Removal and Recovery of Dissolved Oil from High-Salinity Wastewater Using Graphene–Iron Oxide Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil–Water Emulsion: Preparation and Characterization
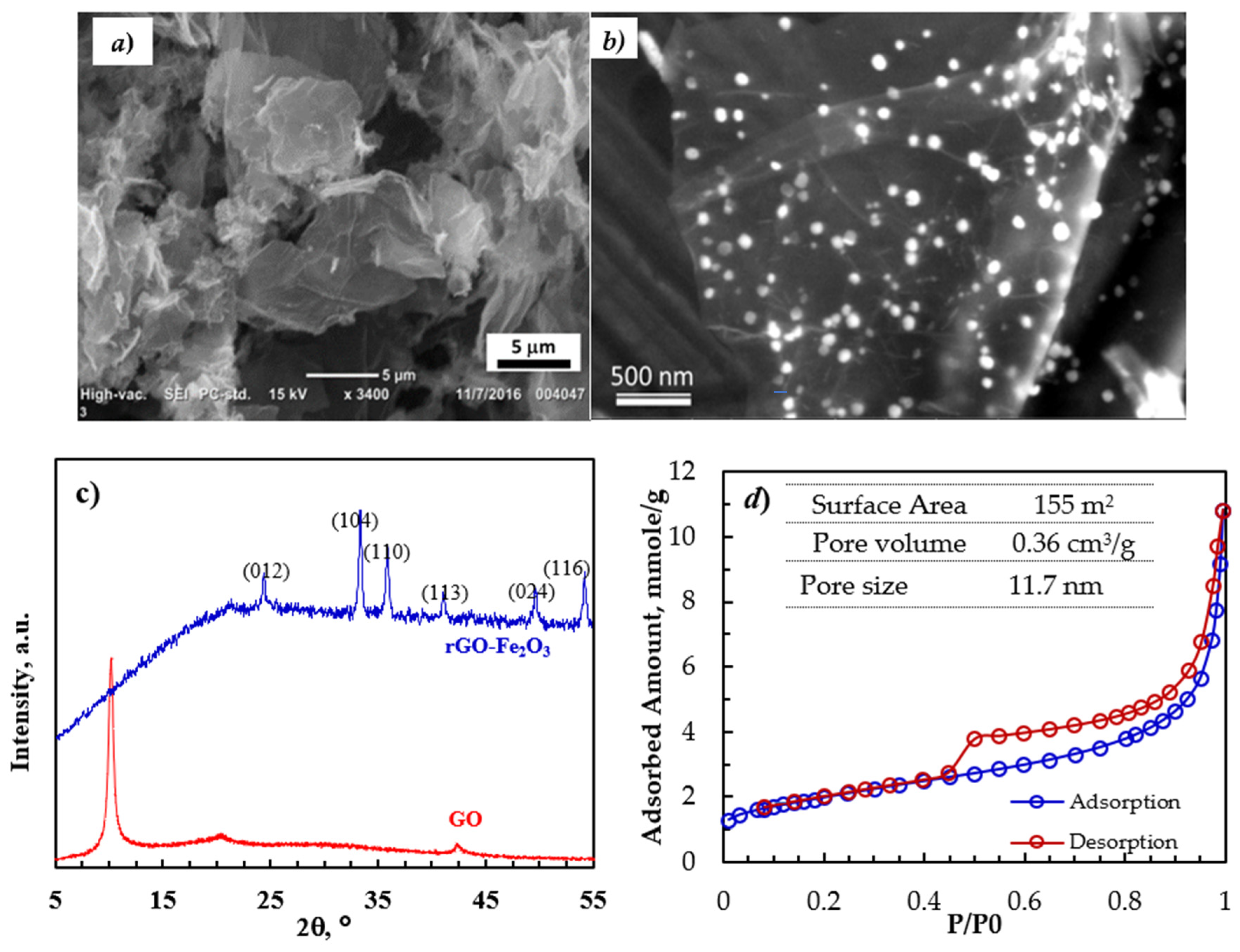
2.3. Characterization of rGO-Fe2O3
2.4. Batch Adsorption
2.5. Breakthrough/Column Experiments
2.6. Adsorption Isotherms
2.7. Adsorption Kinetics
3. Results
3.1. Adsorbent Characterization
3.2. Emulsion Characterization
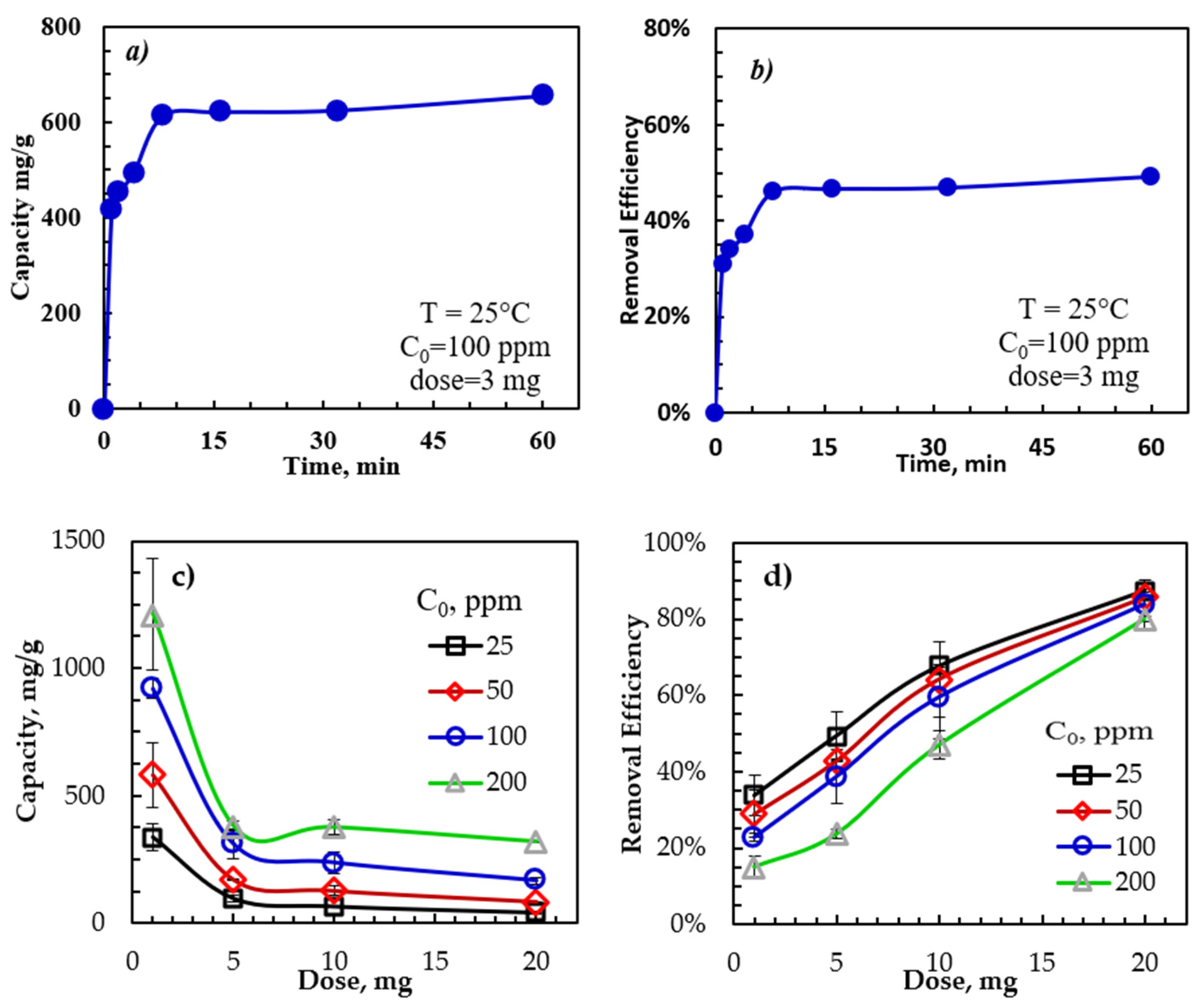
3.3. Effect of Adsorption Parameters
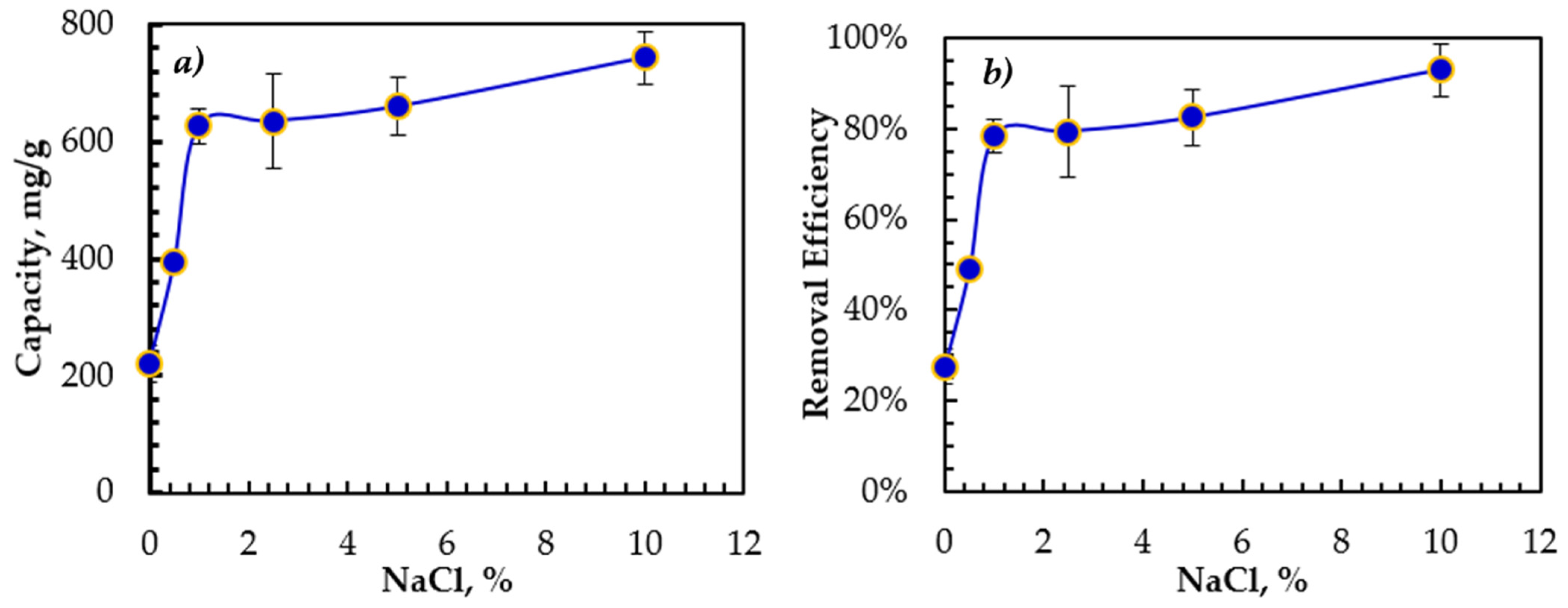
3.4. Effect of Salinity
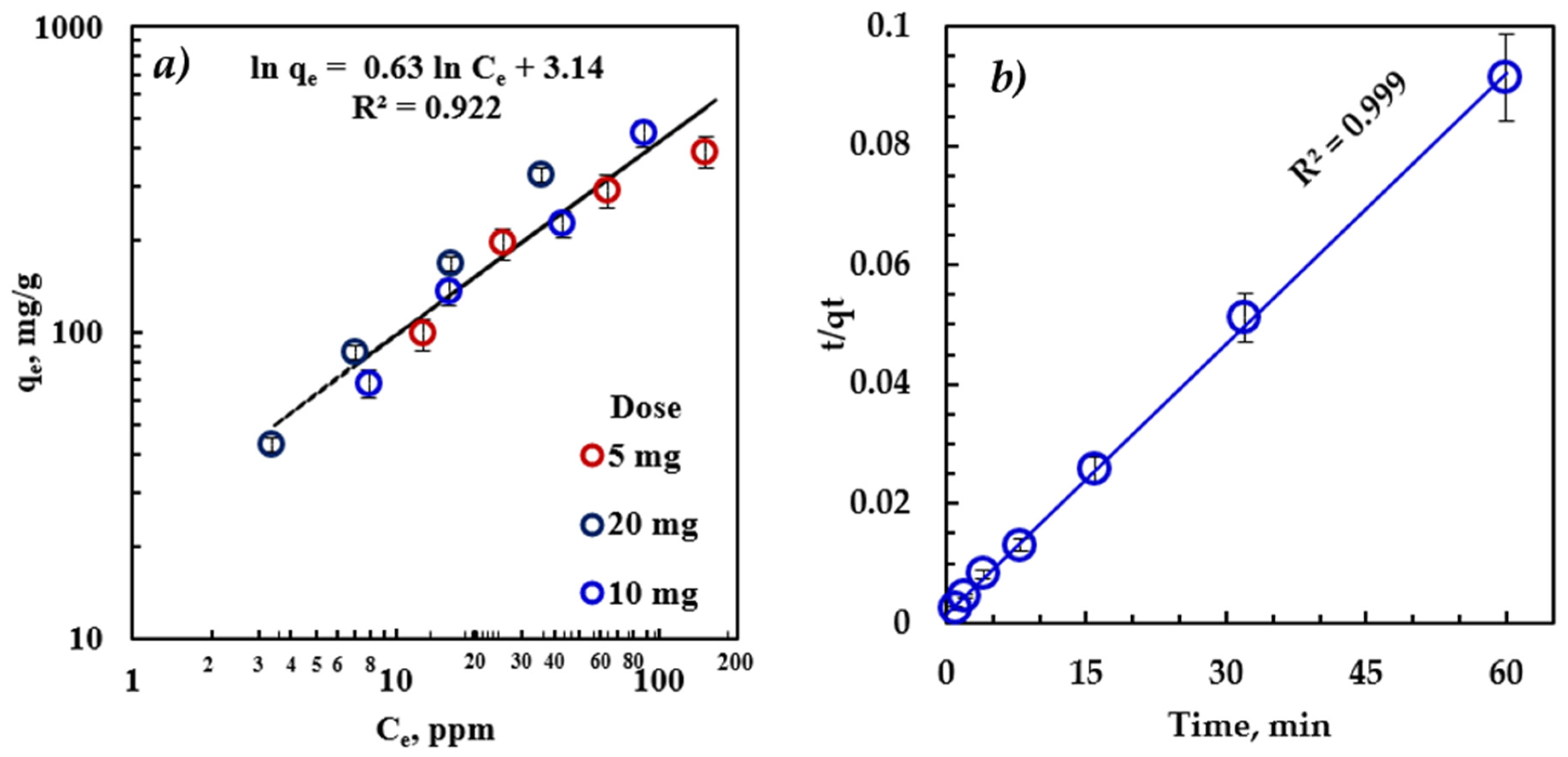
3.5. Adsorption Isotherm and Kinetics
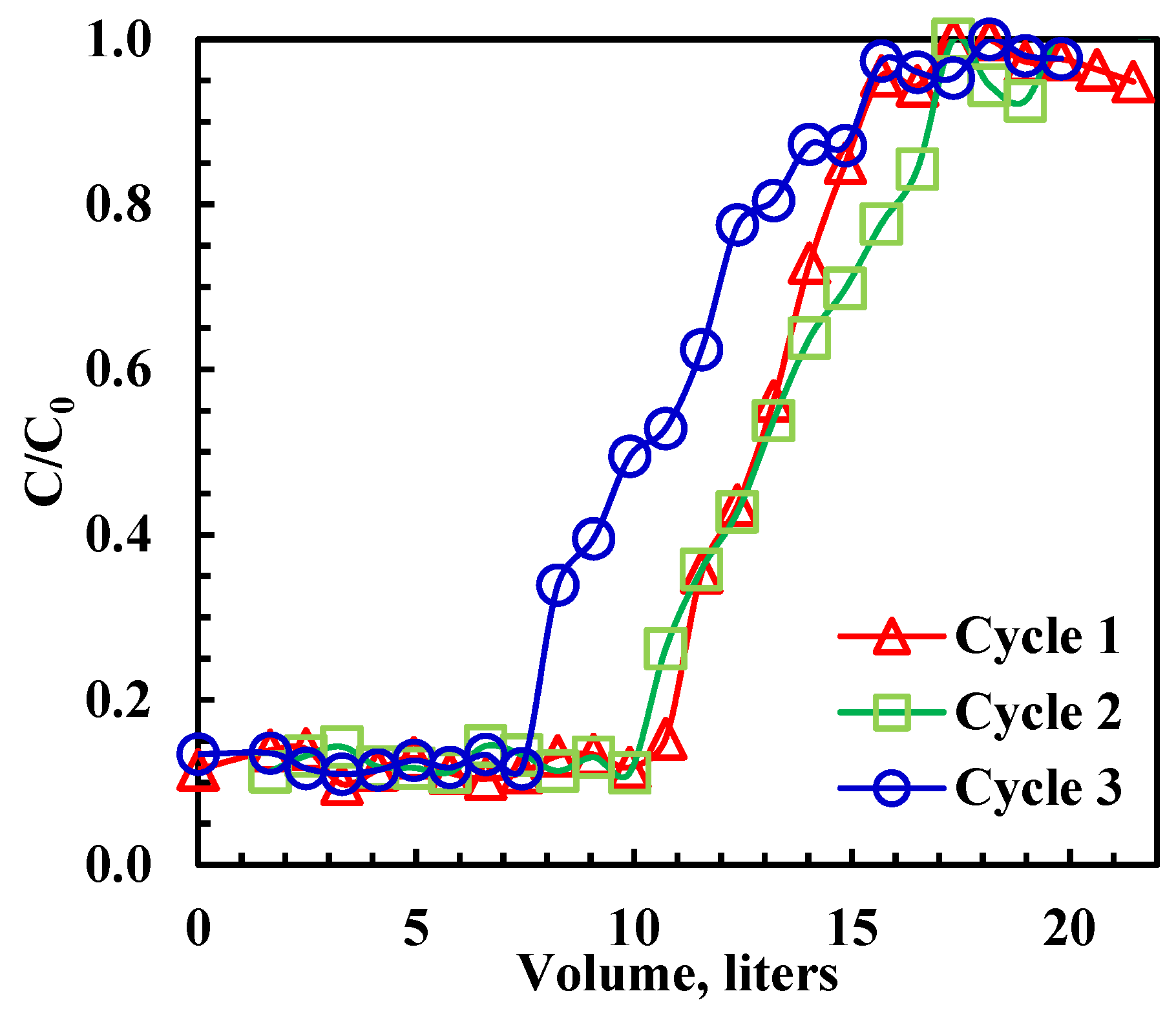
3.6. Column (Breakthrough) Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraus, R. Petroleo: Prospección y Perforación; Instituto Nacional de Seguridad e Higiene en el Trabajo: Madrid, Spain, 2012; pp. 1–16. [Google Scholar]
- Nasiri, M.; Jafari, I. Produced Water from Oil-Gas Plants: A Short Review on Challenges and Opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Veil, J.A.; Puder, M.G.; Elcock, D. A White Paper Describing Produced Water from Production of Crude Oil, Natural Gas, and Coal Bed Methane; Argonne National Laboratory: Chicago, IL, USA, 2004. [Google Scholar]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; All Consulting, LLC: Tulsa, OK, USA, 2005. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef]
- Rickerby, D.; Morrison, M. Nanotechnology and the environment: A European perspective. Sci. Technol. Adv. Mater. 2007, 8, 19. [Google Scholar] [CrossRef]
- Diraki, A.; Mackey, H.R.; McKay, G.; Abdala, A. Removal of emulsified and dissolved diesel oil from high salinity wastewater by adsorption onto graphene oxide. J. Environ. Chem. Eng. 2019, 7, 103106. [Google Scholar] [CrossRef]
- Diraki, A.; Mackey, H.; McKay, G.; Abdala, A.A. Removal of oil from oil–water emulsions using thermally reduced graphene and graphene nanoplatelets. Chem. Eng. Res. Des. 2018, 137, 47–59. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: Application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 2017, 34, 792–802. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.; Tadé, M. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Functional Oxide Nanomaterials and Nanocomposites for the Removal of Heavy Metals and Dyes. Nanomater. Nanotechnol. 2013, 3, 3–20. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Multiwalled carbon nanotubes for speciation of chromium in environmental samples. J. Hazard. Mater. 2007, 147, 219–225. [Google Scholar] [CrossRef]
- Song, H.; Hao, L.; Tian, Y.; Wan, X.; Zhang, L.; Lv, Y. Stable and Water-Dispersible Graphene Nanosheets: Sustainable Preparation, Functionalization, and High-Performance Adsorbents for Pb2+. ChemPlusChem 2012, 77, 379. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.; Kim, H.J.; Jung, D.H. A Comprehensive Review of Graphene Nanocomposites: Research Status and Trends. J. Nanomater. 2013, 2013, 158. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Vasudevan, S.; Shibayama, A.; Yamada, M. Graphene and Graphene-Based Composites: A Rising Star in Water Purification—A Comprehensive Overview. ChemistrySelect 2016, 1, 4358–4385. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, characterization, and adsorption properties of magnetic Fe3O4@ graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.D.M.; Muñoz, G.A.; Arboleda-Faini, X.; Streitwieser, D.A. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption separation of condensate oil from produced water using ACTF prepared of oil palm leaves by batch and fixed bed techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Lei, G.; Mao, P.; He, M.; Wang, L.; Liu, X.; Zhang, A. Combination of column adsorption and supercritical fluid extraction for recovery of dissolved essential oil from distillation waste water ofYulania liliiflora. J. Chem. Technol. Biotechnol. 2016, 91, 1896–1904. [Google Scholar] [CrossRef]
- Meng, L.; Bao, M.; Sun, P. A new perspective of particle adsorption: Dispersed oil and granular materials interactions in simulated coastal environment. Mar. Pollut. Bull. 2017, 122, 100–109. [Google Scholar] [CrossRef]
- Cheu, S.C.; Kong, H.; Song, S.T.; Saman, N.; Johari, K.; Mat, H. High removal performance of dissolved oil from aqueous solution by sorption using fatty acid esterified pineapple leaves as novel sorbents. RSC Adv. 2016, 6, 13710–13722. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Q.; Bian, J. Preparation of a recyclable demulsifier for the treatment of emulsified oil wastewater by chitosan modification and sodium oleate grafting Fe3O4. J. Environ. Chem. Eng. 2021, 9, 105663. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Novel and recyclable demulsifier of expanded perlite grafted by magnetic nanoparticles for oil separation from emulsified oil wastewaters. Chem. Eng. J. 2018, 337, 10–18. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Wu, Y. Adsorption of toluene, ethylbenzene and m-xylene on multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. J. Hazard. Mater. 2011, 192, 1370–1379. [Google Scholar] [CrossRef]
- Javadian, S.; Khalilifard, M.; Sadrpoor, S.M. Functionalized graphene oxide with core-shell of Fe3O4@oliec acid nanospheres as a recyclable demulsifier for effective removal of emulsified oil from oily wastewater. J. Water Process Eng. 2019, 32, 100961. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Huang, P. Graphene-based nanomaterials in bioimaging. Biomed. Appl. Funct. Nanomater. 2018, 247–287. [Google Scholar] [CrossRef]
- Al-Maas, M.; Minier-Matar, J.; Krupa, I.; Al-Maadeed, M.A.A.; Adham, S. Evaluation of polymeric adsorbents via fixed-bed columns for emulsified oil removal from industrial wastewater. J. Water Process Eng. 2022, 49, 102962. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef]
- Gao, R.; Li, F.; Li, Y.; Wu, T. Effective removal of emulsified oil from oily wastewater using in situ generated metallic hydroxides from leaching solution of white mud. Chem. Eng. J. 2017, 309, 513–521. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Sun, D.; Li, Y.; Wu, T. Effective removal of emulsified oil from oily wastewater using surfactant-modified sepiolite. Appl. Clay Sci. 2018, 157, 227–236. [Google Scholar] [CrossRef]
- Waisi, B.I.; Arena, J.T.; Benes, N.E.; Nijmeijer, A.; McCutcheon, J.R. Activated carbon nanofiber nonwoven for removal of emulsified oil from water. Microporous Mesoporous Mater. 2020, 296, 109966. [Google Scholar] [CrossRef]
- Ma, J.; Fu, X.; Xia, W.; Zhang, R.; Fu, K.; Wu, G.; Jia, B.; Li, S.; Li, J. Removal of emulsified oil from water by using recyclable chitosan based covalently bonded composite magnetic flocculant: Performance and mechanism. J. Hazard. Mater. 2021, 419, 126529. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Tang, Q.; Wang, P.; Xu, Z.; Liang, J. Utilization of ultra-light carbon foams for the purification of emulsified oil wastewater and their adsorption kinetics. Chem. Phys. 2019, 516, 139–146. [Google Scholar] [CrossRef]
- Yang, M. Measurement of oil in produced water. In Produced Water; Springer: New York, NY, USA, 2011; pp. 57–88. [Google Scholar]
- Gulistan, A.S. Oil Removal from Produced Water Using Natural Materials. Ph.D. Thesis, American University of Sharjah, Sharjah, United Arab Emirates, 2014. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Al-Meshragi, M.; Ibrahim, H.G.; Aboabboud, M.M. Equilibrium and kinetics of chromium adsorption on cement kiln dust. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2008. [Google Scholar]
- Karhu, M.; Leiviskä, T.; Tanskanen, J. Enhanced DAF in breaking up oil-in-water emulsions. Sep. Purif. Technol. 2014, 122, 231–241. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic Graphene Oxide: Effect of Preparation Route on Reactive Black 5 Adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Adsorption characteristics of industrial solid waste derived activated carbon prepared by microwave heating for methylene blue. Fuel Process. Technol. 2012, 99, 103–109. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Rashid, A.K.; Khalid, K. Linear regression analysis for kinetics and isotherm studies of sorption of manganese (II) ions onto activated palm ash from wastewater. Orient. J. Chem. 2011, 27, 405–415. [Google Scholar]
- Frost, J. Regression Analysis: How Do I Interpret R-Squared and Assess the Goodness-of-Fit; The Minitab Blog: State College, PA, USA, 2013; Volume 30. [Google Scholar]
- Muzic, M.; Sertic-Bionda, K.; Gomzi, Z.; Podolski, S.; Telen, S. Study of diesel fuel desulfurization by adsorption. Chem. Eng. Res. Des. 2010, 88, 487–495. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Banat, F.; Al-Lagtah, N. Separation of Ethanol–Water Mixtures Using Molecular Sieves and Biobased Adsorbents. Chem. Eng. Res. Des. 2004, 82, 855–864. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
| Adsorbent | Oil Type | Oil Conc., ppm | Adsorption Capacity, mg/g | Ref. |
|---|---|---|---|---|
| Chitosan–sodium oleate-grafted Fe3O4 | Diesel | 10,000 | 88% removal | [29] |
| expanded perlite-grafted APTES-Fe3O4 | Crude oil | 3000 | 90% removal | [30] |
| Multi-wall carbon nanotubes | m-xylene | 80 | 40 | [31] |
| GO core–shell Fe3O4@oliec acid | Crude oil | 30,000 | 47 | [32] |
| Multi-wall carbon nanotubes | Toluene | 110 | 80 | [31] |
| Multi-wall carbon nanotubes | Ethylbenzene | 80 | 80 | [31] |
| Bentonite granules | Motor oil | 15,000 | 176 | [33] |
| Sepiolite granules | Motor oil | 15,000 | 184 | [33] |
| Zeolite fine powder | Motor oil | 15,000 | 192 | [33] |
| Activated carbon from cork | Sunflower oil | 150 | 233 | [34] |
| Polymeric adsorbent beads | Crude oil | 30 | 301 | [35] |
| Activated carbon | Oilfield PW | 165 | 334 | [36] |
| Metallic hydroxides from white mud leaching | Crude oil | 1500 | 356 | [37] |
| Bentonite | Oilfield PW | 55 | 378 | [36] |
| Deposited carbon | Oilfield PW | 35 | 386 | [36] |
| Surfactant-modified sepiolite | Crude oil | 3000 | 455 | [38] |
| Graphene aerogel | Diesel–water | 800 | 745 | [34] |
| Graphene nanoplatelets | Diesel | 200 | 805 | [10] |
| Activated carbon nanofiber nonwoven | Crude oil | 10,000 | 1250 | [39] |
| Graphene oxide | Diesel | 200 | 1335 | [9] |
| Thermally reduced graphene | Diesel | 200 | 1554 | [10] |
| Fluorinated graphene | Crude oil | 1000 | 1566 | [40] |
| Ultralight carbon foam | Crude oil | 2020 | 1627 | [41] |
| rGO-Fe2O3 | Diesel | 200 | 1280 | This Study |
| Emulsion Concentration, ppm | ||||
|---|---|---|---|---|
| 25 | 50 | 100 | 200 | |
| Zeta Potential (mV) | −29.2 | −27.4 | −40.6 | −34.4 |
| Droplet size, Population 1 (nm) | 50 | 91 | 142 | 106 |
| Droplet size, Population 2 (nm) | 142 | 295 | 4800 | 712 |
| Parameter | rGO-Fe2O3 |
|---|---|
| n | 1.6 |
| kf | 23 |
| R2 | 0.922 |
| qf | 1301 |
| Cycle (n) | rGO-Fe2O3-NC | GO [9] | ||
|---|---|---|---|---|
| qe,n (mg/g) | qe,n/qe,1 (%) | qe,n (mg/g) | qe,n/qe,1 (%) | |
| 1 | 1279 | 100% | 1140 | 100% |
| 2 | 1208 | 94% | 1138 | 99.8% |
| 3 | 1002 | 83% | 904 | 79% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diraki, A.; Mackey, H.R.; McKay, G.; Abdala, A. Removal and Recovery of Dissolved Oil from High-Salinity Wastewater Using Graphene–Iron Oxide Nanocomposites. Appl. Sci. 2022, 12, 9414. https://doi.org/10.3390/app12199414
Diraki A, Mackey HR, McKay G, Abdala A. Removal and Recovery of Dissolved Oil from High-Salinity Wastewater Using Graphene–Iron Oxide Nanocomposites. Applied Sciences. 2022; 12(19):9414. https://doi.org/10.3390/app12199414
Chicago/Turabian StyleDiraki, Ahmad, Hamish R. Mackey, Gordon McKay, and Ahmed Abdala. 2022. "Removal and Recovery of Dissolved Oil from High-Salinity Wastewater Using Graphene–Iron Oxide Nanocomposites" Applied Sciences 12, no. 19: 9414. https://doi.org/10.3390/app12199414
APA StyleDiraki, A., Mackey, H. R., McKay, G., & Abdala, A. (2022). Removal and Recovery of Dissolved Oil from High-Salinity Wastewater Using Graphene–Iron Oxide Nanocomposites. Applied Sciences, 12(19), 9414. https://doi.org/10.3390/app12199414








