Abstract
Food is a necessity in people’s lives. Equally importantly, alcoholic beverages are also highly demanded globally due to the indispensable role they play in cultural, social, and ritual events. However, the production of food and alcoholic beverages suffers from a variety of contaminants, such as toxins, pesticides, antibiotic residues, and heavy metals, which are seriously harmful to human beings. These urgent threats have raised the awareness of the need to improve product quality and safety via developing effective, rapid, and economical monitoring and detecting methods. Fortunately, due to their numerous advantages, including high sensitivity, short response time, low cost, and easy portability, electrochemistry sensors have made huge contributions to ensuring the quality of food and alcoholic beverages. The purpose of this review is to introduce applications of electrochemical sensors to foods and alcoholic beverages, and to highlight the important role of carbon-based materials (i.e., carbon dots, carbon nanotubes, and graphene) as electrochemical sensors in detecting various contaminants. In addition, the preparation methods of these carbon-based electrochemical sensors and corresponding detection mechanisms are discussed in detail. It is hoped that this review can inspire more innovative detection technologies for ensuring the safety of food and alcoholic beverages.
1. Introduction
Food is a necessity for human beings and the ongoing improvement in living standards leads to increasingly higher requirements for food production. Consequently, food safety is regarded as a major issue involved in social progress, economic development, and public health [,]. Currently, food safety is mainly challenged by the excessive presence of illegal additives, pesticide residues, organic contamination, biological toxins, veterinary drugs, and heavy metals added during the preparation, post-treatment, and storage processes. Similarly, alcoholic beverages are a globally popular drink that contain different constituents, including ethanol, water, peptides, organic acids, and sugars. Possible contaminants, such as carcinogens, methanol, mycotoxin, and amine, pose great threats to human health, thus leading to an increase in the public awareness about the safety of food and alcoholic beverages. Therefore, there is an urgent need to develop advanced analytical techniques characterized by high sensitivity, short response time, low cost, and easy portability [,,,].
In recent decades, numerous analytical methods and equipment have been exploited to meet the increasing need for qualitative or/and quantitative detection of various chemical contaminants and hazardous substances existing in foods and alcoholic beverages. The classical analytical methods can be mainly categorized as chromatography and spectroscopy disciplines. For instance, the commonly used chromatographic techniques include hydrodynamic chromatography (HDC), high-performance liquid chromatography (HPLC), size exclusion chromatography (SEC), gas chromatography (GC), HPLC-mass spectrometry-mass spectrometry (HPLC-MSMS), matrix-assisted laser desorption/ionization (MALDI-TOF), and gas chromatographic-MSMS (GC-MSMS) [,,,]. Although these chromatographic methods have advantages in terms of high separation efficiency, accurate quantitation ability, and excellent detection sensitivity, the extremely high cost of equipment and maintenance, and the complicated operation process involved, have seriously limited their ability to be applied to food and alcoholic beverages in a portable and fast way. Compared with chromatographic techniques, spectroscopic methods such as near-infrared (NIR), ultraviolet-visible (UV-vis), Raman, and fluorescence spectroscopies are more efficiently and conveniently operated and analyzed, even without the destruction of analytes. However, the disadvantages are also evident, such as the requirement of multistage pretreatment and the lack of wide applicability. Because of these shortcomings, novel advanced analysis methods are expected to realize the rapid and efficient analysis of contaminants and residues [,].
Benefiting from numerous advantages, such as rapid response, high selectivity, flexibility, and portability, electrochemical sensors have become powerful candidates via transducing the specific interaction between analytes and sensors into recognizable electrical signals. Moreover, electrochemical sensors are cheap, simple, and applicable for various types of analytes. Generally speaking, electrochemical sensors are composed of sensitive components, conversion components, electronic circuits, and structural accessories []. As one of the important components, the active electrode, which is typically composed of nanomaterials with multiple properties, determines the sensitivity and functionality of electrochemical sensors [,,]. Among various types of electrodes, the advent of carbon nanomaterials-based electrodes involving carbon dots (CDs), carbon nanotubes (CNTs), and graphene have created new possibilities for fast, reliable, and economical detection of contaminants and toxins in foods and alcoholic beverages [,,]. To ensure superior performances, additional ions, molecules, or polymers are usually added for functionalizing the raw carbon-based materials with enhanced electrocatalytic activity, electro-chemiluminescence, photoelectricity, etc. [].
The currently reported literature has demonstrated the superiority of carbon-based electrochemical sensors in the application of food and alcohol detection [,], and some recently published reviews have also summarized the syntheses of carbon nanomaterial-based electrodes and their application performances [,,,]. However, very few reviews have systematically illustrated their design principle and working mechanism []. A deep understanding of the relationship between the structure of carbon-based materials and their detection performances, and their application scopes, is still lacking. Therefore, a timely overview of the above issues is highly desirable, and can offer useful guidance for developing new types of highly efficient electrochemical sensors. In this review, we firstly introduce the structures and characteristics of carbon material-based electrochemical sensors. Then, their working mechanisms are also discussed by presenting some prototype examples. Finally, the perspectives and challenges in this field are provided based on our personal understandings.
2. Electrochemical Techniques
Electrochemical sensors have become a widespread analysis technique with the merits of low cost, high accuracy, fast response, and good reliability, and have drawn substantial attention for the qualitative or/and quantitative analysis of foods and alcoholic beverages [,]. The design of high-quality electrochemical sensors needs a full understanding of their basic detection principles. Figure 1a shows that samples without pretreatment can be first added into the electrochemical cell. Then, upon applying electricity, the electrochemical sensor converts the physical and chemical response into recognizable electrochemical response signals. Through analyzing the correlation between the detected substances and corresponding electronic signals, the contaminants in foods and alcoholic beverages can be quickly identified. As shown in Figure 1b, a typical three-electrode electrochemical system includes a working electrode (WE), a reference electrode (RE) (e.g., Ag/AgCl), and a counter electrode (CE) (e.g., carbon rod, platinum). Recently, functional carbon-based nanomaterials such as CDs, CNTs, graphene oxide (GO), and porous carbon were selected as candidates for the working electrode (WE) due to their excellent conductivity and durability [,]. Of note, the selectivity, linearity, sensitivity, stability, detection limit, dynamic range, and response time of the resultant electrochemical sensors can be dramatically improved by further regulating the composition and the structure of adopted carbon nanomaterials [,,]. Additionally, versatile electrochemical analysis methods are also required to be established for efficiently extracting the unique information from different analytes in food and alcoholic beverages. Specifically, the current-type (generating an amperometric current), potential-type (generating a potentiometric potential), conductivity-type (measurably altering the conductive properties of a medium between electrodes), and impedimetric-type (measuring impedance through an electrochemical impedance spectroscopy method) are the four types of golden protocols [,,,].
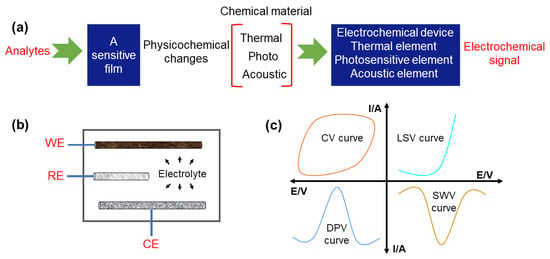
Figure 1.
Principle and test methods of electrochemical sensors. (a) The detection principle of an electrochemical sensor. (b) The basic configuration of an electrochemical sensor. (c) The diagrams of cyclic voltammetry (CV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV) curves.
In practice, cyclic voltammetry (CV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV) (Figure 1c) are most commonly selected according to the properties of the detected substance [,,,]. So, it is necessary to understand the working principles of those electrochemical analysis methods, which is helpful for more rationally and accurately analyzing the contaminants in food and alcohol beverages. First, CV is a commonly used electrochemical analysis method operated by scanning the response current one or more times in a triangular waveform under varyingly applied voltage. The reversibility of electrode reaction, intermediate, phase boundary adsorption, phase transformation, and the mechanism of coupled reactions can be acquired and judged according to the presented shape of CV curves []. Second, LSV is another electrochemical analysis method in which a linear change in voltage is applied on the electrode; that is, the potential changes linearly with the applied voltage recording the current on the working electrode at the same time. The peak current measured in the LSV curve has a linear relationship with the concentration of the measured analytes, which is the basis of quantitative analysis []. Third, DPV is an electrochemical method that involves the superposition of a linearly increasing voltage with a rectangular pulse of constant amplitude. As a result of the rapid development of electronic circuits, pulse voltammetry has been widely used in the field of electrochemical analysis. For instance, the quantitative determination of various analytes and the mechanism of complex electrode reactions can be decoded due to pulse voltammetry’s high sensitivity and low detection limit. Moreover, by combining differential pulse voltammetry with other methods such as stripping voltammetry, the detection sensitivity can be greatly improved []. Finally, SWV is an effective electrochemical technique that can be utilized for electrode mechanism understanding and electro-kinetics measurement. It is worth mentioning that SWV can efficiently suppress the background current, thereby obviously increasing the signal-to-noise ratio and reducing the detection limit [,].
3. Carbon Nanomaterial-Based Electrochemical Sensors
Carbon is one of the most abundant elements in nature, and carbon-based materials (Figure 2) have been continuously studied and utilized in different fields. Generally speaking, according to the dimension of the carbon-based materials, they can be divided into zero-dimensional materials (their dimensions in all directions are in the order of nanometers, e.g., CDs), one-dimensional materials (their dimensions in two directions are in the order of nanometers, e.g., CNTs and carbon nanofibers), and two-dimensional materials (their dimension in only one direction is in the order of nanometers, e.g., graphene and GO) [,,,,,,,]. As early as the 18th century, it was known that graphite was formed by sp2 and diamond was formed by sp3 hybridized carbon atoms. Furthermore, the fullerenes, such as C60 and C70, and CNTs, were discovered in 1985 and 1991, respectively [,]. These studies not only expanded the scope of the carbon material family, but also marked the start of a new era for the research of carbon nanomaterials. In particular, the discovery of graphene in 2004 triggered a new wave of research []. Due to their excellent electrical conductivity, wide potential window, high electrocatalytic activity, and chemical modifiability, carbon-based materials have been accepted as one of the best candidates for the construction of electrochemical sensors []. In the following section, we summarize the exploration and application of typical carbon-based materials, including CDs, CNTs, and graphene, as electrochemical sensors in food and beverage safety due to their low cost, good conductivity, and facile fabrication process.
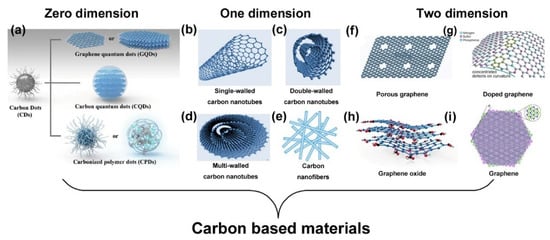
Figure 2.
Classification of carbon-based sensors according to the dimension of materials. (a) Reproduced with permission from []. Copyright American Chemical Society, 2020. (b–d) Reproduced with permission from []. Copyright Springer Nature, 2019. (e) Reproduced with permission from []. Copyright MDPI, 2019. (f) Reproduced with permission from []. Copyright Elsevier, 2012. (g) Reproduced with permission from []. Copyright Wiley, 2016. (h) Reproduced with permission from []. Copyright Elsevier, 20. (i) Reproduced with permission from []. Copyright Springer Nature, 2020.
3.1. CDs
In 2004, Xu et al. discovered that CDs can present fluorescence during the process of electrophoresis []. CDs are defined as zero-dimensional nanoparticles having a size of less than 10 nm that are mainly composed of carbon elements. CDs have been widely used in electroluminescence, medical imaging, environmental monitoring, chemical analysis, photocatalysis, and energy conversion due to their colorful optical properties, good water solubility, low toxicity, environmental friendliness, abundant raw source, low cost, and good biocompatibility [,]. Regarding CDs’ preparation, a variety of methods, including arc discharge, laser ablation, electrochemical oxidation, acidic oxidation, microwave, ultrasonic, calcination, hydrothermal, template synthesis, and hydrosol condensation polymerization, have been reported [,,]. Interestingly, CDs can be functionalized easily with other materials, such as organic, polymeric, and inorganic species, via reacting with surface carboxylic acid moieties. In the following section, we illustrate the applications of CD-based materials in the development of electrochemical sensors for H2O2, dopamine (DA), triclosan, glucose, acetaminophen, Cu2+ ions, uric acid, etc.
Acting as a neuromodulator of ionotropic synapses, DA sets a threshold for the striatal activity involved in many diseases and drug addiction. In addition, DA is available as an intravenous medication acting on the sympathetic nervous system. Hence, the determination of DA in vivo/vitro becomes increasingly important in practice [,]. As shown in Figure 3a, Zhu et al. [] prepared a new type of N-doped CDs by a one-step microwave irradiation method. The N-doped CDs obtained by this method had a highly sensitive electrochemical response to DA, with a linear range of 0.05~8 μM and a detection limit of 1.2 nM. Moreover, in contrast to traditional detection methods requiring complex pretreatment and producing a large quantity of organic waste, the developed electrochemical sensing detection can be performed by directly adding DA to the detection system for qualitative and quantitative analysis. Huang et al. [] designed a new type of DA sensor (Figure 3b) based on a Au@CDs-CS/glassy carbon electrode (GCE), which has high sensitivity and excellent performance stability and can suppress the background interference currents from ascorbic acid (AA) and uric acid (UA). Under the optimum experimental conditions, the linear range of 0.01~100.0 μM and the detection limit of 0.1 nM (S/N = 3) were obtained. Dai and co-workers [] reported that CDs and chitosan were combined to construct a composite electrode. It was found that the signal was significantly higher than that of the bare electrode. Moreover, the composite electrode has higher sensitivity for the detection of triclosan, and also shows good results for the detection of actual samples such as toothpaste and gargle daily water, with the linear range of 10~1.0 mM and the detection limit of 0.92 nM (Figure 3c). As shown in Figure 3d, Sheng et al. [] used CDs-Chitosan (CS)/hemoglobin composite membrane for electrochemical detection of H2O2, which showed good sensitivity and robust usability. The linear current response for H2O2 was from 1 to 118 µM with a detection limit of 0.27 μM at the signal-to-noise ratio of 3, and the apparent Michaelis–Menten constant was 0.067 mM. Their work showed that loading metal nanoparticles can further significantly improve the sensitivity and selectivity of the electrochemical sensors, presenting a superior detection performance to the pure approach. Based on the easy modification of CDs, the rich carboxyl or amino functional groups on their surface are conducive to the grafting of small organic molecules for the detection of special chemicals. For example, Zhang et al. [] selected nitrogen-doped carbon dots (N-CDs) containing amino groups as the raw electrode due to their good conductivity and redox performance. In addition, a Ferrocene (Fc) and β-cyclodextrin (β-CD) host–guest complex was introduced to improve the solubility, electrical stability, and bioavailability. Accordingly, based on the CV and DPV techniques in phosphate buffer solution, this ternary detection system, Fc CD/N-CDs, was found to be a suitable choice for the detection of uric acid via the establishment of a new method. Shao et al. [] used CDs to interact with N-(2-aminoethyl)-N,N′,N′-tris(pyridine-2-yl-methyl)ethane-1,2-diamine (TPEA) for the detection of Cu2+. Benefiting from the good conductivity of CDs and the strong complexing ability of TPEA with Cu2+, the detection of Cu2+ content in the mouse brain was as good as the result of inductively coupled plasma-atomic emission spectrometry (ICP-AES), indicating that this method has good feasibility and application prospects (Figure 3e). Li and coworkers [] successfully synthesized the CDs/octahedral Cu2O nanocomposites, which exhibited good linear response, low detection limit, high selectivity, and wide detection range toward the electrocatalytic oxidation of glucose and the electrocatalytic reduction of H2O2. Yang et al. [] prepared an ultra-sensitive sensor of Au NP/CD nanocomposites protected by Fc derivatives and graphene, which can be used for simultaneous detection of vitamin C, DA, UA, and paracetamol.
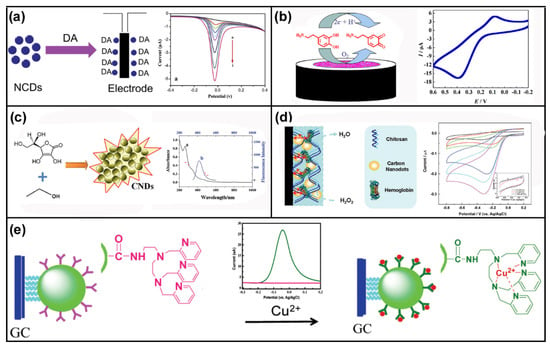
Figure 3.
CD-based electrochemical sensors for food and alcoholic beverage safety. (a) Schematic illustration of N-doped CD synthesis and DA detection. Reprinted with permission from []. Copyright Elsevier, 2015. (b) Schematic illustration of the strategy for DA detection. Reprinted with permission from []. Copyright the Royal Society of Chemistry, 2013. (c) Hydrothermal approach to CNDs and its TEM image, UV-vis absorption, and PL spectra. Reprinted with permission from []. Copyright Elsevier, 2012. (d) Schematic illustration of Hb-CNDs-chitosan system and CVs of Hb-CNDs-chitosan/GC electrode in 5 pH 7.4 PBS containing H2O2. Reprinted with permission from []. Copyright Elsevier, 2014. (e) A schematic illustration of the electrochemical sensor for Cu2+ detection based on CDs-TPEA. Reprinted with permission from [], Copyright American Chemical Society, 2012.
As summarized in Table 1, CDs have been widely used as electrode materials for the detection of chemical substances in bulk food, but their application to alcoholic beverages still needs to be further studied. Modifications such as doping nitrogen, grafting organic molecules, and loading metal nanoparticles can greatly improve the sensitivity, selectivity, durability, and repeatability of CD-based electrochemical sensors. However, the resultant complex electrode systems greatly hinder the exploration of underlying detection mechanisms. Therefore, in the future, more attention should focus on the development of electrode systems with an accurate structure and single components.

Table 1.
Typical applications of electrochemical sensors based on CD materials associated with electrochemical techniques for the detection of different target analytes.
3.2. CNTs
CNTs, also known as bucky tubes, are one-dimensional tubular nanomaterials with special structures. CNTs are mainly coaxial round tubes with a single to dozens of layers composed of sp2 hybridized carbon atoms [,]. In theory, CNTs can be regarded as hollow tubes rolled by graphene sheets. According to the number of rolled layers of the graphene sheets, CNTs can be subdivided into single-walled carbon nanotubes (SWCNT) formed by a hexagonal grid structure, and multi-walled carbon nanotubes (MWCNT) assembled from several to dozens of concentric cylinders with regular layer spacing [,]. Because their unique structure is completely different from that of bulk carbon materials, CNTs can exhibit excellent properties, such as unique electrical, special magnetic, and strong light absorption properties. Because of their excellent electrical conductivity, large specific surface area, good biocompatibility, easily functionalization, and abundant active sites, CNTs are also an advantageous option in the design of electrochemical sensors [,,]. In the following section, we illustrate the applications of CNTs-based materials in the development of electrochemical sensors for food and beverage safety, as summarized in Table 2.
Wine is a complex drink mixed with hundreds of compounds, among which, phenolic compounds exist at much lower levels of concentration than other organics []. To evaluate the antioxidant properties of the wine samples, the gallic acid (GA, as one of the main phenolic components) oxidation process was constructed using 30% (m/m) carbon nanotube-modified electrodes []. The linear range of 0.5–15 µM and the detection limit of 0.3 µM were obtained in an optimized experimental condition. Finally, the proposed procedure was successfully used for estimating the determination of total polyphenols in the samples of red and white wines. Compared with traditional analytic methods, electrochemical analytic methods with CNT-modified electrodes have the advantages of high detection limit, small demand for raw materials, and strong selective identification of specific pollutants []. Furthermore, Ziyatdinova and co-workers [] researched the oxidation peaks of phenolic antioxidants existing in red or white dry wine samples based on MWNT/GCE using differential pulse voltammetry in the phosphate buffer solution of pH 4.0. The evaluation of wine antioxidant capacity (AOC) was established by a one-step chronocoulometric method. The results demonstrated that the AOC of white wine was significantly less than that of red wine (386 ± 112 vs. 1224 ± 184, p < 0.0001). The presented effective measurement, AOC evaluation, made it possible for quality control at different stages in the winemaking process, which usually cannot be accurately performed due to the limitation of common analytic technology. It should be emphasized that CNT-based composite materials via introducing metal oxides and metal nanoparticles have recently shown great success in food and beverage safety testing. The possible reasons for this were that the added metal components dramatically boosted electrical conductivity, enhanced chemical signals, and promoted chemical interaction with the analyte. As shown in Figure 4a, various phenolic compounds in wine samples were determined by CV, DPV, and EIS methods based on Fe3O4/modified carbon paste electrode (MCPE) in a three-electrode system. In their work, the incorporation of Fe3O4/MCPE electrodes as a sensor showed excellent sensitivity, selectivity, repeatability, reproducibility, stability, and low preparation cost via optimizing scan rates and pH values. The detection limits equating to 2.2–10 µM for sinapic acid, 2.6–10 µM for syringic acid, and 0.8–10 µM for rutin were obtained. [] Moreover, various types of metal nanoparticles were added onto the CNTs to generate additional electrocatalytic sites and to increase the sensitivity and detection limits of resultant electrodes []. A CS nanocomposite with Au-NP-decorated CNTs has been developed as an electrochemical sensor for the detection of catechol. Due to highly electrochemical active AuNPs, the excellent reproducibility and repeatability of catechol detection in the range of 0 to 1 mM were obtained by CV analysis, with a detection limit of 3.7 µM []. Ezhil Vilian et al. [] reported Pt/MnO2/functionalized multi-wall CNT (f-MWCNT) electrode material was synthesized by a simple and facile strategy. As shown in Figure 4b, the as-prepared Pt/MnO2/f-MWCNT, having a larger effective surface area, greater porosity, and more reactive sites than bare MWCNTs, exhibited excellent sensitivity for catechin sensing. Under optimized conditions, a very low detection limit (0.02 µM) and the linear range of 2–950 µM were obtained. The developed electrochemical sensor was expected to be useful for industrial applications because of the electrochemical reactivity, excellent reproducibility, and good long-term stability. Similarly, as shown in Figure 4c, a novel electrochemical sensor based on GOX-NFM/MWCNT composite was developed by electrospinning and coating methods to detect glucose in beer samples. The electrochemical response of the sensor was analyzed by CV and CA, and excellent reproducibility and repeatability to glucose detection in the range of 1–3 mM were found with a detection limit of 20 µM [].

Table 2.
Application of electrochemical sensors based on CNT-based materials associated with electrochemical techniques for the detection of different target analytes in foods and beverages.
Table 2.
Application of electrochemical sensors based on CNT-based materials associated with electrochemical techniques for the detection of different target analytes in foods and beverages.
| Analytes | Materials | Electrochemical Techniques | Linear Range | Detection Limit | Sample | Ref. |
|---|---|---|---|---|---|---|
| Gallic acid | MCPE | CV and DPV | 0.5–15 µM | 0.3 µM | Wines | [] |
| Phenolic antioxidants | MWCNT | DPV | ND | ND | Wines | [] |
| Phenolic compounds | Fe3O4/MCPE | CV and DPV and ESI | 0.22–0.26 µM | 0.08 µM | Wines | [] |
| Catechol | AuNP-MWCNT | CV | 0–1.0 mM | 3.7 µM | Wines | [] |
| Catechin | Pt/MnO2/f-MWCNT | SWV | 2–950 µM | 0.02 µM | Wines | [] |
| Glucose | GOX-NFM/MWCNT | CV and CA | 1–3 mM | 20 µM | Beer | [] |
| TBHQ | CuO NFs/NH2-CNTs | DPV | 0.01–3.9 μM | 3 nM | Edible oils | [] |
| Bisphenol A | MWCNTs-βCD/SPCE | CV | 125 nM–2 μM | 13.76 nM | Water | [] |
| Methyl parathion | MWCNT/zirconia | CV | 19.9–176.8 μM | 9 nM | Ethanolic soybean | [] |
| Semicarbazide | MIP/SWNTs-COOH/CS | CV and PDV and ESI | 0.04–0.6 ng mL−1 | 0.025 ng mL−1 | Sheep casings | [] |
MCPE: modified carbon paste electrode; CV: cyclic voltammetry; CA: chronoamperometry; DPV: differential pulse voltammetry; SWV: square wave voltammetry; ESI: electrochemical impedance spectroscopy; mM: millimolar; µM: micromolar; nM: nanomolar; ng: nanogram; MWCNT: multi-walled carbon nanotube; Fe3O4: ferroferric oxide; AuNPs: gold nanoparticles; Pt: platinum; MnO2: manganese dioxide; GOX: glucose oxidase; NFM: nylon nanofibrous membrane; TBHQ: tert-butyl hydroquinone; CuO: copper oxide; NFs: nanoflowers; βCD: β-cyclodextrin; SPCE: screen-printed carbon electrode; MIP: molecularly imprinted polymer; SWNTs: single-walled carbon-nanotubes; CS: chitosan.
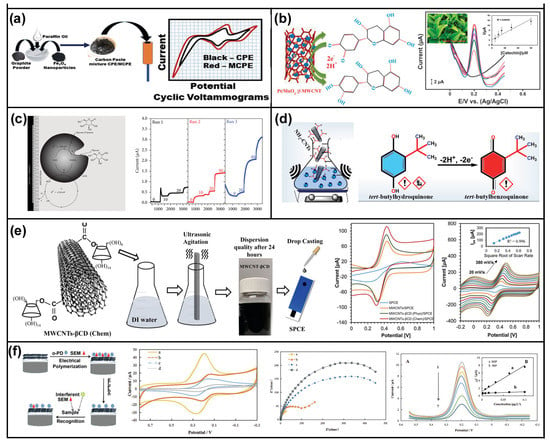
Figure 4.
CNT-based electrochemical sensors for food and alcoholic beverage safety. (a) Schematic diagram of the phenolic compounds’ electrochemical sensor preparation. Reprinted with permission from []. Copyright MDPI, 2021. (b) SWV experiments for various concentrations of 0, 5, 10, 15, 30, and 60 mM of green tea leaves extract diluted with catechin concentration. Reprinted with permission from []. Copyright The Royal Society of Chemistry, 2015. (c) Sensing mechanism. OX and RED are the oxidized and reduced mediator forms, respectively. Reprinted with permission from []. Copyright Hindawi, 2016. (d) Schematic illustration of TBHQ detection. Reprinted with permission from []. Copyright Elsevier, 2021. (e) CV curves of different electrodes and different scan rates in an aqueous electrolyte. Reprinted with permission from []. Copyright Elsevier, 2020. (f) The schematic diagram of the fabrication procedure of the sensor. Reprinted with permission from []. Copyright Elsevier, 2020.
Furthermore, Balram et al. [] reported a novel CuO NF/NH2-CNT composite synthesized using the hydrothermal and ultrasound-assisted method. As shown in Figure 4d, this composite as a working electrode material was used for detecting cytotoxic food preservative tert-butyl hydroquinone (TBHQ). In this work, 3D CuO nanoflowers (NFs) with exceptional chemical stability, non-toxicity, and electron correlation effects could improve the reproducibility, testing repeatability, and stability of electrochemical sensors. An excellent recovery efficiency in the range of 95.90–104.87% and a maximum relative standard deviation (RSD) of 2.71% were obtained using Oils analysis. Recently, Bisphenol A (BPA), one of the most extensively used plasticizers, was detected based on the reversibility and irreversibility of electrochemical redox reactions. Younus Alia and co-workers [] developed a simple, low-cost electrochemical sensor (Figure 4e) for detecting very low concentrations of BPA in water using a WCNTs-βCD decorated screen-printed carbon electrode (SPCE). The electrochemical response of the sensor for detecting BPA showed a completely irreversible process including two electrons and two protons. The sensor showed a two-step linear response from 125 nM to 2 μM and 2 to 30 μM, with a detection limit of 13.76 nM. Moreover, a mesoporous MWCNT/zirconia composite was synthesized by the sol-gel method to detect methyl parathion (MP) in food samples. In this work, the electrochemical responses for MP were obtained by DPV with a detection limit of 9 nM and in a linear interval of 19.9–176.8 μM. Specifically, the developed sensor was applied in ethanolic soybean extract and showed satisfactory recovery and repeatability []. In addition to modifying CNTs with metal or metal oxide nanoparticles, directly installing active molecules on CNTs was also a very effective method for the rapid detection of contaminants in food. Recently, as shown in Figure 4f, Yu et al. [] reported that molecularly imprinted polymer (MIP)/carboxylated single-walled carbon-nanotubes/chitosan (MIP/SWNTs-COOH/CS) was prepared as an electrochemical sensor using MIP as the recognition element. Three different electrochemical techniques, including CV, DPV, and ESI, were applied for the detection of semicarbazide (SEM) in four different real samples. The linear range of 0.04-0.6 ng mL−1 and the low detection limit of 0.025 ng mL−1 were obtained at an optimized experimental condition.
Based on the good conductivity and strong recyclability of carbon nanotubes, we first reviewed the detection and monitoring of chemicals in foods and alcoholic beverages using CNT-based materials as sensor electrodes. Furthermore, a variety of electrochemical analysis methods used for qualitatively and quantitatively detecting chemical substances in foods were also introduced. In addition, compared with CDs, CNTs can be directly used to detect the antioxidant and bioactive substances in alcoholic beverages due to the multiple active sites exposed on CNTs surface. Although a large number of advances have been achieved, more strategies aiming to construct a specific and effective sensor electrode remain to be further studied.
3.3. Graphene
Graphene is a 2D honeycomb lattice structure composed of a single layer of sp2 hybridized carbon atoms. Graphene-based materials have been widely examined by researchers in experiments and theoretical studies due to, among other factors, their high chemical stability, thermal conductivity, and electron mobility [,]. As far as we know, graphene has shown great application potential in the fields of electronics, optics, magnetism, biomedicine, catalysis, and sensors [,]. In particular, graphene is very suitable for the development of sensing materials in electrochemical sensors by taking advantage of its large specific surface area and high electron mobility. In the following section, we illustrate the applications of graphene-based materials in the development of electrochemical sensors for food and beverage safety, as summarized in Table 3.

Table 3.
Application of electrochemical sensors based on graphene-based materials associated with electrochemical techniques for the detection of different target analytes in foods and beverages.
Due to the 2D planar structure of graphene, the fabricated detection system can fully contact the detected substances, which is conducive to accelerating mass transfer and therefore realizing rapid detection. In addition, the detection requirements of different pollutants can be rationally met via grafting organic functional groups or compounding different metal nanomaterials. Recently, Cioates Negut et al. [] reported that oleamide-derivative decorated graphene can be directly coated on carbon electrodes and used to detect amino acids, such as L-histidine, L-tyrosine, L-ornithine, and L-lysine in wine (Figure 5a). In this work, the proposed sensors exhibited excellent sensitivity, reliability, and reproducibility for the quality determination of wines. Furthermore, an amperometric immunosensor for Saccharomyces cerevisiae was constructed using functionalizing and coupling approaches, as shown in Figure 5b. The immunosensor allowed the amperometric detection of the yeast in buffer solution and white wine samples in the range of 10–107 CFU mL−1. Because this propionic acid-functionalized graphene oxide could provide a large number of adsorption and sensing sites, the immunosensor successfully detected Saccharomyces cerevisiae at the refermentation stage, exhibiting a low detection limit and high selectivity, and good reproducibility and storage stability []. The functionalized graphene is not merely able to serve as a platform for aptamer sequencing, but also as the signal-enlarging platform. Goud et al. [] reported an electrochemical apta-sensor based on hexamethylenediamine-functionalized GO as the signal-enlarging platform via carbodiimide amide-bonding. Aflatoxin B1 (AFB1) analyte molecule detection was accomplished in phosphate buffer saline (PBS) solution using methylene blue as a signaling fragment and FGO as the signal-enlarging platform. Under optimized conditions, a very low detection limit was obtained with the linear range of 0.05–6.0 ng mL−1. Heteroatom doped graphene is one of the significant strategies for graphene properties’ regulation. Nitrogen, phosphorus, oxygen, sulfur, and other atom-doped graphene have been extensively studied in the electrochemical catalysis field []. Identification and detection of iron (III) (ferriciron, Fe3+) are challenging in the complex system using traditional detection techniques. As shown in Figure 5c, the Fe3+ electrochemical sensor was constructed by depositing the partially oxidized graphene sheets (po-Gr) on a glassy carbon electrode. The linear response range and the limit of detection of Fe3+ were obtained from 37.5 nM to 21.53 mM and 18.7 nM in the presence of other valence ions, such as Fe2+, Cu2+, Pb2+, Hg2+, Mn2+, Ni2+, Zn2+, Co2+, and Cd2+, respectively. Interestingly, in this work, partially oxidized graphene sheets provided active sites for selectively capturing Fe3+, allowing it to be detected in low concentrations in complex red wine samples [].
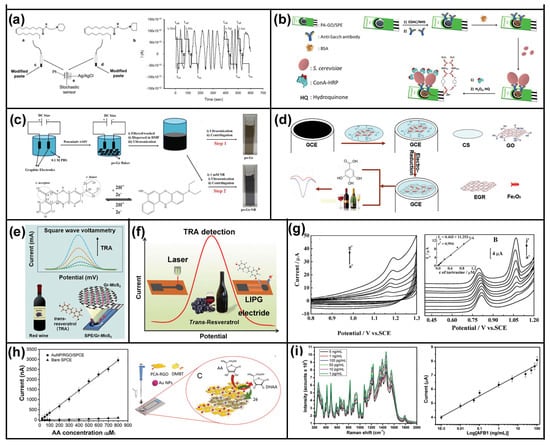
Figure 5.
Graphene-based electrochemical sensors for food and alcoholic beverage safety. (a) The design of the electrochemical platform. Reprinted with permission from []. Copyright Wiley, 2019. (b) Schematic display of the steps involved in the preparation and performance of the immunosensor for Sacch. Reprinted with permission from []. Copyright Springer, 2018. (c) Electrochemical synthesis of po-Gr and treatment of NR with po-Gr. Reprinted with permission from []. Copyright Elsevier, 2017. (d) Illustration for the fabrication of CS–fFe2O3–ERGO/GCE and its application for the detection of GA in wines. Reprinted with permission from []. Copyright Elsevier, 2015. (e) Two-dimensional nanocomposite-based electrochemical sensor for rapid determination of trans-resveratrol. Reprinted with permission from []. Copyright Elsevier, 2020. (f) Schematic illustration of the preparation of LIPG-based electrochemical sensor for the detection of TRA in red wines and grape skins. Reprinted with permission from []. Copyright Elsevier, 2020. (g) CV curves and the possible electrochemical reaction mechanism of sunset yellow and tartrazine. Reprinted with permission from []. Copyright Elsevier, 2013. (h) Sketch of the AuNP/RGO/SPCE-modified nanostructured platform and its application for AA determination in milk. Reprinted with permission from []. Copyright Elsevier, 2020. (i) Raman spectrum and CV curves for different concentrations of AFB1. Reproduced with permission from []. Copyright PLOS, 2019.
Metal oxides are stable heterogeneous catalysts with abundantly exposed active sites, and have been extensively studied in photocatalytic, electrochemical, and traditional thermal catalytic reactions. As shown in Figure 5d, the CS-fishbone-shaped Fe2O3 (fFe2O3)- electrochemically reduced graphene oxide (ERGO), having a large surface area, excellent electronic conductivity, and high stability, was modified on the glassy carbon electrode for detecting GA to estimate the antioxidant capacity of wines. Under the optimal conditions, the good linear range and the low limit of detection of GA were obtained from 1 to 100 µM and 0.15 µM, respectively. In this work, ERGO and fFe2O3 hybrids have a significant synergistic amplification effect on enhancing the electrochemical performance of the electrode. Moreover, the good structural stability of the reported CS-fFe2O3-ERGO can avoid contamination of the detection system []. Similarly, Zhang et al. [] reported an electrochemical sensor that was fabricated using SnO2 decorated graphene (SnO2-RGO) composite, which exhibited excellent selectivity, reproducibility, and stability for caffeic acid (CA) detection in commercial red wine samples.
Two-dimensional disulfide nanosheets, and particularly molybdenum disulfide (MoS2), have aroused tremendous research interest in electrochemical aptasensor applications under complicated conditions [,]. However, the facile aggregation and low electronic conductivities of MoS2 decrease the number of electroactive sites on the electrode surface, thereby restricting electron transfer and related electrochemical kinetics [,]. A highly sensitive electrochemical sensor based on 2D nanocomposite for trans-resveratrol (TRA) was fabricated using graphene-molybdenum disulfide (Gr-MoS2), as shown in Figure 5e. Under optimized conditions, the prepared sensor showed a linear response in TRA concentration from 1.0 to 200 μM with a limit of detection of 0.45 μM due to the synergistic effect between Gr and MoS2 []. Furthermore, Wang et al. [] reported reduced graphene oxide/molybdenum disulfide/polyaniline@gold nanoparticles/chitosan (RGO/MoS2/PANI@AuNPs/Cs), among which Cs acted as an aptasensor and AuNPs were dedicated to signal amplification, was constructed using facile hydrothermal and coating methods. The Au-S bonds formed on the surface of MoS2 facilitate effective installation of the aptamer. As-developed RGO/MoS2/PANI@AuNPs/Apt exhibited a wide linear range from 0.01 fg mL−1 to 1.0 fg mL−1 and a remarkably low detection limit of 0.002 fg mL−1 due to the synergistic effects among distinct components.
In addition to coating catalysts on the surface of carbon electrodes, fabricating self-supported electrodes can effectively increase the contact between the detected substance and the sensor. Based on 3D printing technology, graphene-polylactic acid (graphene-PLA) electrodes were fabricated for detecting atropine in contaminated beverage samples, such as white wine, vodka, whisky, and energy drink. A linear concentration range between 5 and 60 μM associated with a detection limit of 1 μM was obtained using a SWV determination protocol []. Moreover, as shown in Figure 5f, a novel flexible electrochemical sensor using a direct laser-induced graphene (LIG) technique that transforms the commercial Kapton tape into 3D porous graphene was developed for sensitive detection of trans-tesveratrol (TRA) molecules in red wines. The prepared electrochemical sensor with excellent repeatability, stability, reproducibility, and reliability guaranteed an excellent linear response within the TRA concentration range from 0.2 to 50 μM and a low limit of detection of 0.16 μM. Furthermore, the developed sensor can be applied for the evaluation of TRA levels in red wines and grape skins with a satisfactory result [].
Electrochemical sensors based on graphene composite materials have been widely studied in daily food safety and drug monitoring. In order to maximize the detection efficiency and accuracy, porous structures such as Co3O4, TiO2, and MnO2 were loaded onto the surface of graphene to exhibit greater analytic performance in food and alcohol safety []. For instance, a composite of graphene and mesoporous TiO2, as a novel voltammetric sensor, was fabricated by a facile one-pot hydrothermal method. The developed sensor exhibited well-defined and separated SWV peaks (i.e., 272 mV) for detecting sunset yellow FCF and tartrazine in several food sample extracts using CV and SWV techniques, as shown in Figure 5g []. With the aim of enhancing the sensitivity of the electrochemical apta-sensors, Zhou et al. [] reported gold nanoparticles dotted graphene (GNPs/GR) as an efficient aptamer used for bisphenol A (BPA) detection in milk products. The fabricated electrochemical sensor exhibited a wide range of concentrations and a low limit of BPA detection by analyzing the current change of ferricyanide. Similarly, Yang et al. [] reported the composite of layer-by-layer films of graphene (Gr)-gold nanoparticles (Au) and molecularly imprinted polymers (MIPs) as an electrochemical sensor used for efficiently detecting trans-resveratrol by taking advantage of their synergistic effects. In addition to the detection of chemical contaminants in foods, it is also important to monitor the quality of nutrients in food. Au nanoparticles decorated reduced graphene oxide flakes as an efficient detector were developed to monitor the quality control and quantitative assessment of vitamin C in infant food by studying L-ascorbic acid (AA) oxidation. As shown in Figure 5h, the oxidation of AA to dehydroascorbic acid showed a good linear relationship in the range of 50–500 μM and a low detection limit of 17 μM []. Finally, combining electrochemical sensing technology with other technologies, such as Raman spectroscopy, FT-IR spectroscopy, and mass spectrometry, can further improve the detection accuracy and sensitivity. A new Aflatoxin B1 sensor was developed based on Au nanostructures/graphene nanosheets-modified indium tin oxide (ITO) substrate, which could enhance the Raman effect and the electrochemical conductivity (Figure 5i). The presented sensor was a simple, easy, and sensitive sensor for monitoring the low concentrations of AFB1 with a detection limit of about 6.9 pg mL−1. It also allowed the determination of AFB1 in spiked food samples [].
Graphene is an excellent candidate in the design of electrochemical sensors due to its excellent electrical conductivity, large specific surface area, good biocompatibility, easy functionalization, and abundant active sites. Benefiting from the development of functionalization strategies, highly efficient electrochemical sensors can be constructed by doping heteroatoms on graphene. The heteroatom-doped graphene with an accurate structure helps to explore the sensing mechanism at the molecular level. In addition, metal nanoparticles or metal oxide particles are usually loaded on the surface of graphene to improve the sensitivity of electrochemical sensors. However, the added metal salts will greatly increase the cost and even deteriorate the stability of sensors. In future research, it is hoped that researchers will directly use graphene as a 3D self-supported electrode in electrochemical sensors based on graphene’s robust durability, which will provide technical support for further practical industrial applications.
4. Conclusions and Perspectives
In summary, electrochemical sensors constructed by carbon-based composites can acquire a wide linear response range and low detection limit in the detection of food and alcoholic beverage contaminants. CV, LSV, SWV, and DPV are the most widely used electrochemical sensing methods because they can discern the possible intermediates with an extremely low detection limit, decode the nature of coupled chemical reactions, explore the weak adsorption phenomenon, study the mechanism of complex electrode reactions, and suppress the background current, respectively. Due to the good electrical conductivity, wide potential window, and easy chemical modifiability, carbon material (i.e., CDs, CNTs, and graphene)-based composite electrodes have been built with the introduction of metal nanoparticles, polymers, organic acids, doped heteroatoms, metal-oxide nanoparticles, and 2D sulfides to offer high sensitivity, unique selectivity, good durability, and repeatability. The presented electrochemical sensors have been widely used for detecting and monitoring chemical contaminants including dopamine, acetaminophen, H2O2, GA, methyl parathion, Cu2+ and Fe3+, Bisphenol A, sunset yellow FCF, Aflatoxin B1, and amino acids in foods and alcoholic beverages. Furthermore, electrochemical sensors have been practically applied for the detection of chemical contaminants in white wine, red wine, beer, edible oils, water, milk, and medicines.
Although most of the reported sensors exhibited potential as portable instruments, most of them have been assessed only in the laboratory. Thus, developing novel construction methods and revealing the sensing mechanism underlying the molecular precision are the core contents that must be studied continuously in order to realize applications in the industrial model for food and beverage safety in the future. Due to the good electrical conductivity and chemical modifiability of carbon materials, it is hoped that an electrochemical sensor can be constructed based on single-atom functionalized carbon materials, which can further reduce the cost and increase the detection sensitivity. Furthermore, using advanced in situ characterization techniques, such as Fourier-transform infrared spectroscopy (FT-IR), Raman spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) to reveal the sensing mechanism, can provide the theoretical support for the rational design and construction of efficient sensing electrodes. Finally, to realize the online detection under complex working conditions available for industrial applications, the proposed integration of designed electrochemical sensors within a smart device, such as a phone, iPad, and computer, is becoming a popular research topic in this field.
Author Contributions
Z.Y., J.G. and X.Z. all contributed to the collection of data and preparation of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wenzhou Key Laboratory of Biomaterials and Engineering (Grant No: WIUCASSWCL21005) and the Science and Technology Plans of Tianjin (21ZYJDJC00050).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data presented in this manuscript were derived from the indicated articles, which are published in the literature and listed in the Reference section.
Conflicts of Interest
The author declares no conflict of interest.
References
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Yáñez, L.; Ortiz, D.; Calderón, J.; Batres, L.; Carrizales, L.; Mejía, J.; Martínez, L.; García-Nieto, E.; Díaz-Barriga, F. Overview of human health and chemical mixtures: Problems facing developing countries. Env. Health Persp. 2002, 110, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Shenashen, M.A.; Emran, M.Y.; El Sabagh, A.; Selim, M.M.; Elmarakbi, A.; El-Safty, S.A. Progress in sensory devices of pesticides, pathogens, coronavirus, and chemical additives and hazards in food assessment: Food safety concerns. Prog. Mater. Sci. 2021, 124, 100866. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zeng, X.; Cao, J.; Jiang, W. Application of blockchain technology in food safety control: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2020, 62, 2800–2819. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2019, 19, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Alcoholic Beverage Production in Indochina: Local Wisdom, Safety, Quality, and Legal Control. Prod. Mana. Bevera. 2019, 1, 381–407. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Miliadis, G. Development and validation of an easy multiresidue method for the determination of mul-ticlass pesticide residues using GC–MS/MS and LC–MS/MS in olive oil and olives. Talanta 2013, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, Y.; Li, Y.; Fan, X.; Ding, S. Determination of neonicotinoid insecticides residues in eels using subcritical water extraction and ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2013, 777, 32–40. [Google Scholar] [CrossRef]
- Vilchez, J.; El-Khattabi, R.; Fernández, J.; González-Casado, A.; Navalón, A. Determination of imidacloprid in water and soil samples by gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 746, 289–294. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, L.; Cheng, Q.; Cai, J.; Wang, Y.; Yang, M.; Hua, X.; Liu, F. A bare-eye based one-step signal amplified semi-quantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples. Anal. Chim. Acta 2015, 881, 82–89. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2019, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.T.; Durairaj, S.; Prins, S. Aicheng ChenNanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectro. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Adley, C.C. Past, Present and Future of Sensors in Food Production. Foods 2014, 3, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, B.; Wu, A.; Cheng, G.; Li, Z.; Dong, S. A Method to Construct a Third-Generation Horseradish Peroxidase Biosensor: Self-Assembling Gold Nanoparticles to Three-Dimensional Sol−Gel Network. Anal. Chem. 2002, 74, 2217–2223. [Google Scholar] [CrossRef]
- Privett, B.; Shin, J.; Schoenfisch, M. Electrochemical sensors. Anal. Chem. 2010, 78, 3965. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Xiang, Q.; Huang, J.; Huang, H.; Mao, W.; Ye, Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv. 2018, 8, 1820–1825. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.-H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-Based Nanomaterials in Sensors for Food Safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yong, K.; Choi, J.; Cowie, A. Emerging point-of-care technologies for food safety analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Progress in Nanomaterial-Based Optical Aptamer Assay for the Detection of Food Chemical Contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Yang, Y.; Liu, H.; Fang, G.; Lu, X.; Wang, S. Emerging functional nanomaterials for the detection of food con-taminants. Trends Food Sci. Tech. 2018, 71, 94–106. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Hu, Y.; Zhou, C.; Lan, W.; Fu, H.; She, Y. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality and authenticity. Sens. Actuators B Chem. 2020, 329, 129135. [Google Scholar] [CrossRef]
- Arslan, M.; Tahir, H.E.; Zareef, M.; Shi, J.; Rakha, A.; Bilal, M.; Xiaowei, H.; Zhihua, L.; Xiaobo, Z. Recent trends in quality control, discrimination and authentication of alcoholic beverages using nondestructive instrumental techniques. Trends Food Sci. Technol. 2020, 107, 80–113. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.G.; Swain, G.M. Electrochemistry and the environment. J. Appl. Electrochem. 1994, 24, 1077–1091. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Arrigan, D. Bioelectrochemistry. Fundamentals, Experimental Techniques and Applications. Chromatographia 2010, 72, 585. [Google Scholar] [CrossRef][Green Version]
- Moses, P.R.; Wier, L.; Murray, W.R. Chemically modified tin oxide electrode. Anal. Chem. 1975, 47, 1882–1886. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef] [PubMed]
- Bonizzoni, M.; Anslyn, E.V. Combinatorial Methods for Chemical and Biological Sensors. J. Am. Chem. Soc. 2009, 131, 14597–14598. [Google Scholar] [CrossRef]
- Ouyang, J. Application of intrinsically conducting polymers in flexible electronics. SmartMat 2021, 2, 263–285. [Google Scholar] [CrossRef]
- Menon, S.; Jesny, S.; Kumar, K. A voltammetric sensor for acetaminophen based on electropolymerized molecular-ly imprinted poly (oaminophenol) modified gold electrode. Talanta 2018, 179, 668–675. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Lingerfelt, L. Immobilisation of DNA on Chips I; Wittmann, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 161–186. [Google Scholar]
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive monitoring of protein immobilization and antigen–antibody reactions on monomolecular alkylthiol films on gold electrodes. Biosens. Bioelectron. 1997, 12, 977–989. [Google Scholar] [CrossRef]
- Pardakhty, A.; Ahmadzadeh, S.; Avazpour, S.; Gupta, V.K. Highly sensitive and efficient voltammetric determination of ascorbic acid in food and pharmaceutical samples from aqueous solutions based on nanostructure carbon paste electrode as a sensor. J. Mol. Liq. 2016, 216, 387–391. [Google Scholar] [CrossRef]
- Gheibi, S.; Karimi-Maleh, H.; Khalilzadeh, M.A.; Bagheri, H. A new voltammetric sensor for electrocatalytic determination of vitamin C in fruit juices and fresh vegetable juice using modified multi-wall carbon nanotubes paste electrode. J. Food Sci. Technol. 2013, 52, 276–284. [Google Scholar] [CrossRef]
- Trani, A.; Petrucci, R.; Marrosu, G.; Zane, D.; Curulli, A. Selective electrochemical determination of caffeine at a gold-chitosan nanocomposite sensor: May little change on nanocomposites synthesis affect selectivity? J. Electroanal. Chem. 2017, 788, 99–106. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H. Simultaneous electrochemical determination of dopamine, melatonin, methionine and caffeine. Sens. Actuators B Chem. 2015, 208, 195–203. [Google Scholar] [CrossRef]
- Chiappalone, M.; Vato, A.; Tedesco, M.; Marcoli, M.; Davide, F.; Martinoia, S. Networks of neurons coupled to microelectrode arrays: A neuronal sensory system for pharmacological applications. Biosens. Bioelectron. 2003, 18, 627–634. [Google Scholar] [CrossRef]
- Thangavelu, K.; Palanisamy, S.; Chen, S.-M.; Velusamy, V.; Chen, T.-W.; Ramaraj, S.K. Electrochemical Determination of Caffeic Acid in Wine Samples Using Reduced Graphene Oxide/Polydopamine Composite. J. Electrochem. Soc. 2016, 163, B726–B731. [Google Scholar] [CrossRef]
- Lupu, S.; Parenti, F.; Pigani, L.; Seeber, R.; Zanardi, C. Differential pulse techniques on modified conventional-Size and mi-croelectrodes. Electroactivity of poly[4,4′-bis(butylsulfanyl)-2,2′-bithiophene] coating towards dopamine and ascorbic acid oxidation. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2003, 15, 715–725. [Google Scholar]
- Osteryoung, J.; Osteryoung, R. Square wave voltammetry. Anal. Chem. 1985, 57, 101–110. [Google Scholar] [CrossRef]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Q.; Li, T.; Yan, J.; Ren, Y.; Feng, J.; Wei, T. Easy synthesis of porous graphene nanosheets and their use in supercapacitors. Carbon 2012, 50, 1699–1703. [Google Scholar] [CrossRef]
- Ito, Y.; Shen, Y.H.; Hojo, D.; Itagaki, Y.; Fujita, T.; Chen, L.H.; Aida, T.; Tang, Z.; Adschiri, T.; Chen, M.W. Correlation be-tween chemical dopants and topological defects in catalytically active nanoporous graphene. Adv. Mater. 2016, 28, 10644–10651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, H.T.; Shi, Y.N.; Han, J.Y.; Yang, Z.J.; Zhang, Y.; Long, C.; Guo, J.; Zhu, Y.F.; Qiu, X.Y.; et al. Boosting CO2 conversion with terminal alkynes by molecular architecture of gra-phene oxide-supported Ag nanoparticles. Matter 2020, 3, 558–570. [Google Scholar] [CrossRef]
- Cao, Y.; Rodan-Legrain, D.; Rubies-Bigorda, O.; Park, J.M.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Tunable corre-lated states and spin-polarized phases in twisted bilayer-bilayer graphene. Nature 2020, 583, 215–220. [Google Scholar] [CrossRef] [PubMed]
- The different dimensions of nanotechnology. Nat. Nanotechnol. 2009, 4, 135. [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable Photoluminescence Across the Entire Visible Spectrum from Carbon Dots Excited by White Light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974. [Google Scholar] [CrossRef]
- Teradal, N.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Atkin, P.; Daeneke, T.; Wang, Y.; Careya, B.; Berean, K.; Clark, R.; Ou, J.; Trinchi, A.; Cole, I.; Kalantar-zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13563–13571. [Google Scholar] [CrossRef]
- Vercelli, B.; Donnini, R.; Ghezzi, F.; Sansonetti, A.; Giovanella, U.; La Ferla, B. Nitrogen-doped carbon quantum dots obtained hydrothermally from citric acid and urea: The role of the specific nitrogen centers in their electrochemical and optical responses. Electrochim. Acta 2021, 387, 138557. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Stachurski, C.D.; Click, S.M.; Wolfe, K.D.; Dervishogullari, D.; Rosenthal, S.J.; Jennings, G.K.; Cliffel, D.E. Optical and electrochemical tuning of hydrothermally synthesized nitrogen-doped carbon dots. Nanoscale Adv. 2020, 2, 3375–3383. [Google Scholar] [CrossRef]
- Rigodanza, F.; Đorđević, L.; Arcudi, F.; Prato, M. Customizing the electrochemical properties of carbon nanodots with quinones in bottom-up syntheses. Angew. Chem. Int. Ed. 2018, 130, 5156–5161. [Google Scholar] [CrossRef]
- Ji, X.; Palui, G.; Avellini, T.; Na, H.; Yi, C.K.; Knappenberger, L.; Mattoussi, H. On the pH-dependent quenching of quantum dot photoluminescence by redox active dopamine. J. Am. Chem. Soc. 2012, 134, 6006–6017. [Google Scholar] [CrossRef]
- Morgan, L.D.; Baker, H.; Yeoman, M.S.; Patel, B.A. Chromatographic assay to study the activity of multiple enzymes involved in the synthesis and metabolism of dopamine and serotonin. Analyst 2012, 137, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, B.; Meng, F. Microwave-assisted preparation of N-doped carbon dots as a biosensor for electrochemical do-pamine detection. J. Colloid Interface Sci. 2015, 452, 199–202. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Hu, S.; Li, F.; Weng, W.; Chen, J.; Wang, Q.; He, Y.; Zhang, W.; Bao, X. A sensitive and reliable dopamine biosensor was developed based on the Au carbon dots–chitosan composite film. Biosens. Bioelectron. 2013, 52, 277–280. [Google Scholar] [CrossRef]
- Dai, H.; Xu, G.; Gong, L.; Yang, C.; Lin, Y.; Tong, Y.; Chen, J.; Chen, G. Electrochemical detection of triclosan at a glassy carbon electrode modifies with carbon nanodots and chitosan. Electrochim. Acta 2012, 80, 362–367. [Google Scholar] [CrossRef]
- Sheng, M.; Yue, G.; Sun, J.; Feng, G. Carbon nanodots–chitosan composite film: A plat form for protein immobilization, direct electrochemistry and bioelectrocatalysis. Biosens. Bioelectron. 2014, 58, 351–358. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, P.; Huang, L.; Huang, Y.; Huang, Q.; Zhang, W.; Wei, C.; Hu, S. A nitrogen-doped carbon dot/ferrocene@β-cyclodextrin composite as an enhanced material for sensitive and selective determination of uric acid. Anal. Methods 2014, 6, 2687–2691. [Google Scholar] [CrossRef]
- Shao, X.; Gu, H.; Wang, Z.; Chai, X.; Tian, Y.; Shi, G. Highly Selective Electrochemical Strategy for Monitoring of Cerebral Cu2+ Based on a Carbon Dot-TPEA Hybridized Surface. Anal. Chem. 2012, 85, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, Y.; Zhang, Y.; Weng, W.; Li, S. Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuators B Chem. 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Henifehpour, Y.; Joo, S. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; ul Haq Ali Shah, A.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef]
- Liu, L.-P.; Yin, Z.-J.; Yang, Z.-S. A l-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 2010, 79, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Yoon, B.; Toppare, L.; Swager, T. Quaternized polymer-single-walled carbon nanotube scaffolds for a chemire sistive glucose sensor. ACS Sens. 2017, 2, 1123–1127. [Google Scholar] [CrossRef]
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chem. Int. Ed. 2015, 54, 6554–6557. [Google Scholar] [CrossRef]
- Münzer, A.; Melzer, K.; Heimgreiter, M.; Scarpa, G. Random CNT network and regioregular poly(3-hexylthiophen) FETs for pH sensing applications: A comparison. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4353–4358. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Ingkaninan, K. On-line HPLC–MS–DPPH assay for the analysis of phenolic antioxidant compounds in fruit wine: Antidesma thwaitesianum Muell. Food Chem. 2010, 118, 147–152. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Chronocoulometry of wine on multi-walled carbon nanotube modified electrode: Antioxidant capacity assay. Food Chem. 2016, 196, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Pwavodi, P.; Ozyurt, V.; Asir, S.; Ozsoz, M. Electrochemical sensor for determination of various phenolic compounds in wine samples using Fe3O4 nanoparticlesmodified carbon paste electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Rassas, I.; Minot, S.; Bessueille, F.; Arab, M.; Chevallier, V.; Rodriguez-Mendez, M.; Errachid, A.; Jaf-frezic-Renault, N. Voltammetric sensor based on molecularly imprinted chitosan-carbon nanotubes decorated with gold na-noparticles nanocomposite deposited on boron-doped diamond electrodes for catechol detection. Materials 2020, 13, 688. [Google Scholar] [CrossRef]
- Vilian, A.; Madhu, R.; Chen, S.; Veeramani, V.; Sivakumar, M.; Yun, S.; Han, Y. Facile synthesis of MnO2/carbon nanotubes decorated with a nanocomposite of Pt nanoparticles as a new platform for the electrochemical detection of catechin in red wine and green tea samples. J. Mater. Chem. B 2015, 3, 6285–6292. [Google Scholar] [CrossRef]
- Marco, M.; Edoardo, L.; Matteo, S. Monitoring of glucose in beer brewing by a carbon nanotubes based nylon nanofibrous biosensor. J. Nanomater. 2016, 2016, 5217023. [Google Scholar]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J. Hazard. Mater. 2021, 406, 124792. [Google Scholar] [CrossRef]
- Ali, Y.; Alam, A.U.; Howlader, M.M. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Caetano, K.; Rosa, D.; Pizzolatto, T.; Santos, P.A.M.D.; Costa, T. MWCNT/zirconia porous composite applied as electrochemical sensor for determination of methyl parathion. Microporous Mesoporous Mater. 2020, 309, 110583. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Y.; Sang, Y.; Liu, W.; Wang, S.; Wang, X. Preparation of a carboxylated single-walled carbon-nanotube-chitosan func-tional layer and its application to a molecularly imprinted electrochemical sensor to quantify semicarbazide. Food Chem. 2020, 333, 127524. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, L.; Herranz, M.; Martín, N. The chemistry of pristine graphene. Chem. Commun. 2013, 49, 3721–3735. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Ikhsan, N.I.; Pandikumar, A. Doped-Graphene Modified Electrochemical Sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 67–87. ISBN 9780128153949. [Google Scholar]
- Yang, Z.J.; Yang, C.Y.; Han, J.Y.; Zhao, W.S.; Shao, S.X.; Li, S.Y.; Gao, H.W.; Xie, H.J.; Zhang, X.F. Boosting electrochemical CO2 reduction to formate using SnO2/graphene oxide with amide linkages. J. Mater. Chem. A 2021, 9, 19681–19686. [Google Scholar] [CrossRef]
- João, A.F.; Rocha, R.G.; Matias, T.A.; Richter, E.M.; Petruci, J.F.S.; Muñoz, R.A. 3D-printing in forensic electrochemistry: Atropine determination in beverages using an additively manufactured graphene-polylactic acid electrode. Microchem. J. 2021, 167, 106324. [Google Scholar] [CrossRef]
- Negut, C.; Stefan-van Staden, R.I.; Harja, F.; Staden, J. Pattern recognition of amino acids in wines. Electroanalysis 2019, 32, 7–10. [Google Scholar]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An electrochemical sensor for gallic acid based on Fe2O3/electro-reduced graphene oxide composite: Estimation for the antioxidant capacity index of wines. Eng. C Mater Biol Appl. 2015, 57, 279–287. [Google Scholar] [CrossRef]
- Goud, K.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J. An electrochemical aptasensor based on func-tionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Geleta, G.; Zhen, Z.; Wang, Z. Novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite based elec-trochemical aptasensor for detection of aflatoxin B1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Borisova, B.; Sánchez, A.; Soto-Rodríguez, P.E.D.; Boujakhrout, A.; Arévalo-Villena, M.; Pingarrón, J.M. Disposable am-perometric immunosensor for Saccharomyces cerevisiae based on carboxylated graphene oxide-modified electrodes. Anal. Bioanal. Chem. 2018, 410, 7901–7907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ping, J.; Ying, Y. Evaluation of trans-resveratrol level in grape wine using laser-induced porous graphene-based electrochemical sensor. Sci. Total Environ. 2020, 714, 136687. [Google Scholar] [CrossRef]
- Zhang, C.; Ping, J.; Ye, Z.; Ying, Y. Two-dimensional nanocomposite-based electrochemical sensor for rapid determination of trans-resveratrol. Sci. Total Environ. 2020, 742, 140351. [Google Scholar] [CrossRef] [PubMed]
- Jwz, A.; Kpw, A.; Xuan, Z. Fabrication of SnO2 decorated graphene composite material and its application in electrochemical detection of caffeic acid in red wine. Mater. Res. Bull. 2020, 126, 110820. [Google Scholar]
- Sadak, O.; Sundramoorthy, A.K.; Gunasekaran, S. Highly selective colorimetric and electrochemical sensing of iron (III) using Nile red functionalized graphene film. Biosens. Bioelectron. 2017, 89, 430–436. [Google Scholar] [CrossRef]
- Tian, G.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the sim-ultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Yang, J.; Chen, S.; Hu, F.; Hu, Y.; Lin, H. Synergistic effects of layer-by-layer films for highly selective and sensitive electro-chemical detection of trans-resveratrol. Food Chem. 2020, 338, 127851. [Google Scholar] [CrossRef]
- Bettazzi, F.; Ingrosso, C.; Sfragano, P.; Pifferi, V.; Palchetti, I. Gold nanoparticles modified graphene platforms for highly sen-sitive electrochemical detection of vitamin C in infant food and formulae. Food Chem. 2020, 344, 128692. [Google Scholar] [CrossRef] [PubMed]
- AlThagafi, I.I.; Ahmed, S.A.; El-Said, W.A. Fabrication of gold/graphene nanostructures modified ITO electrode as highly sensitive electrochemical detection of Aflatoxin B1. PLoS ONE 2019, 14, e0210652. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancer biomarker detection. Sens. Actuators B Chem. 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Fang, L.; Huang, K.; Liu, Y. Novel electrochemical dual-aptamer-based sandwich biosensor using molybdenum disul-fide/carbon aerogel composites and Au nanoparticles for signal amplification. Biosens. Bioelectron. 2015, 71, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Ng, A.; Siaj, M.; Zourob, M. Label-Free Voltammetric Aptasensor for the Sensitive Detection of Microcystin-LR Using Graphene-Modified Electrodes. Anal. Chem. 2014, 86, 7551–7557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Pang, X.; Du, B.; Wei, Q. Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 255, 125–132. [Google Scholar] [CrossRef]
- Chu, Y.; Cai, B.; Ma, Y.; Zhao, M.; Ye, Z.; Huang, J. Highly sensitive electrochemical detection of circulating tumor DNA based on thin-layer MoS2/graphene composites. RSC Adv. 2016, 6, 22673–22678. [Google Scholar] [CrossRef]
- Li, N.; Liu, G.; Zhen, C.; Li, F.; Zhang, L.; Cheng, H.-M. Battery Performance and Photocatalytic Activity of Mesoporous Anatase TiO2 Nanospheres/Graphene Composites by Template-Free Self-Assembly. Adv. Funct. Mater. 2011, 21, 1717–1722. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).