Microwave Assisted Preparation of Barium Doped Titania (Ba/TiO2) as Photoanode in Dye Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 and Ba/TiO2 Photoanode
2.3. Photoanode Preparation
2.4. Characterization
3. Results and Discussions
3.1. Structural Analysis
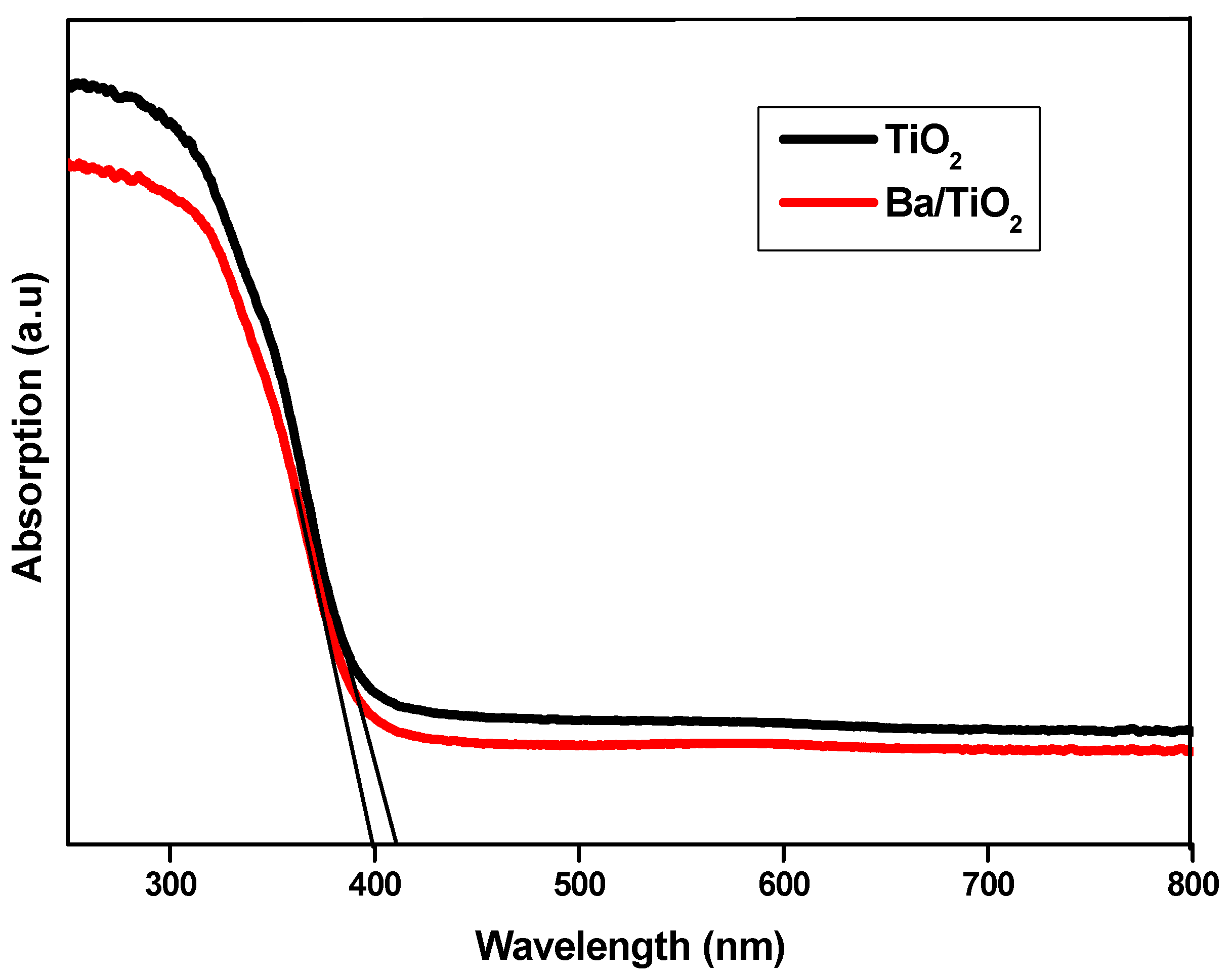
3.2. Optical Properties
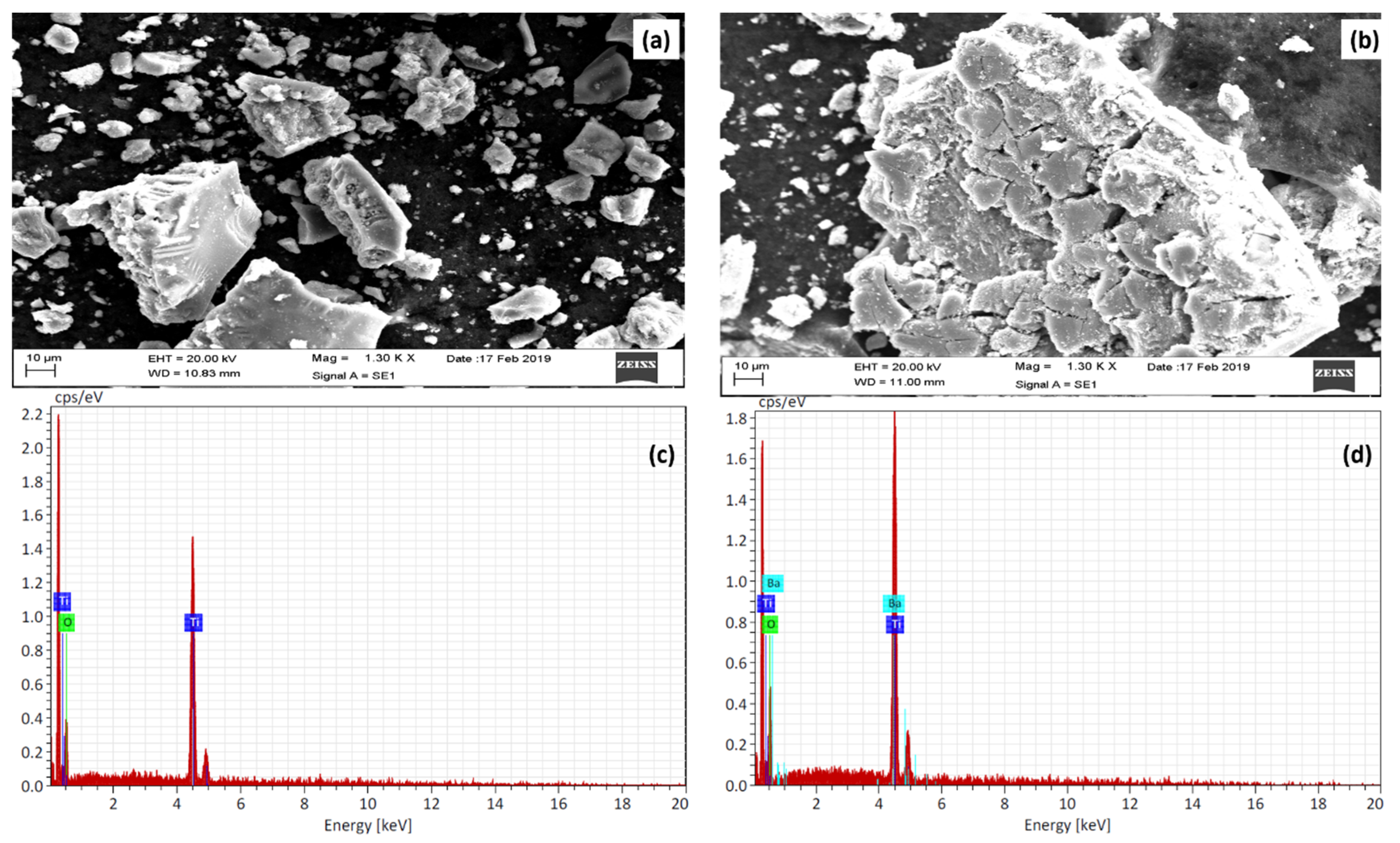
3.3. Morphological Studies
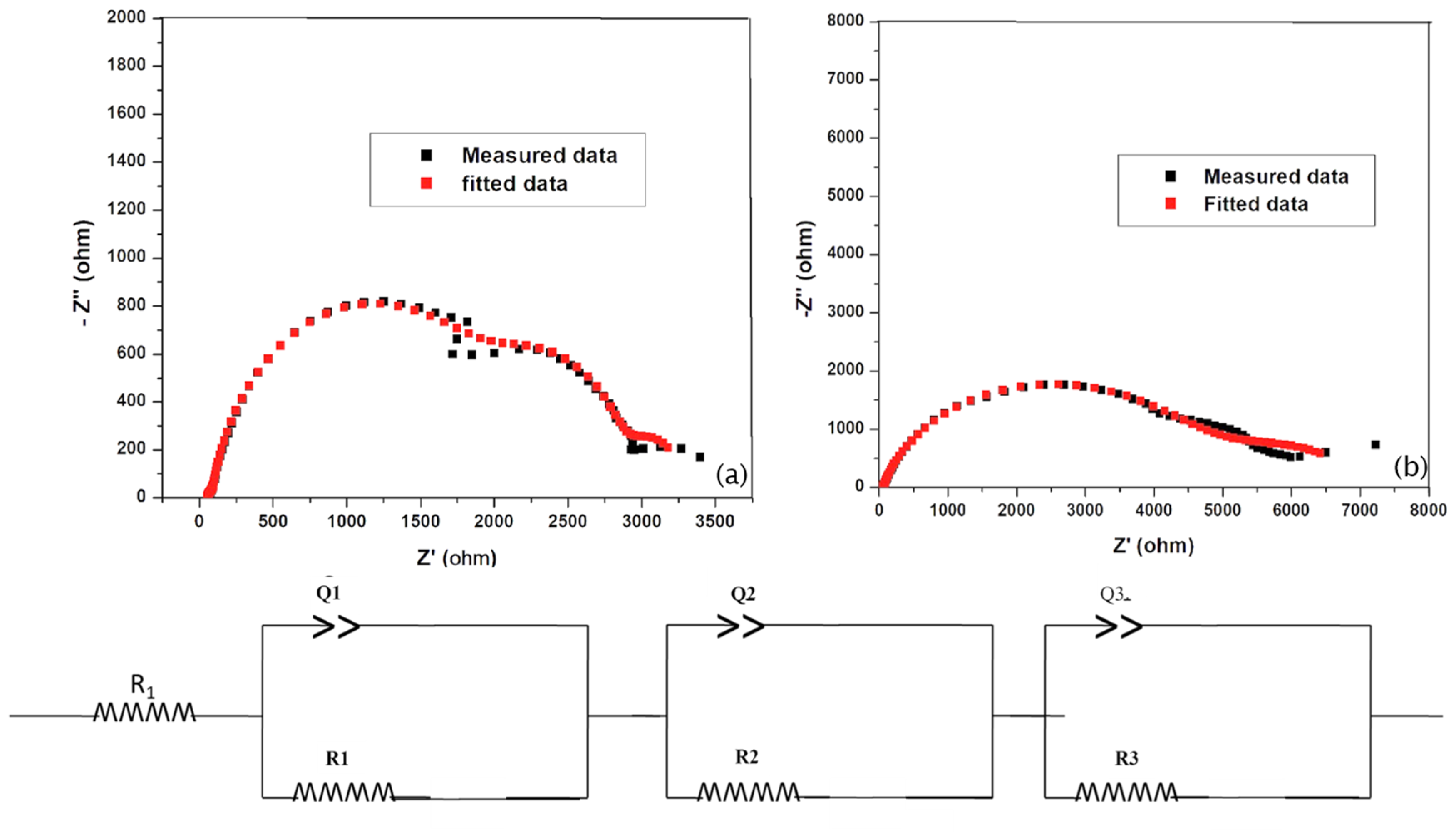
3.4. Electrochemical Behavior of TiO2 and Ba/TiO2
3.5. Photovoltaic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shah, S.S.; Anjum, M.A.R.; Khan, M.R.; Janjua, N.K. Electro-oxidation of ammonia over copper oxide impregnated γ-Al2O3 nanocatalysts. Coatings 2021, 11, 313. [Google Scholar] [CrossRef]
- Tiewsoh, S.L.; Jirásek, J.; Sivek, M. Electricity generation in India: Present state, future outlook and policy implications. Energies 2019, 12, 1361. [Google Scholar] [CrossRef]
- Khan, M.; Janjua, N.K.; Khan, S.; Qazi, I.; Ali, S.; Saad Algarni, T. Electro-oxidation of ammonia at novel Ag2O−PrO2/γ-Al2O3 catalysts. Coatings 2021, 11, 257. [Google Scholar] [CrossRef]
- Saleem, M.; Irfan, M.; Tabassum, S.; Albaqami, M.D.; Javed, M.S.; Hussain, S.; Pervaiz, M.; Ahmad, I.; Ahmad, A.; Zuber, M. Experimental and theoretical study of highly porous lignocellulose assisted metal oxide photoelectrodes for dye-sensitized solar cells. Arab. J. Chem. 2021, 14, 102937. [Google Scholar] [CrossRef]
- Ansir, R.; Shah, S.M.; Ullah, N.; Hussain, M.N. Performance of pyrocatechol violet and carminic acid sensitized ZnO/CdS nanostructured photoactive materials for dye sensitized solar cell. Solid-State Electron. 2020, 172, 107886. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A. Providing all global energy with wind, water, and solar power, Part I: Technologies, energy resources, quantities and areas of infrastructure, and materials. Energy Policy 2011, 39, 1154–1169. [Google Scholar] [CrossRef]
- Irvine, S. Solar Cells and Photovoltaics. In Springer Handbook of Electronic and Photonic Materials; Springer: Cham, The Netherlands, 2017; p. 1. [Google Scholar]
- Gul, M.; Kotak, Y.; Muneer, T. Review on recent trend of solar photovoltaic technology. Energy Explor. Exploit. 2016, 34, 485–526. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Bose, S.; Soni, V.; Genwa, K. Recent advances and future prospects for dye sensitized solar cells: A review. Int. J. Sci. Res. Publ. 2015, 5, 1–8. [Google Scholar]
- Mátravölgyi, B.; Hergert, T.; Thurner, A.; Varga, B.; Sangiorgi, N.; Bendoni, R.; Zani, L.; Reginato, G.; Calamante, M.; Sinicropi, A.; et al. Synthesis and Investigation of Solar-Cell Photosensitizers Having a Fluorazone Backbone. Eur. J. Org. Chem. 2017, 2017, 1843–1854. [Google Scholar] [CrossRef]
- Yu, Q.; Pang, Y.; Jiang, Q. NiS submicron cubes with efficient electrocatalytic activity as the counter electrode of dye-sensitized solar cells. R. Soc. Open Sci. 2018, 5, 180186. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Borbone, F.; Centore, R. Research progress on photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Fischer, M.K.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.M. Dye-sensitized solar cell and photocatalytic performance of nanocomposite photocatalyst prepared by electrochemical anodization. Bull. Mater. Sci. 2016, 39, 1389–1395. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.H. Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8182–8188. [Google Scholar] [CrossRef]
- Labat, F.; Ciofini, I.; Hratchian, H.P.; Frisch, M.J.; Raghavachari, K.; Adamo, C. Insights into working principles of ruthenium polypyridyl dye-sensitized solar cells from first principles modeling. J. Phys. Chem. C 2011, 115, 4297–4306. [Google Scholar] [CrossRef]
- Corrao, R.; D’Anna, D.; Morini, M.; Pastore, L. DSSC-integrated Glassblocks for the construction of Sustainable Building Envelopes. In Advanced Materials Research; Trans Tech Publ: Zurich, Switzerland, 2014. [Google Scholar]
- Wu, C.-G.; Chao, C.-C.; Kuo, F.-T. Enhancement of the photo catalytic performance of TiO2 catalysts via transition metal modification. Catal. Today 2004, 97, 103–112. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Peng, H.; Ying, J.; Zhang, J.; Zhang, X.; Peng, C.; Rao, C.; Liu, W.; Zhang, N.; Wang, X. La-doped Pt/TiO2 as an efficient catalyst for room temperature oxidation of low concentration HCHO. Chin. J. Catal. 2017, 38, 39–47. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Chen, W.; Li, Y. The Progress of TiO2 Nanocrystals Doped with Rare Earth Ions. J. Nanomater. 2012, 2012, 235879. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Momeni, M.M.; Akbarnia, M.; Ghayeb, Y. Preparation of S–W-codoped TiO2 nanotubes and effect of various hole scavengers on their photoelectrochemical activity: Alcohol series. Int. J. Hydrog. Energy 2020, 45, 33552–33562. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Xiao, L.; Chen, F. Properties of carbon and iron modified TiO2 photocatalyst synthesized at low temperature and photodegradation of acid orange 7 under visible light. Appl. Surf. Sci. 2010, 256, 4260–4268. [Google Scholar] [CrossRef]
- Sinhmar, A.; Setia, H.; Kumar, V.; Sobti, A.; Toor, A.P. Enhanced photocatalytic activity of nickel and nitrogen codoped TiO2 under sunlight. Environ. Technol. Innov. 2020, 18, 100658. [Google Scholar] [CrossRef]
- Lu, W.C.; Nguyen, H.D.; Wu, C.Y.; Chang, K.S.; Yoshimura, M. Modulation of physical and photocatalytic properties of (Cr, N) codoped TiO2 nanorods using soft solution processing. J. Appl. Phys. 2014, 115, 144305. [Google Scholar] [CrossRef]
- Liu, R.; Yang, F.; Xie, Y.; Yu, Y. Visible-light responsive boron and nitrogen codoped anatase TiO2 with exposed {0 0 1} facet: Calculation and experiment. Appl. Surf. Sci. 2019, 466, 568–577. [Google Scholar] [CrossRef]
- Jaiswal, R.; Bharambe, J.; Patel, N.; Dashora, A.; Kothari, D.C.; Miotello, A. Copper and Nitrogen co-doped TiO2 photocatalyst with enhanced optical absorption and catalytic activity. Appl. Catal. B Environ. 2015, 168, 333–341. [Google Scholar] [CrossRef]
- Zhang, S. Synergistic effects of C–Cr codoping in TiO2 and enhanced sonocatalytic activity under ultrasonic irradiation. Ultrason. Sonochem. 2012, 19, 767–771. [Google Scholar] [CrossRef]
- Patel, N.; Jaiswal, R.; Warang, T.; Scarduelli, G.; Dashora, A.; Ahuja, B.L.; Kothari, D.C.; Miotello, A. Efficient photocatalytic degradation of organic water pollutants using V–N-codoped TiO2 thin films. Appl. Catal. B Environ. 2014, 150, 74–81. [Google Scholar] [CrossRef]
- Trevisan, V.; Olivo, A.; Pinna, F.; Signoretto, M.; Vindigni, F.; Cerrato, G.; Bianchi, C.L. CN/TiO2 photocatalysts: Effect of co-doping on the catalytic performance under visible light. Appl. Catal. B Environ. 2014, 160, 152–160. [Google Scholar] [CrossRef]
- Cho, I.S.; Lee, C.H.; Feng, Y.; Logar, M.; Rao, P.M.; Cai, L.; Kim, D.R.; Sinclair, R.; Zheng, X. Codoping titanium dioxide nanowires with tungsten and carbon for enhanced photoelectrochemical performance. Nat. Commun. 2013, 4, 1723. [Google Scholar] [CrossRef]
- Yang, X.; Ma, F.; Li, K.; Guo, Y.; Hu, J.; Li, W.; Huo, M.; Guo, Y. Mixed phase titania nanocomposite codoped with metallic silver and vanadium oxide: New efficient photocatalyst for dye degradation. J. Hazard. Mater. 2010, 175, 429–438. [Google Scholar] [CrossRef]
- Devi, R.S.; Venckatesh, D.R.; Sivaraj, D.R. Synthesis of titanium dioxide nanoparticles by sol-gel technique. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15206–15211. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Sivaraj, D. Synergistic antibacterial activity of barium doped TiO2 nanoclusters synthesized by microwave processing. RSC Adv. 2016, 6, 9663–9671. [Google Scholar] [CrossRef]
- Dahiya, M.S.; Tomer, V.K.; Duhan, S. Metal–ferrite nanocomposites for targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 737–760. [Google Scholar]
- Badawi, A.; Althobaiti, M.G.; Alharthi, S.S.; Al-Baradi, A.M. Tailoring the optical properties of CdO nanostructures via barium doping for optical windows applications. Phys. Lett. A 2021, 411, 127553. [Google Scholar] [CrossRef]
- Patel, S.; Gajbhiye, N.; Date, K.S. Ferromagnetism of Mn-doped TiO2 nanorods synthesized by hydrothermal method. J. Alloy. Compd. 2011, 509, S427–S430. [Google Scholar] [CrossRef]
- AlFaify, S.; Ganesh, V.; Haritha, L.; Shkir, M. An effect of La doping on physical properties of CdO films facilely casted by spin coater for optoelectronic applications. Phys. B: Condens. Matter 2019, 562, 135–140. [Google Scholar] [CrossRef]
- N’Konou, K.; Lare, Y.; Haris, M.; Baneto, M.; Amou, K.A.; Napo, K. Influence of barium doping on physical properties of zinc oxide thin films synthesized by SILAR deposition technique. Adv. Mater. 2014, 3, 63–67. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, F.; Stadler, F.J.; Chen, R. A facile and general synthesis strategy to doped TiO2 nanoaggregates with a mesoporous structure and comparable property. RSC Adv. 2015, 5, 64293–64298. [Google Scholar] [CrossRef]
- Moss, T.S. The interpretation of the properties of indium antimonide. Proc. Phys. Soc. 1954, 67, 10. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Son, Y.; Park, M.; Son, Y.; Lee, J.S.; Jang, J.H.; Kim, Y.; Cho, J. Quantum confinement and its related effects on the critical size of GeO2 nanoparticles anodes for lithium batteries. Nano Lett. 2014, 14, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Webb, J.; Williams, D. Absorption edge shift in ZnO thin films at high carrier densities. Solid State Commun. 1981, 39, 1269–1271. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Elangovan, E.; Viegas, J. Effect of La doping on the structural, optical and electrical properties of spray pyrolytically deposited CdO thin films. J. Alloy. Compd. 2017, 708, 804–812. [Google Scholar] [CrossRef]
- Chakraborty, P.; Datta, G.; Ghatak, K. The simple analysis of the Burstein–Moss shift in degenerate n-type semiconductors. Phys. Condens. Matter 2003, 339, 198–203. [Google Scholar] [CrossRef]
- Kumar, R. Structural and optical studies of Mn2+ substituted CdO nano-particles. Appl. Phys. A 2021, 127, 249. [Google Scholar]
- Kumar, K.C.; Rao, N.M.; Kaleemulla, S.; Rao, G.V. Structural, optical and magnetic properties of Sn doped ZnS nano powders prepared by solid state reaction. Phys. B Condens. Matter 2017, 522, 75–80. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Aversa, L.; Tatti, R.; Verucchi, R.; Sanson, A. Spectrophotometric method for optical band gap and electronic transitions determination of semiconductor materials. Opt. Mater. 2017, 64, 18–25. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, J.Y.; Lee, J.A.; Kim, J.S.; Lee, D.K.; Kim, K.; Kim, J.Y.; Kim, B.; Kim, H.; Kim, W.M.; et al. Completely transparent conducting oxide-free and flexible dye-sensitized solar cells fabricated on plastic substrates. ACS Nano 2015, 9, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Kang, H.C.; Yoo, K.; Asiam, F.K.; Lee, J.J.; Shim, J.W. Cosensitization of metal-based dyes for high-performance dye-sensitized photovoltaics under ambient lighting conditions. Dye. Pigment. 2021, 194, 109624. [Google Scholar] [CrossRef]
- Amarsingh Bhabu, K.; Kalpana Devi, A.; Theerthagiri, J.; Madhavan, J.; Balu, T.; Rajasekaran, T.R. Tungsten doped titanium dioxide as a photoanode for dye sensitized solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 3428–3439. [Google Scholar] [CrossRef]
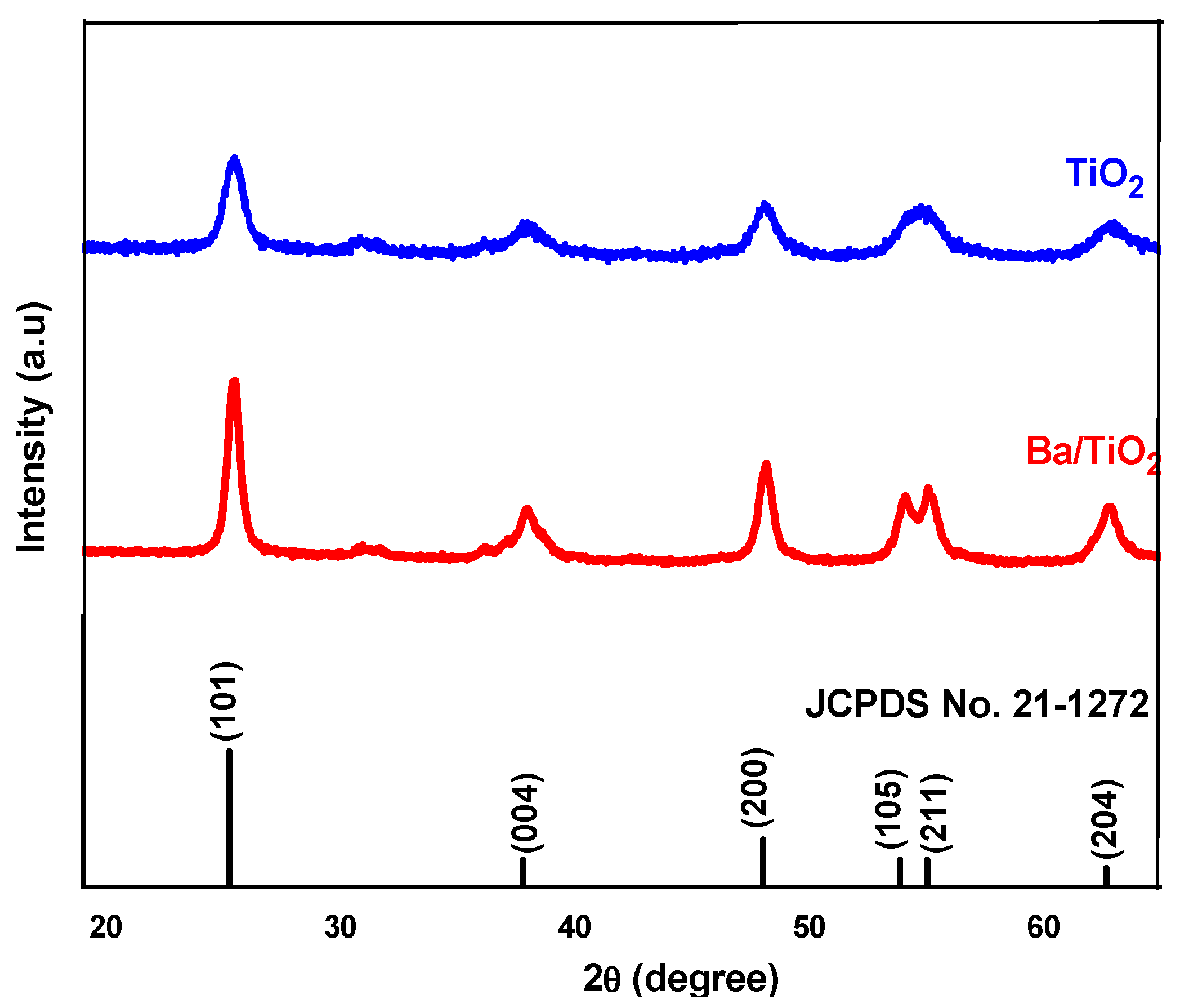


| Photoactive Material | FWHM (2θ) | d-Spacing (Å) | Crystallite Size(D) (nm) | Lattice Constant (Å) | Unit Cell Volume V (Å)3 | Density (ρ) (g/cm3) | Specific Surface Area (m2/g) | |
|---|---|---|---|---|---|---|---|---|
| a = b | C | |||||||
| TiO2 | 0.568 ± 0.026 | 3.494 | 25 | 3.778 ± 0.005 | 9.480 ± 0.012 | 135.31 | 3.9 | 61 |
| Ba/TiO2 | 0.876 ± 0.038 | 3.504 | 16 | 3.781 ± 0.002 | 9.470 ± 0.004 | 135.38 | 3.9 | 95 |
| Sample | Eg (eV) | n Coefficient |
|---|---|---|
| TiO2 | 3.21 | 1.72 ± 0.003 |
| Ba/TiO2 | 3.26 | 1.61 ± 0.003 |
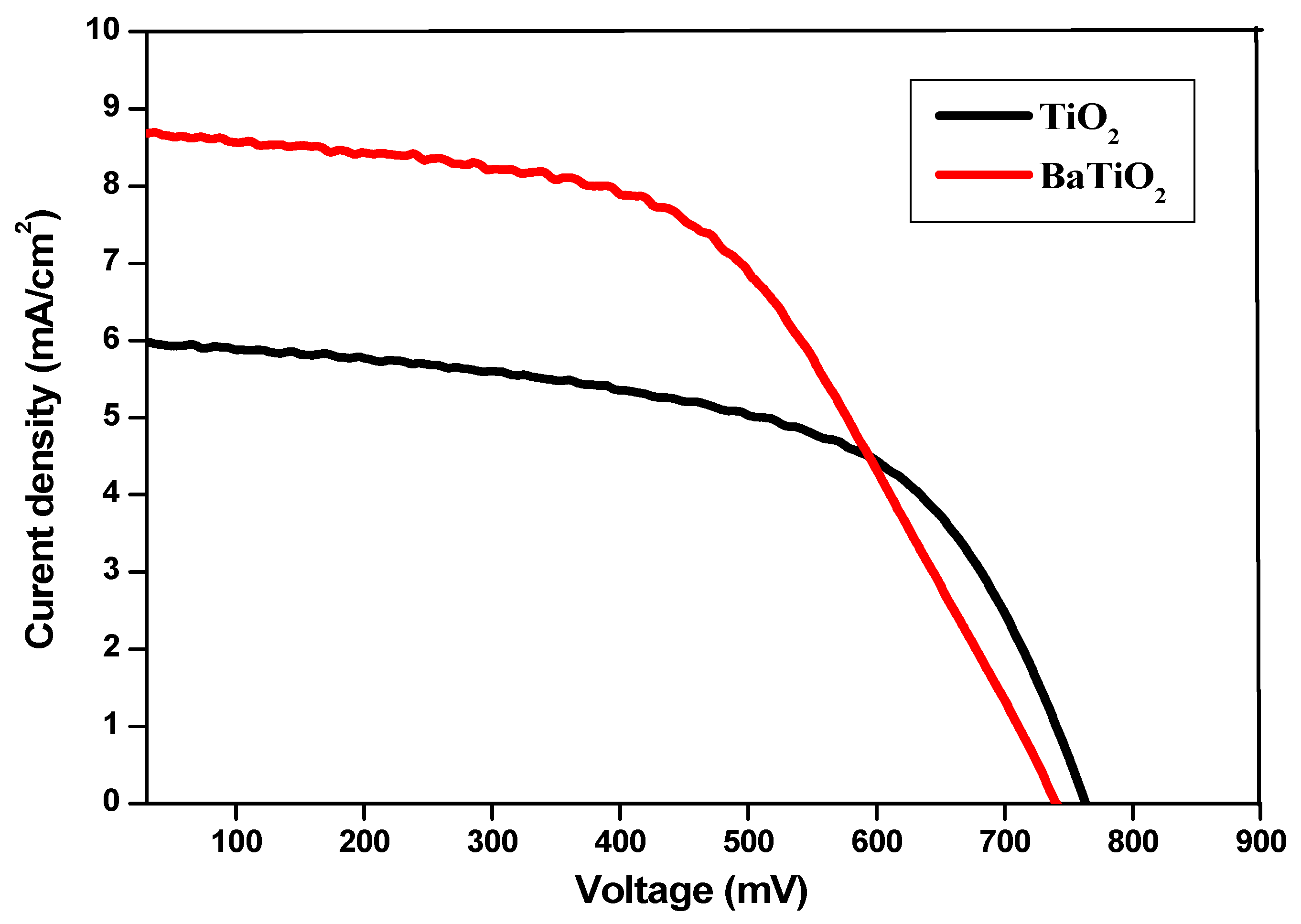
| Sample | Short Circuit Current Density Jsc (mA/cm2) | Open Circuit Voltage Voc (mV) | Fill Factor FF (%) | Efficiency (η) (%) | Ref |
|---|---|---|---|---|---|
| TiO2 | 5.96 | 761 | 48 | 2.1 | Present work |
| Ba/TiO2 | 8.70 | 740 | 53 | 3.4 | Present work |
| TiO2 | 2.53 | 650 | 63 | 1.03 | [57] |
| W-TiO2 | 4.77 | 730 | 71 | 2.47 | [57] |
| Fe-TiO2 | 1.52 | 680 | 0.463 | 0.47 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Khan, S.; Khan, M.; Luque, R.; Jalalah, M.; Alsaiari, M.A. Microwave Assisted Preparation of Barium Doped Titania (Ba/TiO2) as Photoanode in Dye Sensitized Solar Cells. Appl. Sci. 2022, 12, 9280. https://doi.org/10.3390/app12189280
Ahmad A, Khan S, Khan M, Luque R, Jalalah M, Alsaiari MA. Microwave Assisted Preparation of Barium Doped Titania (Ba/TiO2) as Photoanode in Dye Sensitized Solar Cells. Applied Sciences. 2022; 12(18):9280. https://doi.org/10.3390/app12189280
Chicago/Turabian StyleAhmad, Awais, Safia Khan, Mariam Khan, Rafael Luque, Mohammed Jalalah, and Mabkhoot A. Alsaiari. 2022. "Microwave Assisted Preparation of Barium Doped Titania (Ba/TiO2) as Photoanode in Dye Sensitized Solar Cells" Applied Sciences 12, no. 18: 9280. https://doi.org/10.3390/app12189280







