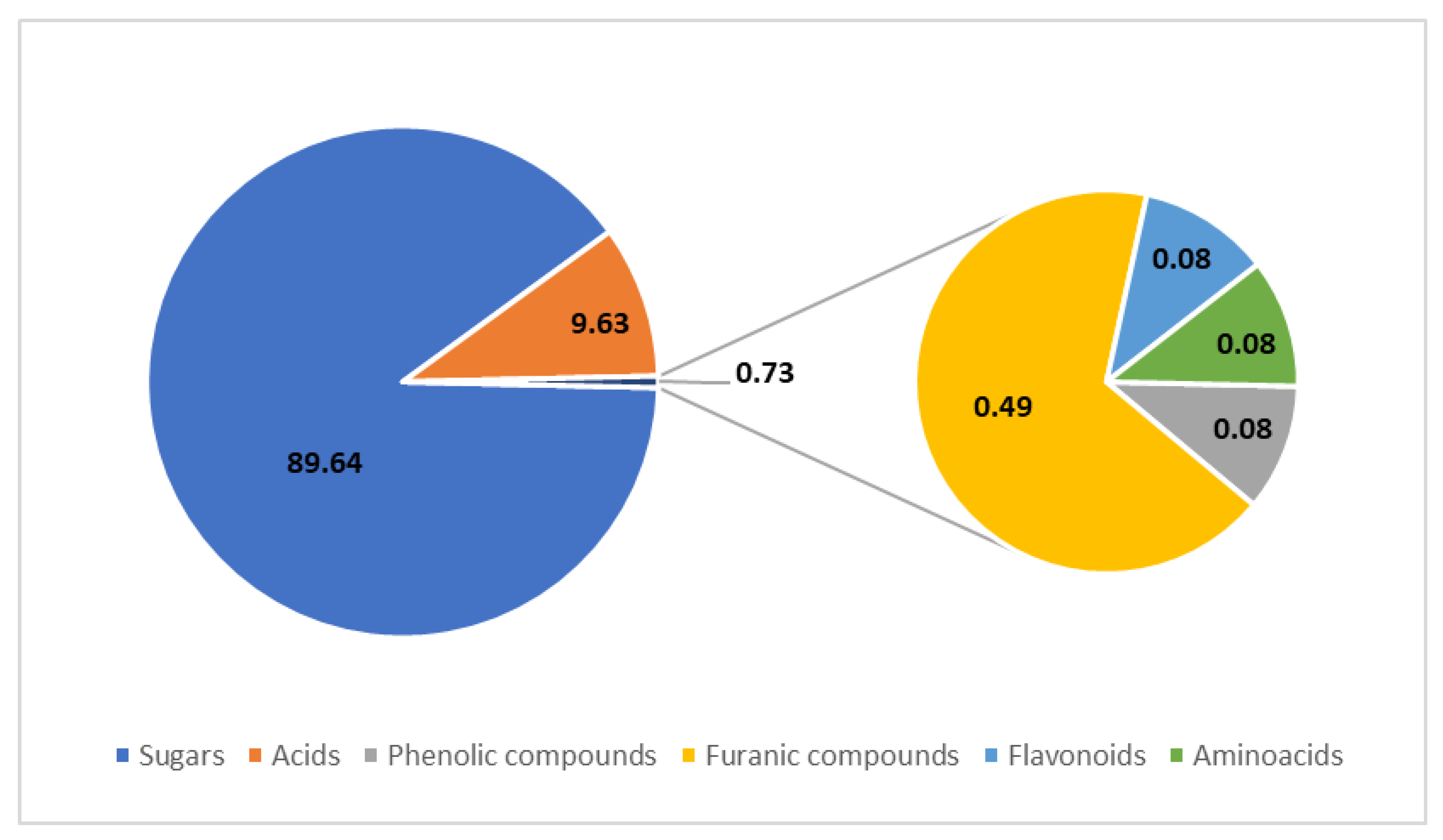
Figure 3.
Profile of organic compounds in balsamic vinegars determined using HPLC, collected from several studies. The values are presented in % (w/w); based on data from [11,12,13,14,15,16].

Table 1.
Table of methods used for GC analysis of balsamic vinegar samples.

Table 2.
Table of methods used for HPLC analysis of balsamic vinegar samples.
2. Separation Methods for Characterization of Balsamic Vinegars
2.1. Gas Chromatography
For samples containing volatile components, gas chromatography is usually the first method of choice, especially coupled to single- or tandem-mass spectrometry (GC-MS, GC-MS/MS). The history of GC dates to the first half of the twentieth century and its invention is inevitably related to the names of Martin and James, who constructed the first chromatograph with an inert carrier gas used as a mobile phase [69]. After decades of research, the technique has matured into the routinely used tool for the analysis of gases, liquids, or suitably dissolved solids. It is usually used for the analysis of organic compounds in the range of molecular weights from 2 to 1000 Da.
The main application area of GC is in the studies of food and edible plants, where a number of groups of compounds are analyzed, including pesticides, endocrine-disrupting chemicals, estrogens, fatty acids, etc. [70]. Balsamic vinegar samples are typically highly complex mixtures containing hundreds of compounds, while most of them are volatile. More than 1500 compounds were detected by GC-MS methods; however, the number of unequivocally identified compounds is usually much lower [38]. Several groups of compounds were quantified using GC, comprising organic acids, esters, alcohols, aldehydes, ketones, furanes, phenols, lactones, odorous compounds, amino acids, and others.
In the case of the analysis of the profile of volatile organic compounds (VOCs) of balsamic vinegar (Figure 4), GC is most often coupled with MS detection, while the choice of extraction techniques can be diverse (Table 1).
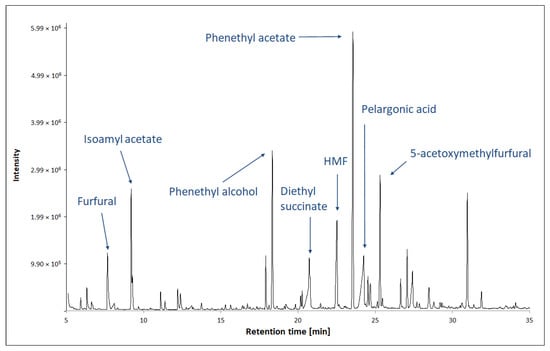
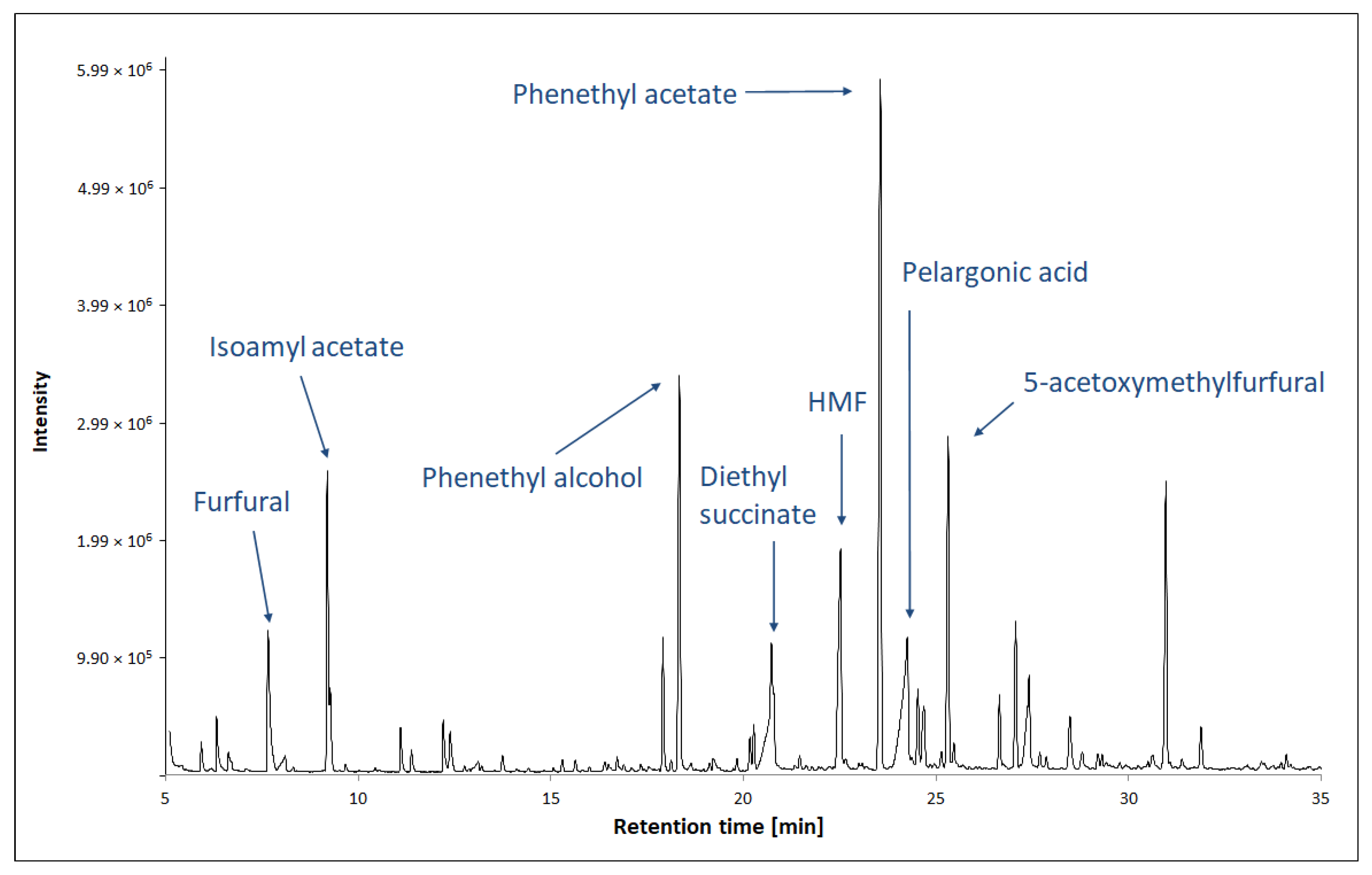
Figure 4.
Analysis of VOCs in TBV by GC-MS method. Figure adapted from [71].
2.2. Liquid Chromatography
In 1952, with the advent of gas chromatography, Martin and Synge suggested the possibility of using a liquid mobile phase for rapid separation, similar to GC. To do this, it was necessary to achieve a small particle size of the stationary phase and the use of pressure on the mobile phase. In 1960, Hamilton, Bogue, and Anderson reported the use of liquid chromatography (LC) for amino acid separation, unfortunately, without the wider attention of other scientists. HPLC was therefore introduced in the late 1960s, when Kirkland, Huber, and Horvath published their work about this method, dealing with the development of uniformed small-particle sorbents [72].
High-performance liquid chromatography is applicable for the separation and analysis of complex chemical mixtures into individual components. Thanks to its almost universal use, it is one of the most widely used analytical methods, especially in food analysis. The most commonly used is the reversed-phase mode, where the stationary phase is less polar than the mobile phase. Gradient elution is often used to speed up the whole analysis process, using two mobile phases (one polar and the other less polar), whose ratio changes during the process [73].
For the application in the food analysis area, the HPLC is typically used for analyses of (poly)phenolic compounds, which usually enter food products as secondary plant metabolites, and exhibit potent antioxidant properties. Together with other non-volatile compounds (such as sugars, amino acids, or amines), the full characterization of complex food samples is sometimes difficult with conventional HPLC, and therefore the application of innovative approaches, for example, multidimensional liquid chromatography [74], or the application of highly efficient columns packed with porous shell particles can be essential [75]. For the study of individual compounds contained in plant products, HPLC is mostly combined with UV/Vis, DAD, and MS detection (Table 2).
2.3. Other Analytical Techniques
Other methods than chromatography for the analysis of balsamic vinegars are less frequently used. There are several studies utilizing nuclear magnetic resonance spectrometric methods for the characterization of balsamic vinegars [76,77,78,79,80,81,82,83], gel electrophoresis for the study of acetic acid bacteria [84,85], and electrochemical methods [86,87,88].
3. Characterization of the Compounds Presented in Balsamic Vinegars
Vinegar is generally an aqueous solution of acetic acid, trace organic acids, and other types of compounds, that is, esters, ketones, and aldehydes, which together contribute to its organoleptic properties. When used as a food ingredient, it also possesses potential health benefits, including antimicrobial, antioxidant, and antitumor activity, due to the content of a wide variety of bioactive compounds, for example, phenolic compounds, vitamins, phytosterols, carotenoids [89]. In the following text, the main classes of compounds determined in balsamic vinegars using chromatographic methods are reviewed and discussed.
3.1. Organic Acids
One of the most abundant groups of VOCs are organic acids, which are formed by the oxidation of alcohols by acetic acid bacteria. Volatile acids with the highest concentration levels typically include acetic acid, benzoic acid, phenylacetic acid, isovaleric acid, isobutyric acid, and octanoic acid [10,38,39]. Cocchi et al. [28] in their study focused on the determination of organic acids by GC using derivatization procedures. The authors identified malic acid as the most abundant organic acid. During the maturation of vinegar, the concentration of organic acids increases significantly, especially for vinegars aged in wooden barrels [90]. Acid content also increases during the storage of vinegar in glass bottles [35].
Morales et al. [61] applied the method of ion exchange chromatography to samples of different vinegars, including one BVM. The authors focused on the main organic acids in vinegars, namely citric, tartaric, malic, lactic, and acetic acid. The method was successfully validated. Of the selected acids, acetic acid had the highest concentration in BVM, followed by malic acid.
Masino et al. [13] used the HPLC method for the characterization of organic acids, sugars, and furans of balsamic vinegar. The highest concentration of acetic acid was detected, which is in agreement with the study by Morales et al. [61]. Succinic acid, malic acid, and gluconic acid were detected as other major organic acids in this study as well.
3.2. Furanic Compounds
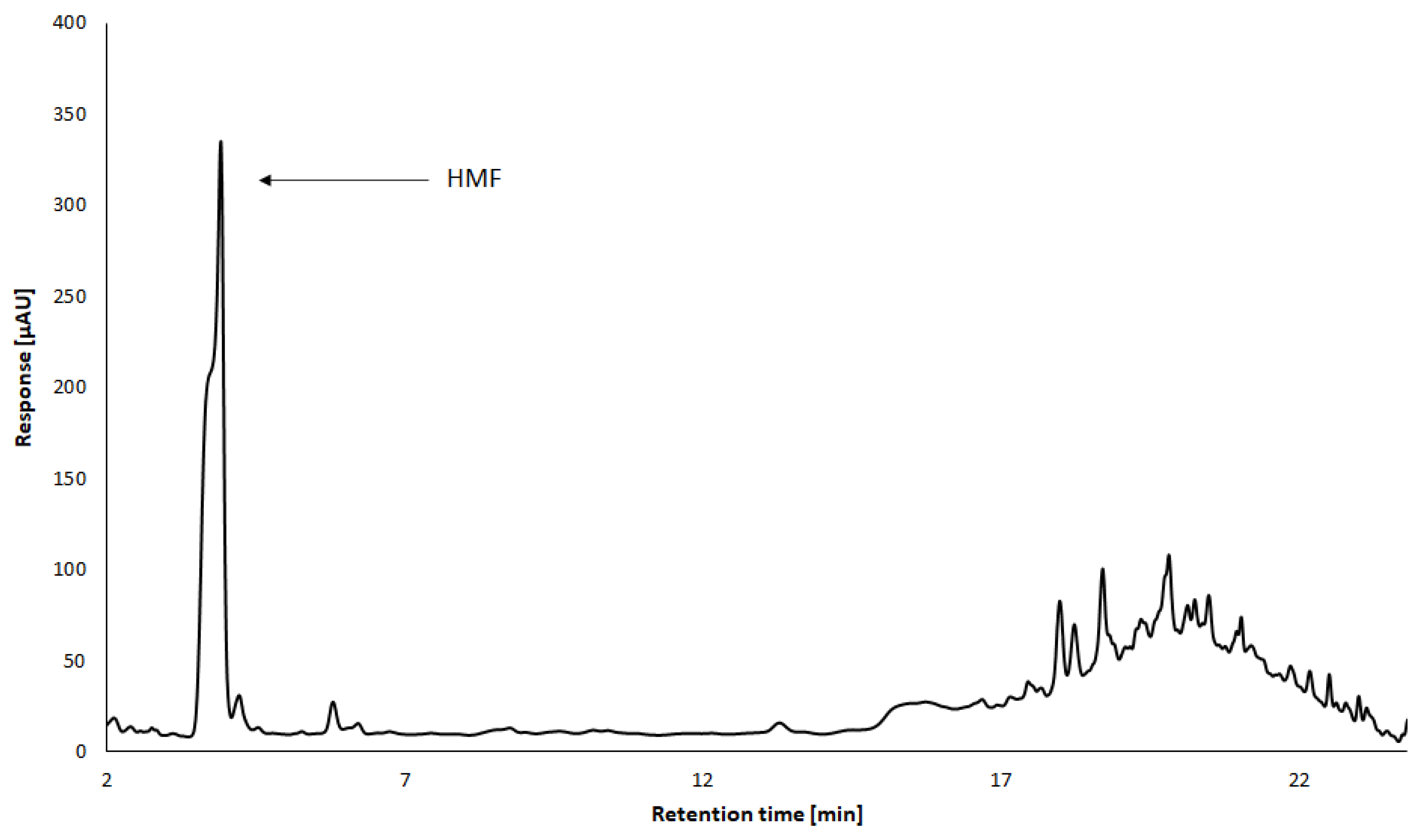
The presence of furanic compounds in balsamic vinegars is associated with the heating of glucose solutions. Furans are generally formed by Maillard reactions between reducing sugars and amino acids, but they can also come from oak barrels, or from the addition of caramel as a frequently used food colorant. The importance of these analytes lies in identifying the organoleptic properties of the products and the possible commercial fraud [10,20,39]. The major furans in balsamic vinegars include hydroxymethylfurfural (HMF), furfural, and 5-acetoxymethylfurfural, with some studies reporting dominance of HMF (Figure 5) and others 5-acetoxymethylfurfural. Other frequently identified furans are 5-methylfurfural and 2-acetylfuran [10,38,39].
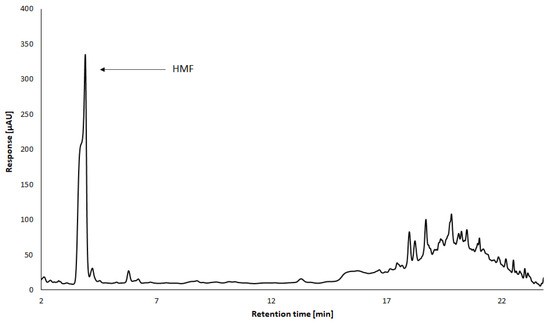
Figure 5.
Analysis of HMF in TBV by HPLC-DAD method. Conditions: Luna Omega 3 µm Polar C18 100 column, mobile phase acetonitrile/water gradient (M. Kašpar, 2022, unpublished data).
Theobald et al. [54] focused their work on the analysis of HMF in various types of vinegar. This compound is a good indicator of age, heat treatment, deterioration, and food adulteration. The HPLC method coupled with the diode array detector (DAD) was used for analysis. The authors concluded that balsamic vinegars, and especially traditional balsamic vinegars, contain the highest concentration of HMF compared to other types of vinegar. The same conclusion was reached by Giordano et al. [20].
Gaspar and Lucena [53] performed the analysis of selected furfurals (HMF, 5-methylfurfural, furfural) and patulin in food products, including balsamic vinegar, using the HPLC-DAD method. Furfural and patulin are good markers of food quality. Regarding patulin, this is the first study to specifically target this mycotoxin in balsamic vinegar. The balsamic vinegar contained all three selected furfurals but did not show signs of patulin. The authors recommend the method for controlling food quality.
Antonelli et al. [66] in their study reported changes in some furanic compounds (HMF, furoic acid, furfural) during the concentration of grape must be used to produce balsamic vinegar. They used the HPLC-DAD method to analyze these compounds. The results of the study show that the furanic content increases during the thermal concentration of must, with the highest increase for HMF.
The determination of furanic compounds was studied by Chinnici et al. [16]. The aim of this work was to create a simple and fast method for the determination of selected furanic compounds in TBVM. They used the method of ion exchange chromatography (IEC) with DAD detection. The presence of HMF, furfural, and 2-furoic acid was detected in all samples. In contrast, no sample contained 4-hydroxy-2,5-dimethyl-3-(2H)-furanone (DHMF), 2-acetylfuran, or furfuryl alcohol.
Several other works have dealt with the LC method and furanic compounds [13,51,52,55,67,91,92]. However, their list is beyond the scope of this paper.
3.3. Phenolic Compounds
Phenolic compounds belong to the group of substances called antioxidants [93], as they help fight oxidative stress, which can be the cause of liver, neurodegenerative, and cardiovascular diseases [94,95]. Their presence in vinegars is associated with original raw material, aging in wooden barrels (e.g., siringol, eugenol), or with the biological decarboxylation of acids (e.g., ethylphenol, ethylguaiacol) [10]. Although some phenols may have a beneficial effect on the overall aroma of vinegar (e.g., vanillin, eugenol), yeast-formed phenols have a negative impact on organoleptic properties, and their aroma is characterized as a spicy, animal, etc. [96,97].
Various volatile phenolic compounds have been identified in balsamic vinegars [10,35,39,41,96]. The main phenols in vinegars are phenolic acids, which, because of their lower volatility, cannot be identified by GC without derivatization procedures. Therefore, HPLC predominates in their analysis, but phenols can be determined by GC after derivatization with bis(trimethylsilyl)trifluoroacetamide [98].
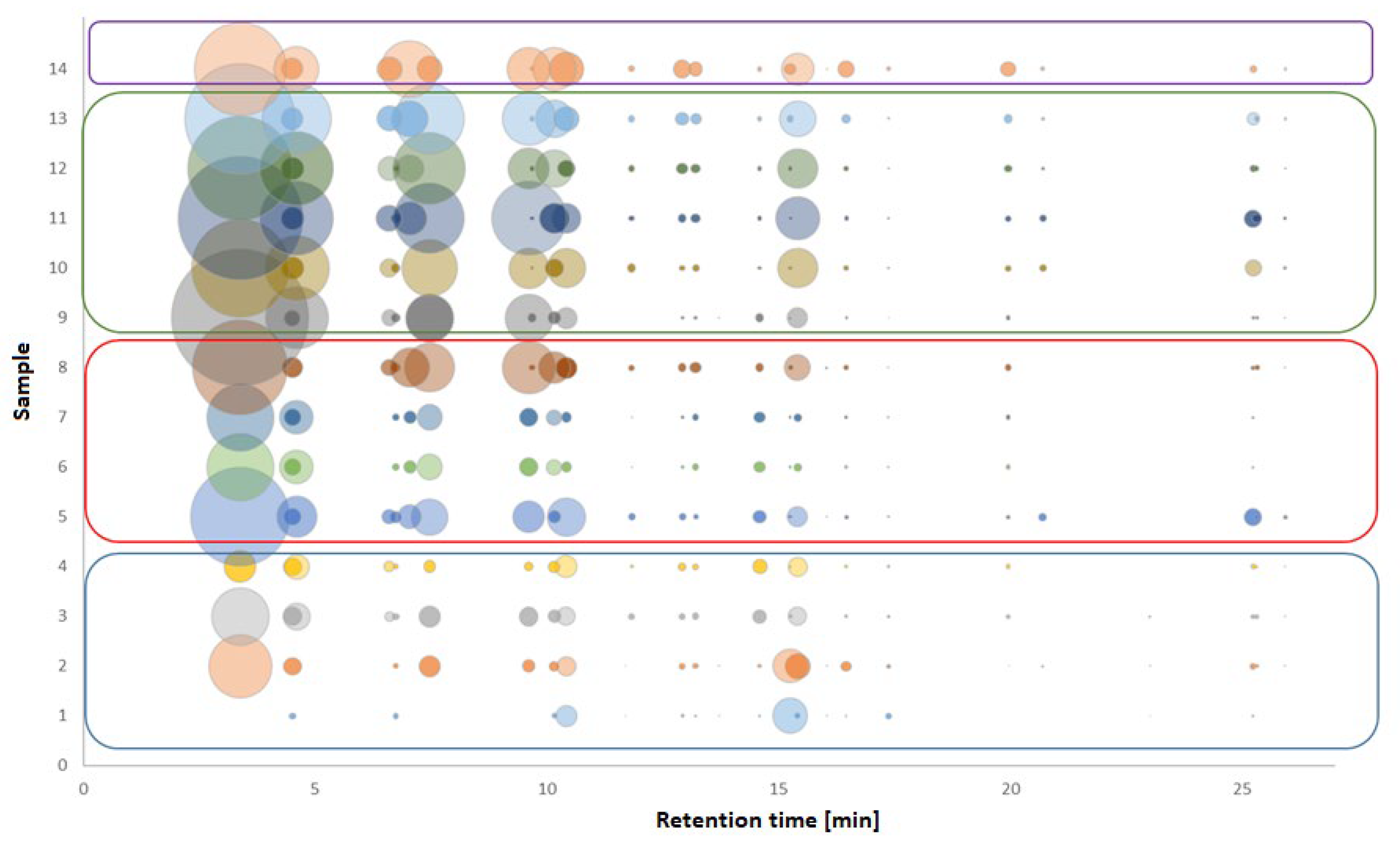
HPLC coupled with DAD or MS detection is the most commonly used method for the analysis of phenolic compounds in samples with a complicated matrix, such as vinegars [11,62,63,64,65,96]. The main phenolic compound is gallic acid (Figure 6), whose content increases during maturation in chestnut barrels [64]. On the contrary, in other types of vinegar, gallic acid belongs to substances with a minor content. At the same time, balsamic vinegars have the highest antioxidant activity compared to other vinegars [62,63]. Phenolic acids generally have higher content in balsamic vinegars than other groups of phenols. In addition to gallic acid, other major acids are protocatechuic acid, caffeic acid, and p-coumaric acid. Of the other substances, protocatechuic aldehyde, ethyl gallate, and tyrosol should be referred to [11,65,96].
Figure 6.
Comparison of the phenolic profile of balsamic vinegar samples obtained by HPLC analysis. Blue box—Greek balsamic vinegar; Red box—BVM; Green box—“premium” BVM; Purple box—TBVM. The first compound is gallic acid. Figure adapted from [96].
3.4. Amino Acids and Amines
Erba and Brückner [25], in their study, focused on the analysis of chiral amino acids in various vinegar samples, including balsamic vinegar, using chiral gas chromatography in combination with mass spectrometry. The results of the study show that balsamic vinegars have an increased content of D-Pro and D-Ala during maturation. At the same time, the amino acids D-Ala, D-Asp, and D-Glu originate from microbial activity and may be useful markers for distinguishing between fermentative and synthetically produced vinegars.
Chinnici et al. [14] analyzed 23 amino acids and 11 amines using the HPLC-DAD method in their work. In addition to BVM and TBVM vinegars, the authors also analyzed the well-known Jerez vinegar (VJ). The authors concluded that balsamic vinegars may contain higher concentrations of amino acids than Jerez vinegars. They identified proline, glycine, and γ-aminobutyric acid as the main amino acids. The lowest concentration of amines was found in TBVM.
Ordóñez et al. [47] dealt with the analysis of biogenic amines in samples of various vinegars, including balsamic vinegar. With a few exceptions, balsamic vinegar showed a higher qualitative and quantitative amount of biogenic amines compared to other vinegars. The highest concentrations of histamine, agmatine, and putrescine were found.
3.5. Esters
Esters are another group of VOCs identified in vinegars. They are formed during alcoholic fermentation or by reactions between organic acids and alcohols during maturation in wooden barrels [36]. Ethyl 3-hydroxybutyrate, ethyl hydrogen succinate, and ethyl octanoate have been identified as the main esters present in balsamic vinegars [10,38,39]. Compared to traditional Spanish vinegars, balsamic vinegars have a higher content of long-chain esters. Dihydroxymethyl jasmonate has been recommended as a possible marker to distinguish between Italian and Spanish vinegars [39].
3.6. Acetates
A separate group of esters are acetic acid esters (or acetates). In balsamic vinegars, they are formed by esterification between acetic acid and alcohols. 1,2,3-propanetriol diacetate, 2-phenylethyl acetate, ethyl acetate and 4-methyl-2-pentyl acetate were identified with the highest content in balsamic vinegars [10,39]. Neryl acetate was recommended in a study by Marrufo-Curtido et al. [39] as a discriminant parameter to distinguish TBVM-extravecchio from other types of balsamic vinegar.
3.7. Alcohols
Due to the high residual ethanol content, alcohols are among the main groups in vinegars in terms of concentrations [35]. Other alcohols with a higher content in balsamic vinegars are benzyl alcohol, furfuryl alcohol, butoxyethoxyethanol, and phenylethyl alcohol [10,35,38,39]. During the maturation of vinegar, the content of some alcohols, such as isoamyl alcohol and ethoxy-1-propanol, can decrease [40].
3.8. Aldehydes and Ketones
Aldehydes originate in vinegars from alcohol oxidation or are extracted from wooden barrels, and therefore their concentration is expected to be higher in vinegars produced by traditional methods [46]. Among aldehydes, siringaldehyde and octanal occur in balsamic vinegars at the highest concentration levels [10,35,38,39]. However, the major aldehydes are furfurals which also belong to the group of furan derivatives and are discussed in detail in Section 3.2. Acetoin is often identified as the main volatile ketone and its concentration increases during the acetification process [99].
3.9. Lactones
The presence of lactones in balsamic vinegars is due to sugar degradation and cyclization of hydroxyacids during fermentation, but also from wooden barrels [35]. α-methyl-γ-crotonolactone and δ-2-decenolactone have been identified as typical lactones for TBVM. The lactones with the highest concentration levels are pantalactone, γ-butyrolactone, angelicalactone, whiskeylactone and δ-2-decenolactone [10,39].
3.10. Odorous Compounds
Corsini et al. [26] in their work used the GC method in combination with olfactometry (GC-O) for the determination of active odor compounds in TBVM and BVM. This is the first study to apply the GC-O method to balsamic vinegars. Several active odor compounds were identified in the study, of which 14 compounds are considered by the authors of high importance (e.g., 2,3-butanedione, acetic acid, furan-2-carbaldehyde). The authors mention the possibility of using this method to distinguish TBVM from BVM.
Ugliano et al. [27] in their work used GC-MS and GC-O coupled with AEDA (aroma extract dilution analysis) methods for analysis and comparison of the main odorous compounds in TBVM, BVM, and other common wine vinegars. The authors identified acetic acid, diacetyl, 3-methylbutanoic acid, and unidentified compounds with balsamic, caramel, and honey aroma as the main components of the odor in TBVM. For BVM, they identified acetic acid, 3-methylbutanoic acid, 2-phenylethanol, and an unidentified compound with caramel aroma as the main odor components. For common wine vinegars, the main odor components were acetic acid, 3-methylbutanoic acid, 2-phenylethanol, and an unidentified compound with a licorice aroma.
3.11. Sugars
Lalou et al. [12] aimed to determine the physicochemical and sensory properties of balsamic vinegars produced in Greece and to compare the results with available data on balsamic vinegars produced in Italy. They used the HPLC method to determine glucose, fructose, glycerol (RI detection), and HMF (UV/VIS detection). Relatively large differences in the content of these compounds were founded, probably depending on the starting material and the production process.
Masino et al. [13] focused their study on the relationship between the physical and chemical profiles of balsamic vinegar and its sensory quality to determine which parameters are suitable for determining the quality of vinegar and which are unnecessary. They used the HPLC method for the analysis of acids, sugars, and furanic compounds. Of these compounds analyzed by HPLC, the analysis of sugars and HMF is important for determining the quality of vinegar.
From both mentioned studies that deal with the analysis of sugars [12,13] and compared to other studies [10,14,15,16,50], it is obvious that glucose and fructose are among the compounds with the highest concentration in balsamic vinegar.
3.12. Dicarbonyl Compounds
Papetti et al. [49] studied the effect of in vitro digestion on free α-dicarbonyl compounds (glyoxal, methylglyoxal, and diacetyl) in balsamic vinegars using the HPLC-DAD method. Previous studies have proven the toxicity of these compounds [100,101]. The authors conducted an in vitro exposure of selected α-dicarbonyl compounds to digestive enzymes. They concluded that digestive enzymes significantly change the concentration of α-dicarbonyl compounds, depending on the compound.
Another study focusing on the analysis of α-dicarbonyl compounds in balsamic vinegars was carried out by Daglia et al. [50]. The presence of glyoxal, methylglyoxal, 2,3-butanedione, 3-deoxyglucosone, 3,4-dideoxyglucosone-3-ene, hydroxypyruvaldehyde, and dihydroxyacetone was confirmed by the HPLC-DAD-MS/MS method. Furthermore, the authors evaluated the cytotoxicity of glyoxal, methylglyoxal, 2,3-butanedione in vitro. The authors concluded that the dietary intake of selected α-dicarbonyl compounds is not a significant source of toxicity.
3.13. Other Compounds Identified by GC
Cunha et al. [18] used the GC-MS method to determine 4-methylimidazole (4-MEI), which is a known neurotoxic agent and is formed in balsamic vinegars during the targeted coloring by caramel. 4-MEI was extracted with bis-2-ethylhexylphosphate and derivatized by isobutylchloroformate for identification. The concentration of 4-MEI in balsamic vinegar was higher than in various sauces or dark beer, and the authors recommend a targeted control of these substances in food.
Cirlini et al. [23] in their study focused on the identification of fructose and glucose acetates. The presence of these acetates has never been demonstrated in vinegars before, although it could be assumed that these esters are formed from sugars in starting materials and acetic acid, which is one of the most basic compounds in vinegars. They used GC-MS and nuclear magnetic resonance (NMR) spectroscopy methods for identification. The authors confirmed the presence of both acetates and found a higher concentration of glucose acetate in TBVM than in BVM.
There are other studies focused on GC analysis of balsamic vinegar dealing with the determination of the 13C/12C glycerol isotope ratio [24], the determination of 2,3-butanediol and 2-hydroxybutanone [17], the analysis of volatile aldehydes [34], the development of new analytical methods for the characterization, authentication, and quality evaluation of BVM [102], the determination of preservatives by the SBSE-TD-GC-MS method [33], and the determination of pesticides by SBSE extraction [30].
3.14. Other Compounds Identified by LC
Hillmann et al. [48] decided in their study to perform a qualitative and quantitative analysis of taste-active and taste-modulating substances in BVM and TBVM. They also focused on the changes in these compounds during the vinegar production process. They used the HPLC-MS/MS method to determine phenolic acids, phenolic acid esters, vanillin, and HMF. They quantified monosaccharides, alditols, organic acids, cations, and inorganic ions by ion chromatography. They determined the content of 15 amino acids by hydrophilic interaction chromatography (HILIC) coupled with MS/MS. A total of 59 compounds were analyzed and other techniques, such as NMR, were used. The authors concluded that the concentrations of some taste-active compounds in BVM and TBVM may be different. For example, BVM contains 2x more acetic acid, which is characterized by its acetic taste, compared to TBVM. TBVM, on the other hand, contains almost three times more sweet-tasting glycerol. According to the authors, during the maturing of vinegar, there is a decrease in acidity and, conversely, a slight increase in sweet taste.
Falcone and Giuidici [57] performed the first semiquantitative determination of molecular weight and molecular weight distribution for TBVM using gel permeation chromatography with a refractive index detector (RI) and UV/VIS detection. The tested samples were determined as a compositionally and structurally heterogeneous mixture of copolymers with a wide range of molecular weights from 2 to more than 2000 kDa. The authors propose the use of the molecular weight distribution parameter as a marker of the aging of balsamic vinegars.
Other LC papers for the study of balsamic vinegars deal with the distribution of molecular weight by gel permeation chromatography [58], determination of ochratoxin A [59], identification and quantification of β-carboline alkaloids in food [68], determination of 4-methylimidazole and 2-acetyl-4(5)-tetrahydroxybutylimidazole [56] and technological parameters that affect the maturation and maturation of BVM [103]. However, their more detailed description is beyond the scope of this text.
4. Conclusions
Chromatographic methods play an important role in the study of balsamic vinegars. Because of their highly complex matrix, LC and GC methods are an ideal choice for studying food or plant products, such as vinegars. Gas chromatography is used more to characterize the overall profile of volatile organic compounds, furfurals, and organic acids. Liquid chromatography is used for the analysis of furfurals, organic acids, sugars, phenolic compounds, and amino acids. Despite the high number of studies on balsamic vinegar, there are only a few works that combine both chromatographic methods, which could provide a better overview of the chemical composition of balsamic vinegars. Furthermore, the use of two-dimensional techniques could provide further useful information in the field of balsamic vinegar studies. Especially in liquid chromatography, the detection of individual compounds should be performed by more sensitive and selective MS and MS/MS techniques instead of using UV/Vis or DAD detection.
Author Contributions
Conceptualization, P.Č., investigation, M.K.; resources, M.K. and P.Č.; writing—original draft preparation, M.K.; writing—review and editing, P.Č.; supervision, P.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Czech Science Foundation, grant number 18-14893S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oster, M. Herbal Vinegar: Flavored Vinegars, Mustards, Chutneys, Preserves, Conserves, Salsas, Cosmetic Uses, Household Tips, 1st ed.; Storey Publishing: North Adams, MA, USA, 2018. [Google Scholar]
- Mattia, G. Balsamic vinegar of Modena: From product to market value: Competitive strategy of a typical Italian product. Br. Food J. 2004, 106, 722–745. [Google Scholar] [CrossRef]
- European Council Regulation (EC) 813/2000 of 17 April 2000 Supplementing the Annex to Commission Regulation (EC) No 1107/96 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid down in Article 17 of Regulation (EEC) No 2081/92. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000R0813 (accessed on 22 June 2022).
- Giudici, P.; Lemmetti, F.; Mazza, S. The Balsamic Family. In Balsamic Vinegars: Tradition, Technology, Trade, 1st ed.; Springer International Publishing: New York, NY, USA, 2015; pp. 33–59. [Google Scholar]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Giudici, P.; Gullo, M.; Solieri, L. Traditional Balsamic Vinegar. In Vinegars of the World, 1st ed.; Solieri, L., Giudici, P., Eds.; Springer Milan: Milano, Italy, 2009; pp. 157–177. [Google Scholar]
- Torija, M.J.; Mateo, E.; Vegas, C.A.; Jara, C.; González, A.; Poblet, M.; Reguant, C.; Guillamon, J.M.; Mas, A. Effect of wood type and thickness on acetification kinetics in traditional vinegar production. Int. J. Wine Res. 2009, 1, 155–160. [Google Scholar]
- Iburg, A. Lexikon Octů a Olejů: Původ, Chuť, Použití, Recepty, 1st ed.; Rebo: Dobřejovice, Czech Republic, 2008. [Google Scholar]
- European Commission Regulation (EC) 583/2009 of 3 July 2009 Entering a Name in the Register of Protected Designations of Origin and Protected Geographical Indications [Aceto Balsamico di Modena (PGI)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R0583 (accessed on 22 June 2022).
- Chinnici, F.; Durán Guerrero, E.; Sonni, F.; Natali, N.; Natera, R.; Riponi, C. Gas Chromatography−Mass Spectrometry (GC−MS) Characterization of Volatile Compounds in Quality Vinegars with Protected European Geographical Indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Natera, R.; Castro, R.; Hernández, M.J.; García-Barroso, C. Chemometric Studies of Vinegars from Different Raw Materials and Processes of Production. J. Agric. Food Chem. 2003, 51, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Lalou, S.; Hatzidimitriou, E.; Papadopoulou, M.; Kontogianni, V.G.; Tsiafoulis, C.G.; Gerothanassism, I.P.; Tsimidou, M.Z. Beyond traditional balsamic vinegar: Compositional and sensorial characteristics of industrial balsamic vinegars and regulatory requirements. J. Food Compos. Anal. 2015, 43, 175–184. [Google Scholar] [CrossRef]
- Masino, F.; Chinnici, F.; Bendini, A.; Montevecchi, G.; Antonelli, A. A study on relationships among chemical, physical, and qualitative assessment in traditional balsamic vinegar. Food Chem. 2008, 106, 90–95. [Google Scholar] [CrossRef]
- Chinnici, F.; Durán-Guerrero, E.; Riponi, C. Discrimination of some European vinegars with protected denomination of origin as a function of their amino acid and biogenic amine content. J. Sci. Food Agric. 2016, 96, 3762–3771. [Google Scholar] [CrossRef]
- Yun, J.-H.; Kim, Y.-J.; Koh, K.-H. Investigation into factors influencing antioxidant capacity of vinegars. Appl. Biol. Chem. 2016, 59, 495–509. [Google Scholar] [CrossRef]
- Chinnici, F.; Masino, F.; Antonelli, A. Determination of Furanic Compounds in Traditional Balsamic Vinegars by Ion-Exclusion Liquid Chromatography and Diode-Array Detection. J. Chromatogr. Sci. 2003, 41, 305–310. [Google Scholar] [CrossRef]
- Caligiani, A.; Silva, G.; Palla, G. Determination of 2,3-Butanediol and 2-Hydroxybutanone Stereoisomers in Batteries of Traditional Balsamic Vinegar. J. Agric. Food Chem. 2007, 55, 7810–7815. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Senra, L.; Fernandes, J.O.; Cunha, S.C. Gas Chromatography–Mass Spectrometry Analysis of 4-Methylimidazole in Balsamic Vinegars and Processed Sauces. Food Anal. Methods 2014, 7, 1519–1525. [Google Scholar] [CrossRef]
- Bononi, M.; Tateo, F. Determination of furan by headspace solid-phase microextraction–gas chromatography–mass spectrometry in balsamic vinegars of Modena (Italy). J. Food Compos. Anal. 2009, 22, 79–82. [Google Scholar] [CrossRef]
- Giordano, L.; Calabrese, R.; Davoli, E.; Rotilio, D. Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry using isotope dilution. J. Chromatogr. A 2003, 1017, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Manzini, S.; Durante, C.; Baschieri, C.; Cocchi, M.; Sighinolfi, S.; Totaro, S.; Marchetti, A. Optimization of a Dynamic Headspace—Thermal Desorption—Gas Chromatography/Mass Spectrometry procedure for the determination of furfurals in vinegars. Talanta 2011, 85, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.M.S.M.; Lopes, J.F. Simple gas chromatographic method for furfural analysis. J. Chromatogr. A 2009, 1216, 2762–2767. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, G. Formation of glucose and fructose acetates during maturation and ageing of balsamic vinegars. Food Chem. 2009, 112, 51–56. [Google Scholar] [CrossRef]
- Sighinolfi, S.; Baneschi, I.; Manzini, S.; Lorenzo, T.; Dallai, L.; Marchetti, A. Determination of glycerol carbon stable isotope ratio for the characterization of Italian balsamic vinegars. J. Food Compos. Anal. 2018, 69, 33–38. [Google Scholar] [CrossRef]
- Erbe, T.; Brückner, H. Chiral amino acid analysis of vinegars using gas chromatography—Selected ion monitoring mass spectrometry. Int. J. Food Res. Technol. 1998, 207, 400–409. [Google Scholar] [CrossRef]
- Corsini, L.; Castro, R.; Barroso, C.G.; Durán-Guerrero, E. Characterization by gas chromatography-olfactometry of the most odour-active compounds in Italian balsamic vinegars with geographical indication. Food Chem. 2019, 272, 702–708. [Google Scholar] [CrossRef]
- Ugliano, M.; Squillante, E.; Genovese, A.; Moio, L. Investigation on aroma compounds of Modena balsamic vinegars. In Flavour Research at the Dawn of the Twenty-First Century, Proceedings of the 10th WeurmanFlavour Research Symposium, Beaune, France, 25–28 June 2002; Editions Tec & Doc: Paris, France, 2003; pp. 733–736. [Google Scholar]
- Cocchi, M.; Lambertini, P.; Manzini, D.; Marchetti, A.; Ulrici, A. Determination of Carboxylic Acids in Vinegars and in Aceto Balsamico Tradizionale di Modena by HPLC and GC Methods. J. Agric. Food Chem. 2002, 50, 5255–5526. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Durante, C.; Grandi, M.; Lambertini, P.; Manzini, D.; Marchetti, A. Simultaneous determination of sugars and organic acids in aged vinegars and chemometric data analysis. Talanta 2006, 69, 1166–1175. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro Mejías, R.; Marín, R.N.; Barroso, C.G. Optimization of stir bar sorptive extraction applied to the determination of pesticides in vinegars. J. Chromatogr. A 2007, 1165, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Plessi, M.; Bertelli, D.; Miglietta, F. Extraction and identification by GC-MS of phenolic acids in traditional balsamic vinegar from Modena. J. Food Compos. Anal. 2006, 19, 49–54. [Google Scholar] [CrossRef]
- Sinanoglou, V.; Zoumpoulakis, P.; Fotakis, C.; Kalogeropoulos, N.; Sakellari, A.; Karavoltsos, S.; Strati, I. On the Characterization and Correlation of Compositional, Antioxidant and Colour Profile of Common and Balsamic Vinegars. Antioxidants 2018, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Sasamoto, K.; Takino, M.; Yamashita, S. Simultaneous determination of preservatives in beverages, vinegar, aqueous sauces, and quasi-drug drinks by stir-bar sorptive extraction (SBSE) and thermal desorption GC–MS. Anal. Bioanal. Chem. 2002, 373, 56–63. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Chinnici, F.; Natali, N.; Riponi, C. Evaluation of volatile aldehydes as discriminating parameters in quality vinegars with protected dikar geographical indication. J. Sci. Food Agric. 2015, 95, 2395–2403. [Google Scholar] [CrossRef]
- Callejón, R.M.; Torija, M.J.; Mas, A.; Morales, M.L.; Troncoso, A.M. Changes of volatile compounds in wine vinegars during their elaboration in barrels made from different woods. Food Chem. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Marín, R.N.; Mejías, R.C.; Barroso, C.G. Stir bar sorptive extraction of volatile compounds in vinegar: Validation study and comparison with solid phase microextraction. J. Chromatogr. A 2007, 1167, 18–26. [Google Scholar] [CrossRef]
- Cocchi, M.; Durante, C.; Grandi, M.; Manzini, D.; Marchetii, A. Three-way principal component analysis of the volatile fraction by HS-SPME/GC of aceto balsamico tradizionale of modena. Talanta 2008, 74, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; De Carvalho-Silva, S.; Trovatti Uetanabaro, A.; Villas-Boas, S. Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry. Metabolites 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Marrufo-Curtido, A.; Cejudo-Bastante, M.J.; Durán-Guerrero, E.; Castro-Mejías, R.; Natera, R.; Chinnici, F.; García-Barroso, C. Characterization and differentiation of high quality vinegars by stir bar sorptive extraction coupled to gas chromatography-mass spectrometry (SBSE–GC–MS). LWT Food Sci. Technol. 2012, 47, 332–341. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, L.; Palla, G. HS-SPME/GC–MS and chemometrics for the classification of Balsamic Vinegars of Modena of different maturation and ageing. Food Chem. 2011, 124, 1678–1683. [Google Scholar] [CrossRef]
- Del Signore, A. Chemometric analysis and volatile compounds of traditional balsamic vinegars from Modena. J. Food Eng. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Jeong, E.-J.; Jeon, S.-Y.; Baek, J.-H.; Cha, Y.-J. Volatile Flavor Compounds in Commercial Vinegar Beverages Derived from Fruits. J. Life Sci. 2011, 21, 292–299. [Google Scholar] [CrossRef]
- Pinu, F.; Villas-Boas, S.G. Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites 2017, 7, 37. [Google Scholar] [CrossRef]
- Jang, Y.K.; Lee, M.Y.; Kim, H.Y.; Lee, S.; Yeo, S.H.; Baek, S.Y.; Lee, C.H. Comparison of traditional and commercial vinegars based on metabolite profiling and antioxidant activity. J. Microbiol. Biotechnol. 2015, 25, 217–226. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Díez, I.; Sáenz-González, C.; González-Sáiz, J.M. Vinegar classification based on feature extraction and selection from headspace solid-phase microextraction/gas chromatography volatile analyses: A feasibility study. Anal. Chim. Acta 2008, 608, 38–47. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Callejón, R.M.; Morales, M.L.; García-Parrilla, M.C. A survey of biogenic amines in vinegars. Food Chem. 2013, 141, 2713–2719. [Google Scholar] [CrossRef]
- Hillmann, H.; Mattes, J.; Brockhoff, A.; Dunkel, A.; Meyerhof, W.; Hofmann, T. Sensomics Analysis of Taste Compounds in Balsamic Vinegar and Discovery of 5-Acetoxymethyl-2-furaldehyde as a Novel Sweet Taste Modulator. J. Agric. Food Chem. 2012, 60, 9974–9990. [Google Scholar] [CrossRef] [PubMed]
- Papetti, A.; Mascherpa, D.; Marrubini, G.; Gazzani, G. Effect of In Vitro Digestion on Free α-Dicarbonyl Compounds in Balsamic Vinegars. J. Food Sci. 2013, 78, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Amoroso, A.; Rossi, D.; Mascherpa, D.; Maga, G. Identification and quantification of α-dicarbonyl compounds in balsamic and traditional balsamic vinegars and their cytotoxicity against human cells. J. Food Compos. Anal. 2013, 31, 67–74. [Google Scholar] [CrossRef]
- Masino, F.; Chinnici, F.; Franchini, G.; Ulrici, A.; Antonelli, A. A study of the relationships among acidity, sugar and furanic compound concentrations in set of casks for Aceto Balsamico Tradizionale of Reggio Emilia by multivariate techniques. Food Chem. 2005, 92, 673–679. [Google Scholar] [CrossRef]
- Cocchi, M.; Durante, C.; Lambertini, P.; Manzini, S.; Marchetii, A.; Sighinolfi, S.; Totaro, S. Evolution of 5-(hydroxymethyl)furfural and furfural in the production chain of the aged vinegar Aceto Balsamico Tradizionale di Modena. Food Chem. 2011, 124, 822–832. [Google Scholar] [CrossRef]
- Gaspar, E.M.S.M.; Lucena, A.F.F. Improved HPLC methodology for food control—furfurals and patulin as markers of quality. Food Chem. 2009, 114, 1576–1582. [Google Scholar] [CrossRef]
- Theobald, A.; Müller, A.; Anklam, E. Determination of 5-Hydroxymethylfurfural in Vinegar Samples by HPLC. J. Agric. Food Chem. 1998, 46, 1850–1854. [Google Scholar] [CrossRef]
- Lin, S.-M.; Wu, J.-Y.; Su, C.; Ferng, S.; Lo, C.-Y.; Chiou, R.Y.-Y. Identification and Mode of Action of 5-Hydroxymethyl-2-furfural (5-HMF) and 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid (MTCA) as Potent Xanthine Oxidase Inhibitors in Vinegars. J. Agric. Food Chem. 2012, 60, 9856–9862. [Google Scholar] [CrossRef]
- Kim, T.R.; Kim, S.U.; Shin, Y.; Kim, J.Y.; Lee, S.M.; Kim, J.H. Determination of 4-Methylimidazole and 2-Acetyl-4(5)-tetrahydroxybutylimidazole in Caramel Color and Processed Foods by LC-MS/MS. Prev. Nutr. Food Sci. 2013, 18, 263–268. [Google Scholar] [CrossRef][Green Version]
- Falcone, P.M.; Giudici, P. Molecular Size and Molecular Size Distribution Affecting Traditional Balsamic Vinegar Aging. J. Agric. Food Chem. 2008, 56, 7057–7066. [Google Scholar] [CrossRef]
- Falcone, P.M.; Boselli, E.; Frega, N.G. Structure-composition relationships of the Traditional Balsamic Vinegar close to jamming transition. Food Res. Int. 2011, 44, 1613–1619. [Google Scholar] [CrossRef]
- Markaki, P.; Delpont-Binet, C.; Grosso, F.; Dragacci, S. Determination of Ochratoxin A in Red Wine and Vinegar by Immunoaffinity High-Pressure Liquid Chromatography. J. Food Prot. 2001, 64, 533–537. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Hao, W.; Zhu, H.; Liang, N.; Liu, J.; Zhang, C.; Ma, K.Y.; He, W.-S.; Yang, Y.; et al. Vinegars but not acetic acid are effective in reducing plasma cholesterol in hamsters fed a high-cholesterol diet. Food Funct. 2020, 11, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Gonzalez, A.G.; Troncoso, A.M. Ion-exclusion chromatographic determination of organic acids in vinegars. J. Chromatogr. A 1998, 822, 45–51. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Devecioglu, D.; Kayacan, S.; Toydemir, G.; Karbancioglu-Guler, F.; Capanoglu, E. Investigating the antioxidant and antimicrobial activities of different vinegars. Eur. Food Res. Technol. 2017, 243, 2083–2094. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Tesfaye, W.; Soria-Díaz, M.E.; Torija, M.J.; Mateo, E.; Garcia-Parrilla, M.C.; Troncoso, A.M. Effect of wood on the phenolic profile and sensory properties of wine vinegars during ageing. J. Food Compos. Anal. 2010, 23, 175–184. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction—Ultrahigh-performance liquid chromatography—Quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef]
- Antonelli, A.; Chinnici, F.; Masino, F. Heat-induced chemical modification of grape must as related to its concentration during the production of traditional balsamic vinegar: A preliminary approach. Food Chem. 2004, 88, 63–68. [Google Scholar] [CrossRef]
- Cocchi, M.; Ferrari, G.; Manzini, D.; Marchetti, A.; Sighinolfi, S. Study of the monosaccharides and furfurals evolution during the preparation of cooked grape musts for Aceto Balsamico Tradizionale production. J. Food Eng. 2007, 79, 1438–1444. [Google Scholar] [CrossRef]
- Diem, S.; Herderich, M. Reaction of tryptophan with carbohydrates: Identification and quantitative determination of novel β-carboline alkaloids in food. J. Agric. Food Chem. 2001, 49, 2486–2492. [Google Scholar] [CrossRef]
- James, A.T.; Martin, A.J.P. Gas-liquid partition chromatography: The separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem. J. 1952, 50, 679. [Google Scholar] [CrossRef] [PubMed]
- Parys, W.; Dolowy, M.; Pyka-Pajak, A. Current Strategies for Studying the Natural and Synthetic Bioactive Compounds in Food by Chromatographic Separation Techniques. Processes 2021, 9, 1100. [Google Scholar] [CrossRef]
- Kašpar, M. Analysis of Volatile Compounds in Balsamic Vinegars. Ph.D. Thesis, University Pardubice, Pardubice, Czech Republic, 2020. [Google Scholar]
- Touchstone, J.C. History of chromatography. J. Liq. Chromatogr. 1993, 16, 1647–1665. [Google Scholar] [CrossRef]
- Dong, M.W. Modern HPLC for Practicing Scientists; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Cacciola, F.; Rigano, F.; Dugo, P.; Mondello, L. Comprehensive two-dimensional liquid chromatography as a powerful tool for the analysis of food and food products. Trends Anal. Chem. 2020, 127, 115894. [Google Scholar] [CrossRef]
- Arigo, A.; Česla, P.; Šilarová, P.; Calabro, M.L.; Česlová, L. Development of extraction method for characterization of free and bonded polyphenols in barley (Hordeum vulgare L.) growth in Czech Republic using liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 245, 829–837. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R.; Benevelli, F.; Spraul, M.; Humpfer, E.; Stocchero, M. NMR and Chemometric methods: A powerful combination for characterization of Balsamic and Traditional Balsamic Vinegar of Modena. Anal. Chim. Acta 2008, 61, 31–40. [Google Scholar] [CrossRef]
- Bertelli, D.; Maietti, A.; Papotti, G.; Tedeschi, P.; Bonetti, G.; Graziosi, R.; Brandolini, V.; Plessi, M. Antioxidant Activity, Phenolic Compounds, and NMR Characterization of Balsamic and Traditional Balsamic Vinegar of Modena. Food Anal. Methods 2015, 8, 371–379. [Google Scholar] [CrossRef]
- Camin, F.; Simoni, M.; Hermann, A.; Thomas, F.; Perini, M. Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study. Molecules 2020, 25, 2932. [Google Scholar] [CrossRef]
- Caligiani, A.; Acquotti, D.; Palla, G.; Bocchi, V. Identification and quantification of the main organic components of vinegars by high resolution 1H NMR spectroscopy. Anal. Chim. Acta 2007, 585, 110–119. [Google Scholar] [CrossRef]
- Consonni, R.; Gatti, A. 1 H NMR Studies on Italian Balsamic and Traditional Balsamic Vinegars. J. Agric. Food Chem. 2004, 52, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R. NMR relaxation data for quality characterization of Balsamic vinegar of Modena. Talanta 2007, 73, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, R.; Bertelli, D.; Marchetti, L.; Papotti, G.; Rossi, M.C.; Plessi, M. Novel 2D-NMR Approach for the Classification of Balsamic Vinegars of Modena. J. Agric. Food Chem. 2017, 65, 5421–5426. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Graziosi, R.; Maietti, A.; Tedeschi, P.; Marchetti, A.; Plessi, M. Traditional balsamic vinegar and balsamic vinegar of Modena analyzed by nuclear magnetic resonance spectroscopy coupled with multivariate data analysis. LWT Food Sci. Technol. 2015, 60, 1017–1024. [Google Scholar] [CrossRef]
- De Vero, L.; Gala, E.; Gullo, M.; Solieri, L.; Landi, S.; Giudici, P. Application of denaturing gradient gel electrophoresis (DGGE) analysis to evaluate acetic acid bacteria in traditional balsamic vinegar. Food Microbiol. 2006, 23, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; De Vero, L.; Giudici, P. Succession of Selected Strains of Acetobacter pasteurianus and Other Acetic Acid Bacteria in Traditional Balsamic Vinegar. Appl. Environ. Microbiol. 2009, 75, 2585–2589. [Google Scholar] [CrossRef]
- Cetó, X.; Pérez, S. Voltammetric electronic tongue for vinegar fingerprinting. Talanta 2020, 219, 121253. [Google Scholar] [CrossRef]
- Kotani, A.; Miyaguchi, Y.; Harada, D.; Kusu, F. A Disposable Voltammetric Cell for Determining the Titratable Acidity in Vinegar. Anal. Sci. 2003, 19, 1473–1476. [Google Scholar] [CrossRef]
- Lo Coco, F.; Novelli, V.; Ceccon, L.; Monotti, P.; Ciraolo, L. Determination of Zinc (II), Cadmium (II), Lead (II) and Copper (II) in Common and Balsamic Vinegar by Stripping Chronopotentiometry, Proceedings of the Euroconference on University and Enterprise, “A Partnership for Training, Research, Employment and Social Development”, Rome, Italy, 26–28 September 2002; Facoltà di Economia, Università la Sapienza: Rome, Italy, 2002; pp. 643–653. [Google Scholar]
- Urbinati, E.; Di Nunzio, M.; Picone, G.; Chiarello, E.; Bordoni, A.; Capozzi, F. The effect of balsamic vinegar dressing on protein and carbohydrate digestibility is dependent on the food matrix. Foods 2021, 10, 411. [Google Scholar] [CrossRef]
- González-Marco, A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Concentration of volatile compounds in Chardonnay wine fermented in stainless steel tanks and oak barrels. Food Chem. 2008, 108, 213–219. [Google Scholar] [CrossRef]
- Kurtbay, H.; Kaynak, I.; Bozkurt, S.; Merdivan, M. Densitometric HPTLC analysis of the 5-hydroxymethylfurfural content of Turkish fruit wines and vinegars. J. Planar Chromatogr. Mod. TLC 2009, 22, 363–366. [Google Scholar] [CrossRef]
- Masino, F.; Antonelli, A.; Chinnici, F. Furanic compound profiles in set of casks for the Traditional Balsamic Vinegar of Reggio Emilia production. Ind. Bevande 2004, 33, 19–23. [Google Scholar]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab. J. Gastroenterol. 2018, 19, 56–64. [Google Scholar] [CrossRef]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Morales, M.; Wendu Tesfaye, L.; García-Parrilla, M.C.; Casas, J.A.; Troncoso, A.M. Evolution of the Aroma Profile of Sherry Wine Vinegars during an Experimental Aging in Wood. J. Agric. Food Chem. 2002, 50, 3173–3178. [Google Scholar] [CrossRef]
- More, S.S.; Raza, A.; Vince, R. The Butter Flavorant, Diacetyl, Forms a Covalent Adduct with 2-Deoxyguanosine, Uncoils DNA, and Leads to Cell Death. J. Agric. Food Chem. 2012, 60, 3311–3317. [Google Scholar] [CrossRef]
- More, S.S.; Vartak, A.P.; Vince, R. The Butter Flavorant, Diacetyl, Exacerbates β-Amyloid Cytotoxicity. Chem. Res. Toxicol. 2012, 25, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M. Development of New Analytical Methods for the Characterization, Authentication and Quality Evaluation of Balsamic Vinegar of Modena. Ph.D. Thesis, Università degli Studi di Parma, Parma, Italy, 2009. [Google Scholar]
- Del Signore, A. Technological parameters affecting aging and refining of balsamic vinegar from Modena in wood receptacles related to the new ec regulation n. 583/2009. Ital. J. Food Sci. 2011, 23, 228. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).