Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies
Abstract
1. Introduction
2. Polymeric Nanoparticles—From Shape to Application
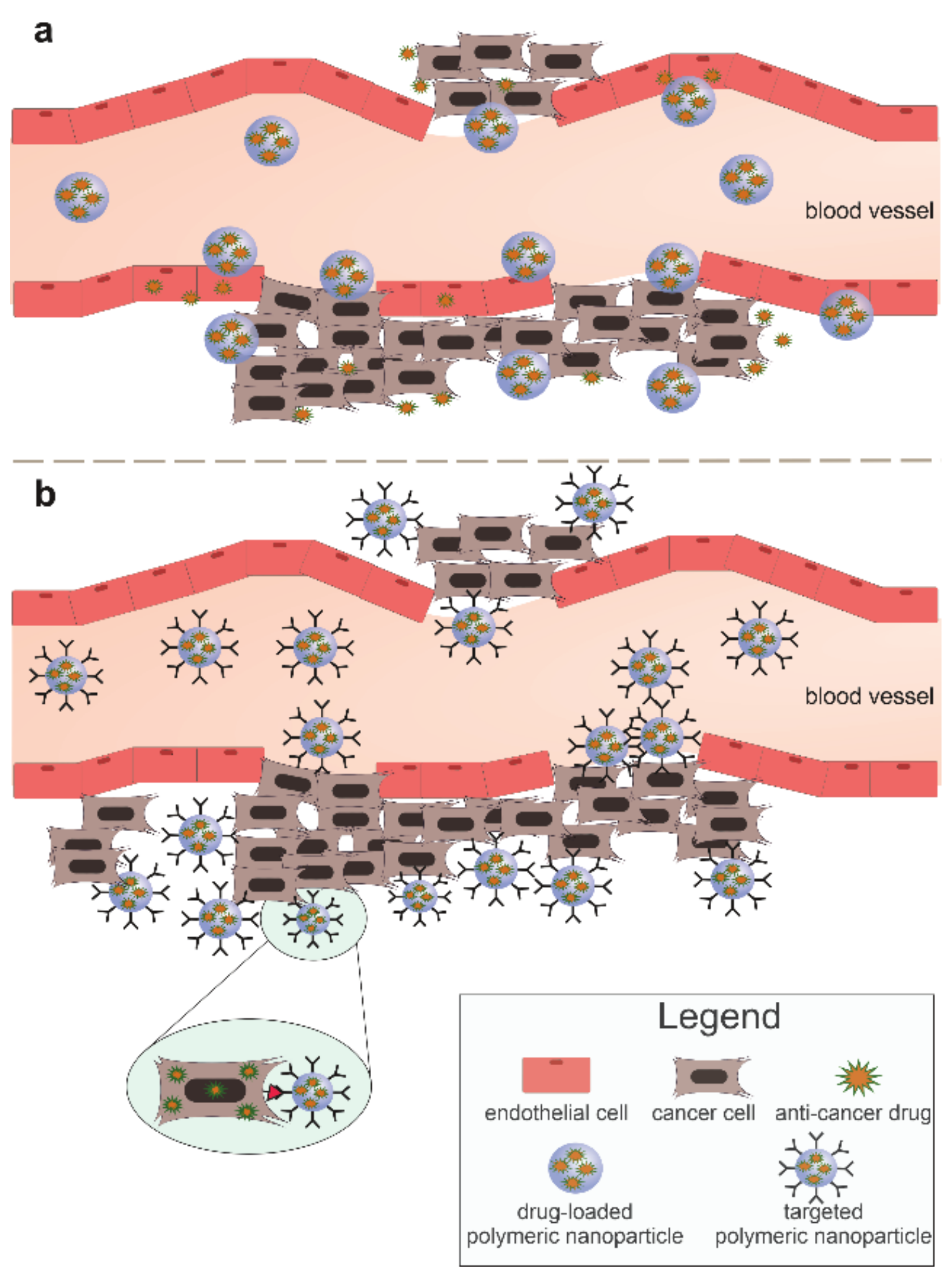
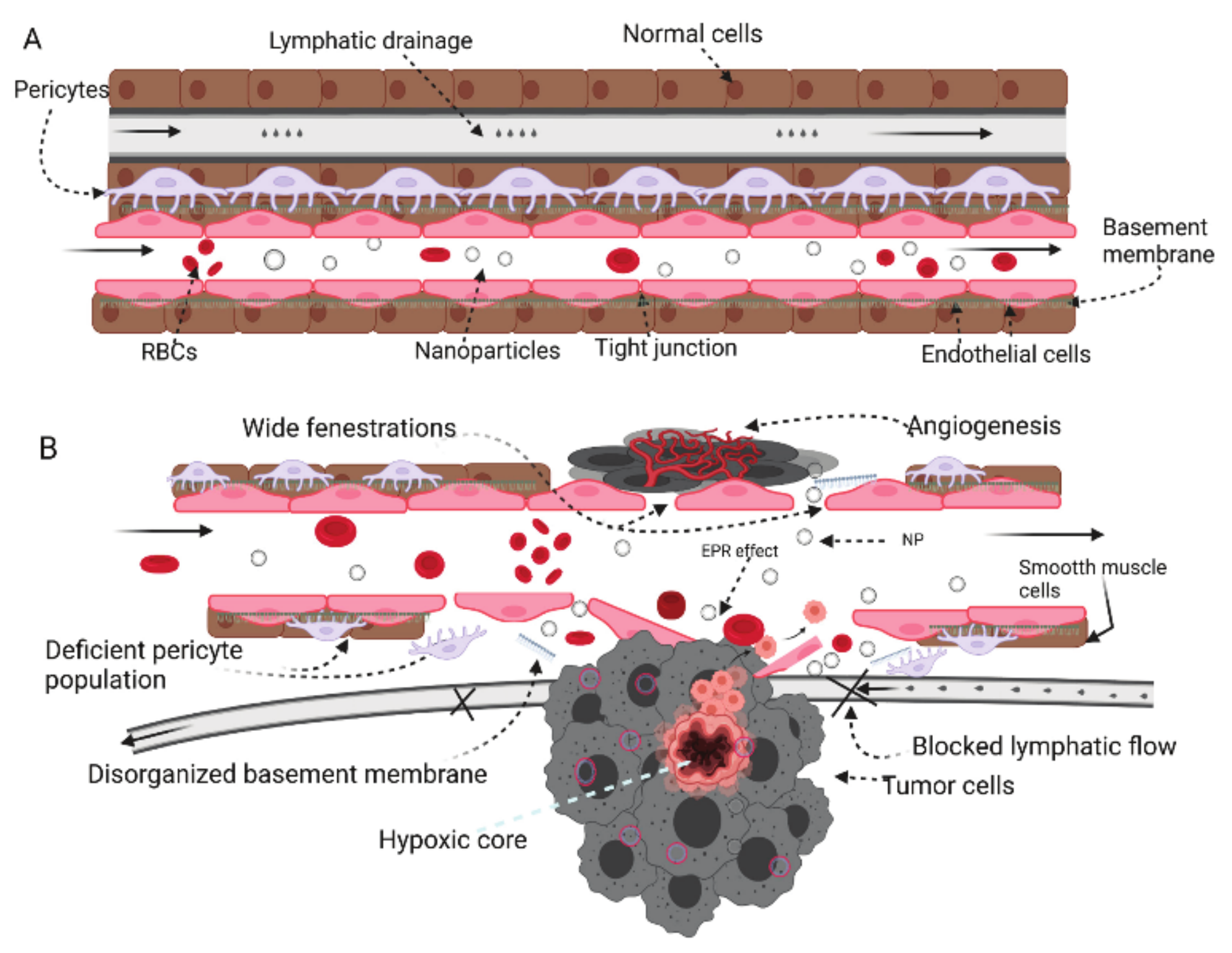
3. Nanoparticles in Cancer Therapy—Passive and Active Mechanisms
| Methods of Formulation | Description | Advantages | Disadvantages | Example | Cancer | Ref. |
|---|---|---|---|---|---|---|
| Nanoprecipitation | Use of two mixing solutions, which results in the displacement of solvent and precipitation of nanoparticles | Single step Formulation of nanospheres and nanocapsules (~200 nm) | Only hydrophobic drugs can be encapsulated Solvent residues | BLC-PTX-loaded d,l-PLGA NPs | Human lung cancer lines | [7,24,25,26,27] |
| Solvent evaporation | PNPs are obtained by evaporation of the solvent from the polymer followed by diffusion through the continuous phase. Single or double emulsion can be carried out | Formulates nanospheres Simplicity | Requires homogenizer and heating Residual solvent may remain For lipid-dissolved drugs | DTX-loaded FA/d,l-PLGA NPs | Breast cancer lines | [3,7,11,24,25,26] |
| Salting out | Water with salt is rapidly added to a polymer solution with a drug and a water-soluble solvent, leading to the diffusion of the solvent and the formation of nanoparticles | For heat-sensitive drugs No heating required Encapsulates nucleic acid and protein molecules | Only for lipophobic drugs Time-consuming Stabilizer removal required | Meloxicam-loaded d,l-PLGA NPs | Human colorectal cancer lines | [7,19,26,28] |
| Dialysis | Uses a dialysis tube inside which a polymer dissolved in a solvent. Suspension of nanoparticles results in displacement of the solvent | Simplicity Easy manipulation of nanoparticle size | Time-consuming Does not require advanced equipment | DTX-loaded poly (N-vinyl- caprolactam) chitosan NPs | TNBC (in vivo) | [3,11,20] |
| Supercritical fluid technology | In this method, supercritical liquid is used and is based on two phenomena: the rapid expansion of the supercritical solution and the rapid expansion of the supercritical solution into the solvent | High purity of nanoparticles Environmentally friendly Possibility of formulating very small sizes (<20 nm) | Technique rarely used Limited solubility of compounds in supercritical fluid | CXB-loaded d,l-PLGA NPs | Metastatic cancers | [11,29,30] |
3.1. Passive Mechanism
3.2. Active Mechanism
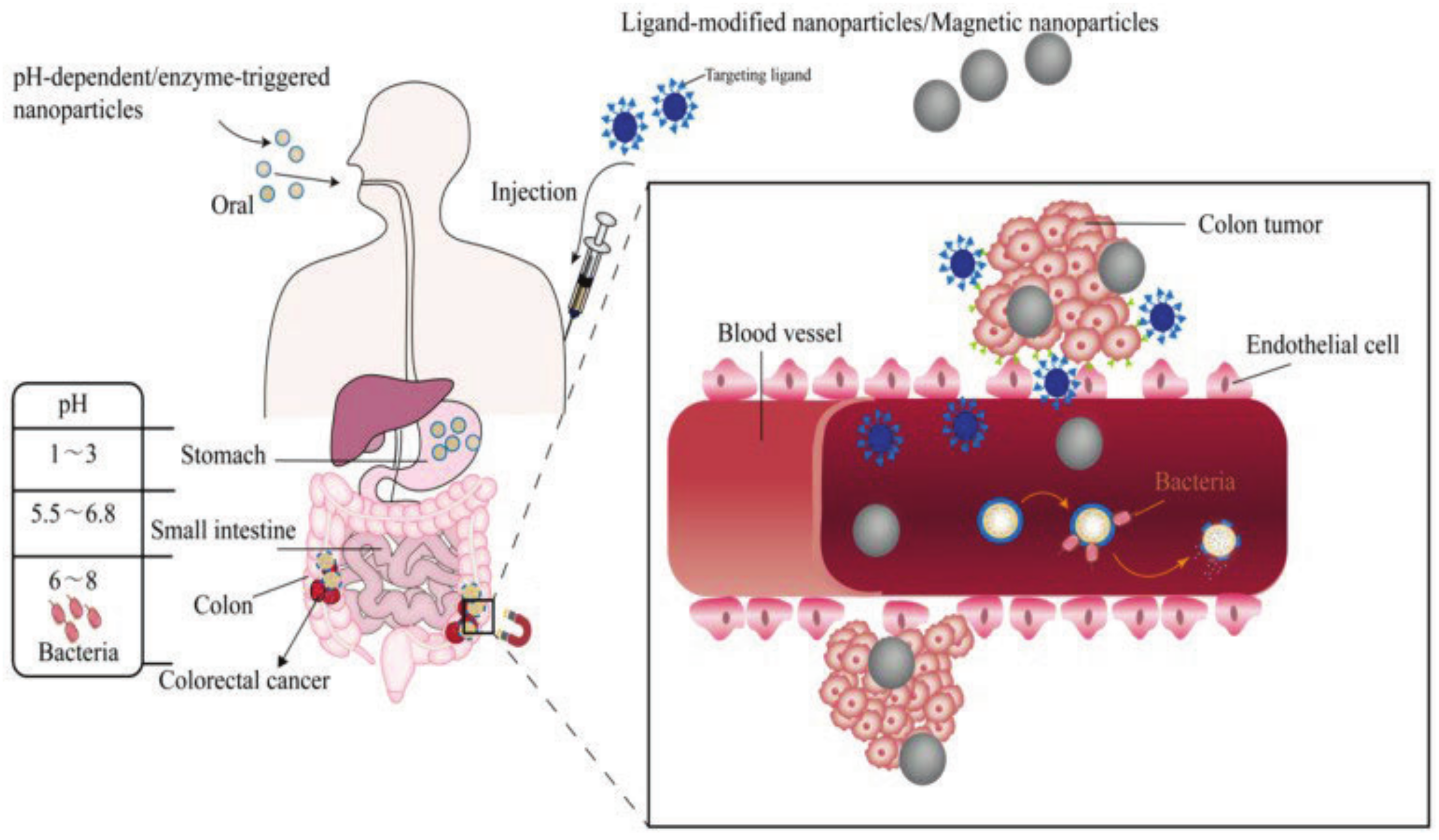
4. Polymeric Nanoparticles in Colorectal Cancer Treatment
5. Polymeric Nanoparticles in Breast and Ovarian Therapy
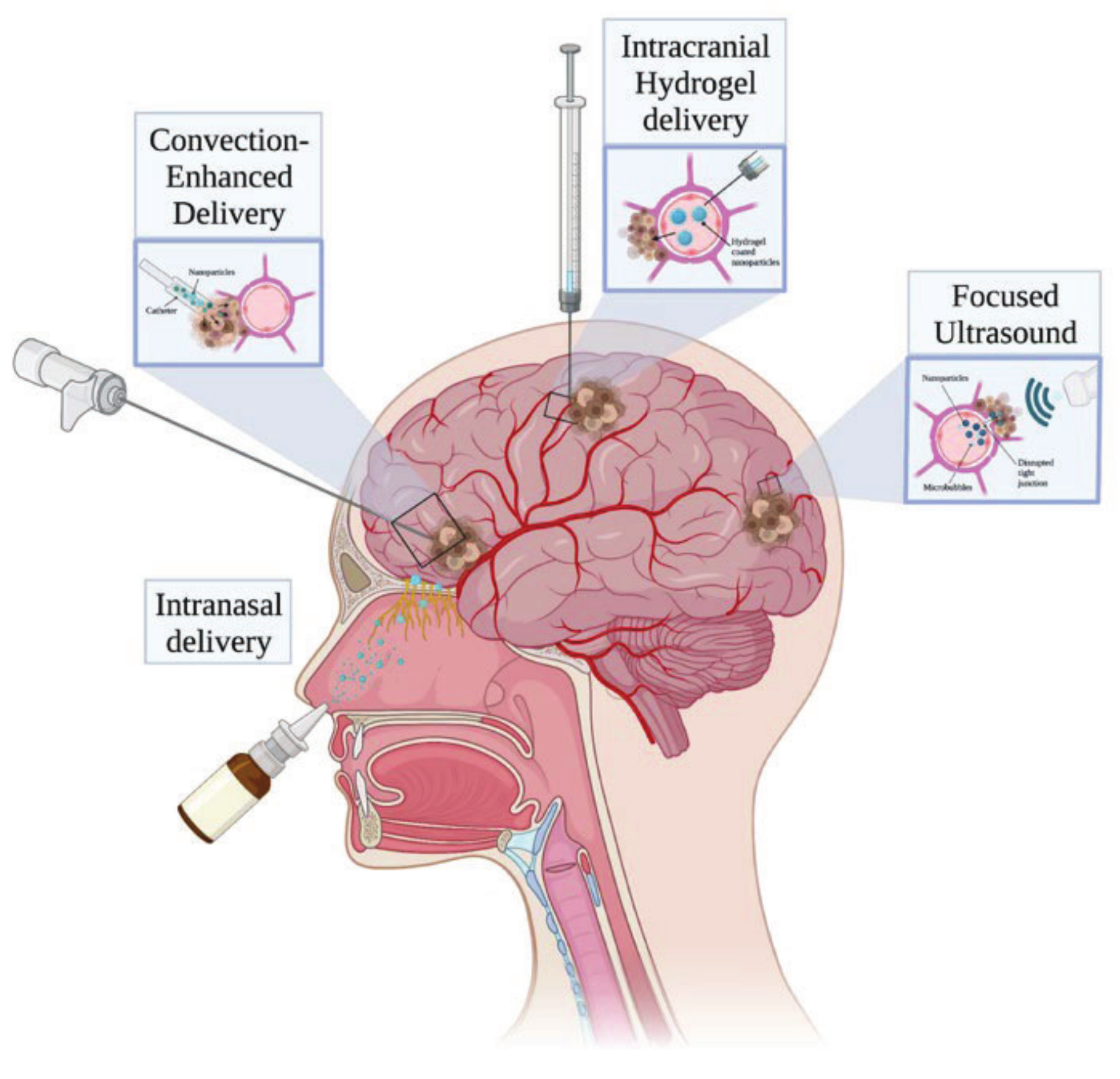
6. Polymeric Nanoparticles in Glioblastoma Multiforme Therapy
7. PNPs as a Drug Delivery System in Cancer-Associated Gene Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Li, Q.; Mo, J.; Dai, H. Drug-loaded polymeric nanoparticles for cancer stem cell targeting. Front. Pharmacol. 2017, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Novel tumor-targeting nanoparticles for cancer treatment-a review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Sartaj, A.; Qamar, Z.; Qizilbash, F.F.; Annu, M.S.; Alhakamy, N.A.; Baboota, S.; Ali, J. Polymeric nanoparticles: Exploring the current drug development and therapeutic insight of breast cancer treatment and recommendations. Polymers. 2021, 13, 4400. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging trends in colorectal cancer: Dysregulated signaling pathways (review). Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.S.; AlAmri, A.H.; McConville, C. Polymeric nanoparticles for the treatment of malignant gliomas. Cancers 2020, 12, 175. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Nie, S.C.; Xu, Z.Y.; Fan, Y.R.; Jiao, M.N.; Miao, H.J.; Liang, S.X.; Yan, Y.B. Advanced polymeric nanoagents for oral cancer theranostics: A mini review. Front. Chem. 2022, 10, 927595. [Google Scholar] [CrossRef]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.A.; Sudesh, K. Exploring various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Cruz-Nova, P.; Ancira-Cortez, A.; Ferro-Flores, G.; Ocampo-García, B.; Gibbens-Bandala, B. Controlled-release nanosystems with a dual function of targeted therapy and radiotherapy in colorectal cancer. Pharmaceutics 2022, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Jiang, X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 2006, 310, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Ocaña, A.; Castro-Osma, J.A.; Bravo, I.; Alonso-Moreno, C. Polyester polymeric nanoparticles as platforms in the development of novel nanomedicines for cancer treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Solanki, P.; Mitra, S. Curcuminoid-loaded poly(methyl methacrylate) nanoparticles for cancer therapy. Int. J. Nanomed. 2018, 13, 101–105. [Google Scholar] [CrossRef]
- Chen, T.H.; Bae, Y.; Furgeson, D.Y. Intelligent biosynthetic nanobiomaterials (IBNs) for hyperthermic gene delivery. Pharm. Res. 2008, 25, 683–691. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for delivery of pentacyclic triterpenoids in anticancer therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Neerooa, B.N.H.M.; Ooi, L.T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy: An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Gad, A.; Kydd, J.; Piel, B.; Rai, P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int J Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Şengel-Türk, C.T.; Hasçiçek, C.; Dogan, A.L.; Esendagli, G.; Guc, D.; Gönül, N. Preparation and in vitro evaluation of meloxicam-loaded PLGA nanoparticles on HT-29 human colon adenocarcinoma cells. Drug Dev. Ind. Pharm. 2012, 38, 1107–1116. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]
- Margulis, K.; Neofytou, E.A.; Beygui, R.E.; Zare, R.N. Celecoxib nanoparticles for therapeutic angiogenesis. ACS Nano 2015, 9, 9416–9426. [Google Scholar] [CrossRef]
- Kim, S.J.; Khadka, D.; Seo, J.H. Interplay between solid tumors and tumor microenvironment. Front. Immunol. 2022, 13, 882718. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New challenges in the use of nanomedicine in cancer therapy. Bioengineered 2022, 13, 759–773. [Google Scholar] [CrossRef]
- Naki, T.; Aderibigbe, B.A. Efficacy of polymer-based nanomedicine for the treatment of brain cancer. Pharmaceutics 2022, 14, 1048. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, M.; Zhang, J.; Lu, W. Preparation and cellular targeting study of VEGF-conjugated PLGA nanoparticles. J. Microencapsul. 2015, 32, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular mechanisms of colon cancer progression and metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Al-Joufi, F.A.; Setia, A.; Salem-Bekhit, M.M.; Sahu, R.K.; Alqahtani, F.Y.; Widyowati, R.; Aleanizy, F.S. Molecular pathogenesis of colorectal cancer with an emphasis on recent advances in biomarkers, as well as nanotechnology-based diagnostic and therapeutic approaches. Nanomaterials 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Reichmann, R.; Kaaks, R.; Jenab, M.; Bueno-de-Mesquita, H.B.; Dahm, C.C.; Eriksen, A.K.; Tjønneland, A.; Artaud, F.; Boutron-Ruault, M.C.; et al. Development and validation of a lifestyle-based model for colorectal cancer risk prediction: The LiFeCRC score. BMC Med. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, R.; Meng, T.; Hu, F.; Yuan, H. Nano-drug delivery systems based on different targeting mechanisms in the targeted therapy of colorectal cancer. Molecules 2022, 27, 2981. [Google Scholar] [CrossRef] [PubMed]
- Ying, K.; Bai, B.; Gao, X.; Xu, Y.; Wang, H.; Xie, B. Orally administrable therapeutic nanoparticles for the treatment of colorectal cancer. Front. Bioeng. Biotechnol. 2021, 9, 670124. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Dorkoosh, F.A. PHBV/PLGA nanoparticles for enhanced delivery of 5-fluorouracil as promising treatment of colon cancer. Pharm. Dev. Technol. 2020, 25, 206–218. [Google Scholar] [CrossRef]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-fluorouracil and chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef]
- Alshaman, R.; Alattar, A.; El-Sayed, R.M.; Gardouh, A.R.; Elshaer, R.E.; Elkazaz, A.Y.; Eladl, M.A.; El-Sherbiny, M.; Farag, N.E.; Hamdan, A.M.; et al. Formulation and characterization of doxycycline-loaded polymeric nanoparticles for testing antitumor/antiangiogenic action in experimental colon cancer in mice. Nanomaterials 2022, 12, 857. [Google Scholar] [CrossRef]
- Akl, M.A.; Kartal-Hodzic, A.; Oksanen, T.; Ismael, H.R.; Afouna, M.M.; Yliperttula, M.; Samy, A.M.; Viitala, T. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016, 32, 10–20. [Google Scholar] [CrossRef]
- Essa, S.; Daoud, J.; Lafleur, M.; Martel, S.; Tabrizian, M. SN-38 active loading in poly(lactic-co-glycolic acid) nanoparticles and assessment of their anticancer properties on COLO-205 human colon adenocarcinoma cells. J. Microencapsul. 2015, 32, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F. Development of a novel nanoformulation against the colorectal cancer. Life Sci. 2021, 281, 119772. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Fabrication and characterization of chitosan-based polymeric nanoparticles of imatinib for colorectal cancer targeting application. Int. J. Biol. Macromol. 2020, 151, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.C.S.L.; Zerillo, L.; Cruz, L.J.; Schomann, T.; Chan, A.B.; de Carvalho, T.G.; Souza, S.V.P.; Araújo, A.A.; de Geus-Oei, L.F.; de Araújo Júnior, R.F. Maximizing the potency of oxaliplatin coated nanoparticles with folic acid for modulating tumor progression in colorectal cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111678. [Google Scholar] [CrossRef]
- Colpan, R.D.; Erdemir, A. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. 2021, 38, 381–393. [Google Scholar] [CrossRef]
- Baião, A.; Sousa, F.; Oliveira, A.V.; Oliveira, C.; Sarmento, B. Effective intracellular delivery of bevacizumab via PEGylated polymeric nanoparticles targeting the CD44v6 receptor in colon cancer cells. Biomater. Sci. 2020, 8, 3720–3729. [Google Scholar] [CrossRef]
- Zu, M.; Ma, L.; Zhang, X.; Xie, D.; Kang, Y.; Xiao, B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy. Colloids. Surf. B Biointerfaces. 2019, 177, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Silva, A.M.; de Caland, L.B.; do Nascimento, E.G.; Oliveira, A.L.C.S.L.; de Araújo-Júnior, R.F.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Self-assembled benznidazole-loaded cationic nanoparticles containing cholesterol/sialic acid: Physicochemical properties, in vitro drug release and in vitro anticancer efficacy. Int. J. Mol. Sci. 2019, 20, 2350. [Google Scholar] [CrossRef] [PubMed]
- Phung, C.D.; Le, T.G.; Nguyen, V.H.; Vu, T.T.; Nguyen, H.Q.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. PEGylated-paclitaxel and dihydroartemisinin nanoparticles for simultaneously delivering paclitaxel and dihydroartemisinin to colorectal cancer. Pharm. Res. 2020, 37, 129. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, L.; Liu, C. A convergent synthetic platform for polymeric nanoparticle for the treatment of combination colorectal cancer therapy. J. Biomater. Sci. Polym. Ed. 2021, 32, 1835–1848. [Google Scholar] [CrossRef]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H.; et al. Co-administration of iRGD enhances tumor-targeted delivery and anti-tumor effects of paclitaxel-loaded PLGA nanoparticles for colorectal cancer treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, N.; Xu, B.; Wu, M.; Yan, X.; Zhong, L.; Cai, H.; Wang, T.; Wang, Q.; Long, F.; et al. Polymer-based nanoparticles for chemo/gene-therapy: Evaluation its therapeutic efficacy and toxicity against colorectal carcinoma. Biomed. Pharmacother. 2019, 118, 109257. [Google Scholar] [CrossRef]
- Cabeza, L.; Perazzoli, G.; Mesas, C.; Jiménez-Luna, C.; Prados, J.; Rama, A.R.; Melguizo, C. Nanoparticles in colorectal cancer therapy: Latest in vivo assays, clinical trials, and patents. AAPS PharmSciTech. 2020, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Lozza, I.; Torres-Suárez, A.I. Actively targeted nanomedicines in breast cancer: From pre-clinal investigation to clinic. Cancers 2022, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Bottosso, M.; Griguolo, G.; Dieci, M.V.; Guarneri, V. Major advancements in metastatic breast cancer treatment: When expanding options means prolonging survival. ESMO Open 2022, 7, 100409. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global increase in breast cancer incidence: Risk factors and preventive measures. Biomed. Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. On Behalf Of Ncd Global Health Research Group Of Association Of Pacific Rim Universities Apru. worldwide burden, risk factors, and temporal trends of ovarian cancer: A global study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Rahman, M.A.; Hasan, M.N.; Akhter, H.; Noor, P.; Islam, R.; Shin, Y.; Rahman, M.D.H.; Gazi, M.S.; Huda, M.N.; et al. Recent advances in ovarian cancer: Therapeutic strategies, potential biomarkers, and technological improvements. Cells 2022, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y. Olaparib and paclitaxel in combination with carboplatin in treatment of ovarian cancer: Influence on disease control. Am. J. Transl Res. 2022, 14, 468–475. [Google Scholar]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the anticancer activity of sunitinib malate in breast cancer through lipid polymer hybrid nanoparticles approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles loaded with docetaxel and resveratrol as an advanced tool for cancer therapy. Biomedicines 2022, 10, 1187. [Google Scholar] [CrossRef]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Xu, X.; Chen, X.; Xin, Y.; Xu, L.; Lai, C.H.N.; Oh, J.; Wong, W.K.R.; Wang, X.; Han, S.; et al. Manipulation of the nanoscale presentation of integrin ligand produces cancer cells with enhanced stemness and robust tumorigenicity. Nano Lett. 2021, 21, 3225–3236. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Alsadat Vakilinezhad, M.; Alipour, S.; Montaseri, H. Fabrication and in vitro evaluation of magnetic PLGA nanoparticles as a potential methotrexate delivery system for breast cancer. J. Drug Deliv. Sci. Technol. 2018, 44, 467–474. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Maher, D.M.; Sundram, V.; Bell, M.C.; Jaggi, M.; Chauhan, S.C. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic acid-decorated PLGA-PEG nanoparticles for targeted delivery of SN-38 to ovarian cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Shen, S.; Du, X.J.; Liu, J.; Sun, R.; Zhu, Y.H.; Wang, J. Delivery of bortezomib with nanoparticles for basal-like triple-negative breast cancer therapy. J. Control. Release 2015, 208, 14–24. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Anjum, M.M.; Patel, K.K. Gemcitabine cationic polymeric nanoparticles against ovarian cancer: Formulation, characterization, and targeted drug delivery. Drug Deliv. 2022, 29, 1060–1074. [Google Scholar] [CrossRef]
- Li, S.Y.; Sun, R.; Wang, H.X.; Shen, S.; Liu, Y.; Du, X.J.; Zhu, Y.H.; Jun, W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J. Control. Release 2015, 205, 7–14. [Google Scholar] [CrossRef]
- Esnaashari, S.S.; Muhammadnejad, S.; Amanpour, S.; Amani, A. A combinational approach towards treatment of breast cancer: An analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020, 21, 166. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, Y.; Wen, Q.; Luo, J.; Lu, Y.; Wu, Z.; Wang, B.; Chen, Y.; Zhao, L.; Fu, S. Co-delivery of paclitaxel and curcumin by biodegradable polymeric nanoparticles for breast cancer chemotherapy. Int. J. Pharm. 2020, 589, 119875. [Google Scholar] [CrossRef]
- Rad, J.G.; Hoskin, D.W. Delivery of apoptosis-inducing piperine to triple-negative breast cancer cells via co-polymeric nanoparticles. Anticancer Res. 2020, 40, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Tian, J.; Zhu, H.; Hong, L.; Mao, Z.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185. [Google Scholar] [CrossRef]
- Alassaif, F.R.; Alassaif, E.R.; Kaushik, A.K.; Dhanapal, J. Enhanced anti-proliferative effect of carboplatin in ovarian cancer cells exploiting chitosan-poly (lactic glycolic acid) nanoparticles. Recent Pat. Nanotechnol. 2022. [Google Scholar] [CrossRef]
- Yao, S.; Li, L.; Su, X.T.; Wang, K.; Lu, Z.J.; Yuan, C.Z.; Feng, J.B.; Yan, S.; Kong, B.H.; Song, K. Development and evaluation of novel tumor-targeting paclitaxel-loaded nano-carriers for ovarian cancer treatment: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2018, 37, 29. [Google Scholar] [CrossRef]
- Khanna, V.; Kalscheuer, S.; Kirtane, A.; Zhang, W.; Panyam, J. Perlecan-targeted nanoparticles for drug delivery to triple-negative breast cancer. Future Drug Discov. 2019, 1, FDD8. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Roger, E.; Toti, U.; Niu, L.; Ohlfest, J.R.; Panyam, J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J. Control. Release 2013, 171, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Shahrousvand, M.; Hajikhani, M.; Nazari, L.; Aghelinejad, A.; Shahrousvand, M.; Irani, M.; Rostami, A. Preparation of colloidal nanoparticles PVA-PHEMA from hydrolysis of copolymers of PVAc-PHEMA as anticancer drug carriers. Nanotechnology 2022, 33, 275603. [Google Scholar] [CrossRef]
- Ram Prasad, S.; Jayakrishnan, A.; Sampath Kumar, T.S. Hydroxyapatite-poly(vinyl alcohol) core-shell nanoparticles for dual delivery of methotrexate and gemcitabine for bone cancer treatment. J. Drug Deliv. Sci. Technol. 2019, 51, 629–638. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, N.; Strzalka-Mrozik, B.; Madej, M.; Pająk, K.; Kruszniewska-Rajs, C.; Kaspera, W.; Gola, J.M. Differences in the expression patterns of TGFβ isoforms and associated genes in astrocytic brain tumors. Cancers 2022, 14, 1876. [Google Scholar] [CrossRef]
- Turek, A.; Stoklosa, K.; Borecka, A.; Paul-Samojedny, M.; Kaczmarczyk, B.; Marcinkowski, A.; Kasperczyk, J. Designing biodegradable wafers based on poly(L-lactide-co-glycolide) and poly(glycolide-co-ε-caprolactone) for the prolonged and local release of idarubicin for the therapy of glioblastoma multiforme. Pharm. Res. 2020, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, V.; Islam, M.M.; Yang, M.; Slaby, R.; Ramirez, H.M.; Mirza, S.P. Glioblastoma: Pathogenesis and current status of chemotherapy and other novel treatments. Cancers 2020, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric nanoparticles in brain cancer therapy: A review of current approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Z.; Yuan, H.; Wang, L.; Gao, L.H. Poly(p-phenylenevinylene) nanoparticles modified with antiEGFRvIII for specific glioblastoma therapy. Sci. Rep. 2021, 11, 4449. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Hosseini Najafabadi, M.R.; Webster, T.J.; Hadjighassem, M.R.; Sadroddiny, E.; Ghanbari, H.; Khosravani, M.; Adabi, M. Effect of paclitaxel/etoposide co-loaded polymeric nanoparticles on tumor size and survival rate in a rat model of glioblastoma. Int. J. Pharm. 2021, 604, 120722. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef]
- Jiang, X.; Xin, H.; Sha, X.; Gu, J.; Jiang, Y.; Law, K.; Chen, Y.; Chen, L.; Wang, X.; Fang, X. PEGylated poly(trimethylene carbonate) nanoparticles loaded with paclitaxel for the treatment of advanced glioma: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 420, 385–394. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, M.; Li, Y.; Sun, Y.; Teng, Y.; Wang, Y.; Duan, Y. The synergic antitumor effects of paclitaxel and temozolomide co-loaded in mPEG-PLGA nanoparticles on glioblastoma cells. Oncotarget 2016, 7, 20890–20901. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Vasey, C.E.; Cavanagh, R.J.; Taresco, V.; Moloney, C.; Smith, S.; Rahman, R.; Alexander, C. Polymer pro-drug nanoparticles for sustained release of cytotoxic drugs evaluated in patient-derived glioblastoma cell lines and in situ gelling formulations. Pharmaceutics 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Sevin, E.; Gosselet, F.; Lima, J.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018, 545, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ananta, J.S.; Paulmurugan, R.; Massoud, T.F. Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: A biophysical and cell culture evaluation. Neurol. Res. 2016, 38, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hamimed, S.; Jabberi, M.; Chatti, A. Nanotechnology in drug and gene delivery. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Cruz, J.C.; Reyes, L.H. Delivery systems for nucleic acids and proteins: Barriers, cell capture pathways and nanocarriers. Pharmaceutics 2021, 13, 428. [Google Scholar] [CrossRef]
- Wong, W.K.; Yin, B.; Lam, C.Y.K.; Huang, Y.; Yan, J.; Tan, Z.; Wong, S.H.D. The interplay between epigenetic regulation and CD8+ T cell differentiation/exhaustion for T cell immunotherapy. Front. Cell Dev. Biol. 2022, 9, 783227. [Google Scholar] [CrossRef]
- Wong, W.K.; Yin, B.; Rakhmatullina, A.; Zhou, J.; Wong, S.H.D. Engineering advanced dynamic biomaterials to optimize adoptive T-cell immunotherapy. Eng. Regen. 2021, 2, 70–81. [Google Scholar] [CrossRef]
- Mi, Y.; Smith, C.C.; Yang, F.; Qi, Y.; Roche, K.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z. A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy. Adv. Mater. 2018, 30, e1706098. [Google Scholar] [CrossRef]
- Risnayanti, C.; Jang, Y.S.; Lee, J.; Ahn, H.J. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci. Rep. 2018, 8, 7498. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, J.W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D.; et al. CD44-targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef]
- Li, T.; Deng, N.; Xu, R.; Fan, Z.; He, J.; Zheng, Z.; Deng, H.; Liao, R.; Lv, X.; Pang, C. NEAT1 siRNA packed with chitosan nanoparticles regulates the development of colon cancer cells via lncRNA NEAT1/miR-377-3p Axis. Biomed. Res. Int. 2021, 2021, 5528982. [Google Scholar] [CrossRef]
- Sadreddini, S.; Safaralizadeh, R.; Baradaran, B.; Aghebati-Maleki, L.; Hosseinpour-Feizi, M.A.; Shanehbandi, D.; Jadidi-Niaragh, F.; Sadreddini, S.; Kafil, H.S.; Younesi, V.; et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol. Lett. 2017, 181, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pho-Iam, T.; Punnakitikashem, P.; Somboonyosdech, C.; Sripinitchai, S.; Masaratana, P.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Wongwan, C.; Nguyen, K.T.; Srisawat, C. PLGA nanoparticles containing α-fetoprotein siRNA induce apoptosis and enhance the cytotoxic effects of doxorubicin in human liver cancer cell line. Biochem. Biophys. Res. Commun. 2021, 553, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, D.; Hu, J.; Ding, P.; Chen, M. Hyaluronic acid-coated pH sensitive poly (β-amino ester) nanoparticles for co-delivery of embelin and TRAIL plasmid for triple negative breast cancer treatment. Int. J. Pharm. 2020, 573, 118637. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Lim, J.; Slack, F.J.; Braddock, D.T.; Bahal, R. Next generation miRNA inhibition using short anti-seed PNAs encapsulated in PLGA nanoparticles. J. Control. Release 2020, 327, 406–419. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, L.; Pan, C.C.; Wang, J.Z.; Lu, G.R.; Yang, S.X.; Xue, Z.X.; Wang, F.Y.; Xu, C.L. Targeted delivery of miRNA-204-5p by PEGylated polymer nanoparticles for colon cancer therapy. Nanomedicine 2018, 13, 769–785. [Google Scholar] [CrossRef]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Eskandani, M.; Barar, J.; Omidi, Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine 2018, 13, 2729–2758. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ansari, M.M.; Verma, R.K.; Khan, R. Aminocellulose-grafted polymeric nanoparticles for selective targeting of CHEK2-deficient colorectal cancer. ACS Appl. Bio. Mater. 2021, 4, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Aravind, A.; Varghese, S.H.; Veeranarayanan, S.; Mathew, A.; Nagaoka, Y.; Iwai, S.; Fukuda, T.; Hasumura, T.; Yoshida, Y.; Maekawa, T.; et al. Aptamer-labeled PLGA nanoparticles for targeting cancer cells. Cancer Nanotechnol. 2012, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
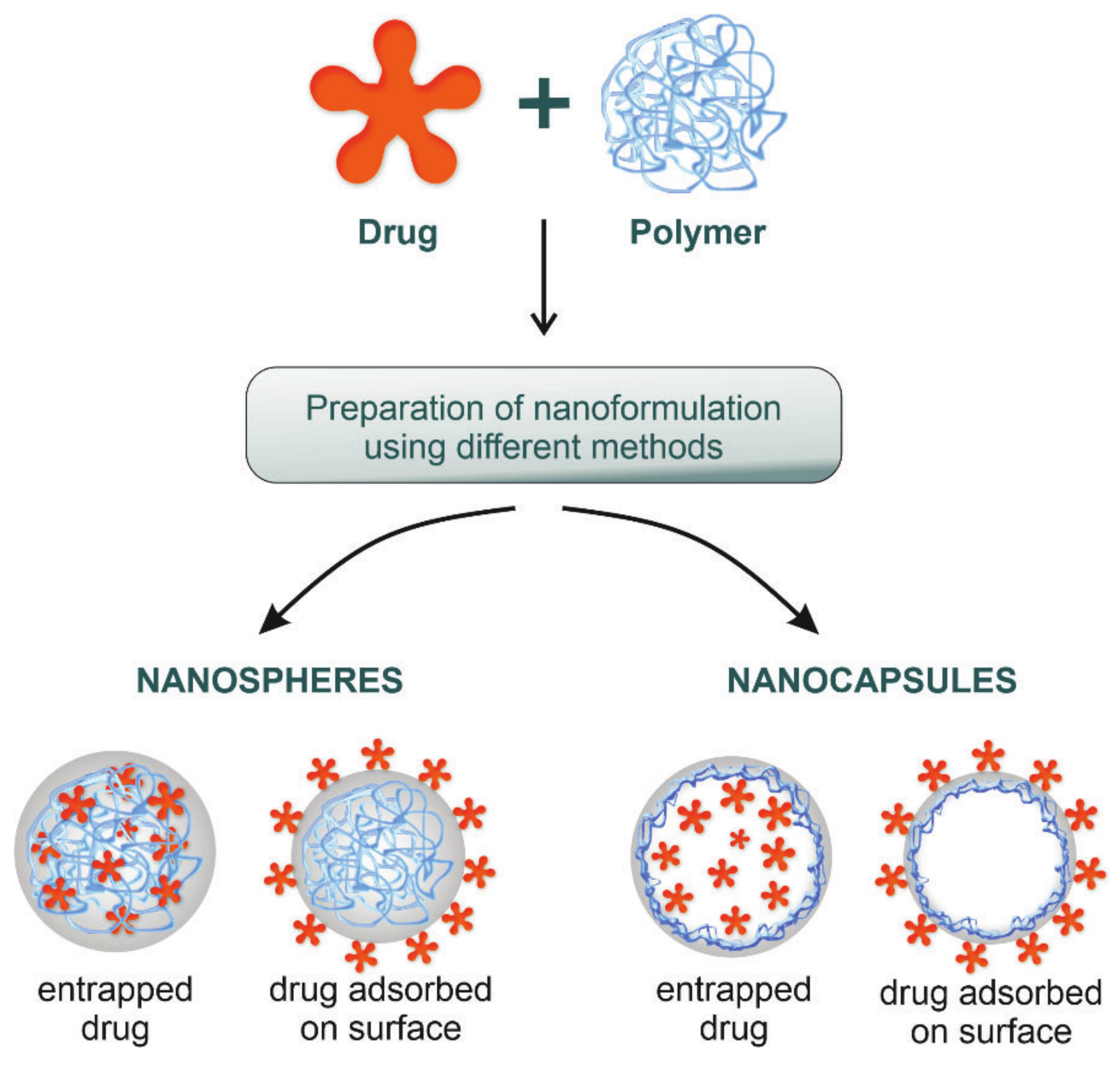
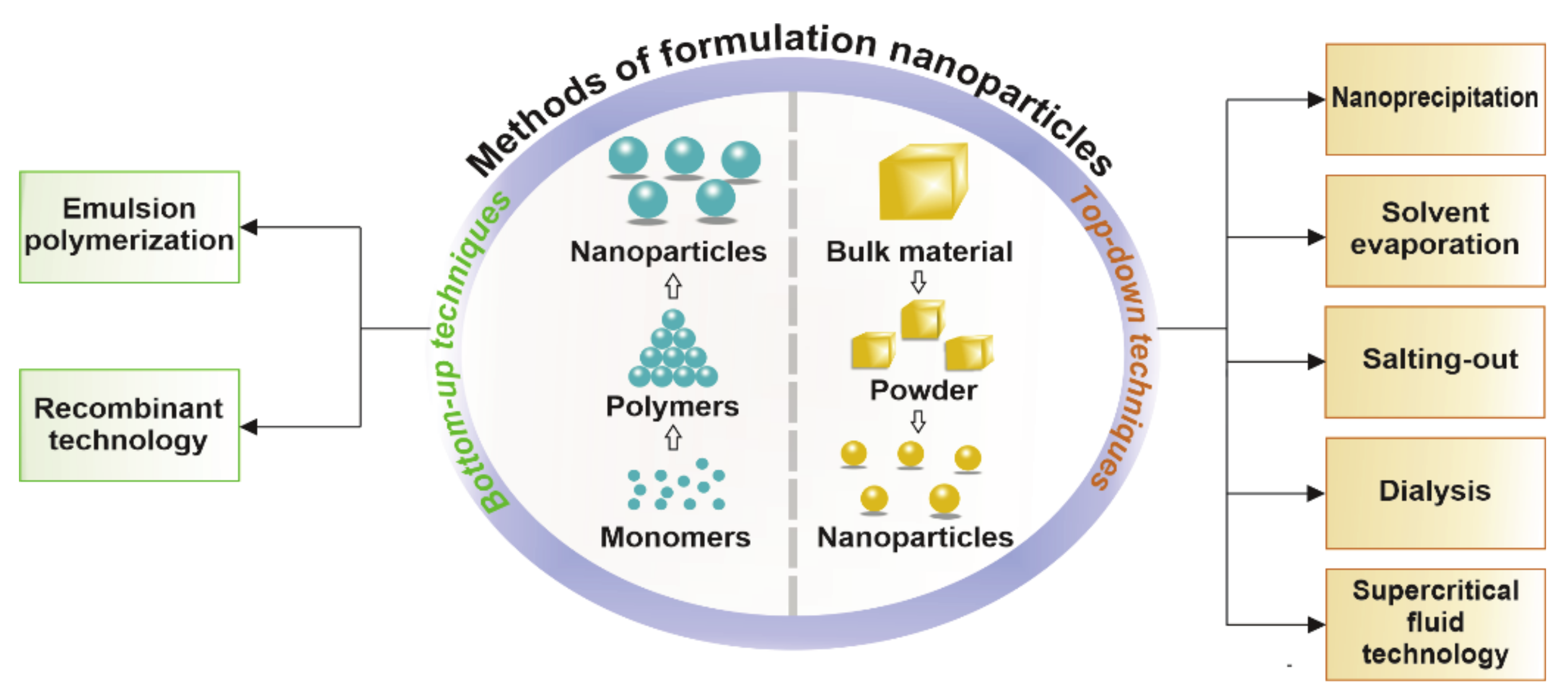

| Methods of Formulation | Description | Advantages | Disadvantages | Example | Cancer | Ref. |
|---|---|---|---|---|---|---|
| Emulsion polymerization | Two subgroups may be distinguished: using a continuous organic phase and using an aqueous phase. Dispersion of the monomer into an emulsion occurs, or the monomer is dissolved in an aqueous solution without surfactants, respectively | PNPs with a high molar mass Often used Does not require surfactants when using a continuous aqueous phase | For the continuous organic phase, it requires the use of surfactants and toxic solvents High cost Time-consuming | Cur-loaded PMMA NPs | Human lung cancer lines | [7,10,11,15,16] |
| Recombinant technology | The latest techniques, based on the use of living organisms, e.g., Escherichia coli, to produce a specific biopolymer by altering the expression of genes, result in various amino acid compositions and particle properties | Efficient method Formulates small sizes For gene delivery | Necessary use of living organisms Method under development | K8-ELP /pDNA | Human breast cancer lines | [11,17] |
| PNPs | Formulation Method | Size (nm) | Drug | Dose | In Vitro/In Vivo | Ref. |
|---|---|---|---|---|---|---|
| PHBV/PLGA | Double emulsion solvent evaporation | ~150 | 5-FU | 3 mg/mL | In vitro—HT-29, CT-26 In vivo—BALB/c mice | [44] |
| PLGA-PEG-PLGA | Double emulsion solvent evaporation | ~40 | 5-FU/Chrysin | 10 mg/mL | In vitro—HT-29 | [45] |
| HPMC phthalate | Nanoprecipitation | ~478 | Doxycycline | 5 mg/kg, 10 mg/kg | In vivo—Swiss albino mice | [46] |
| d,l-PLGA | Single emulsion solvent evaporation | ~191 | Curcumin | 10 mg, 20 mg | In vitro—HT-29 | [47] |
| d,l-PLGA | Spontaneous emulsification | ~310 | SN-38 | 15 mg | In vitro—COLO-205 | [48] |
| Poly-UA | Nanoprecipitation | ~171 | Mith-A | 3 mg | In vitro—CT-26 In vivo—BALB/c mice | [49] |
| Chitosan polymeric | Ionic gelation technique | ~200 | Imatinib | 5 mg | In vitro—CT-26 In vivo—Wistar rat | [50] |
| d,l-PLGA-PEG-FA | Double emulsion solvent evaporation | ~201 | Oxaliplatin | 5 mg/kg | In vitro—CT-26 In vivo—BALB/c mice | [51] |
| d,l-PLGA | Single emulsion solvent evaporation | ~237 | Quercetin and CAPE | 5 mg and 15 mg | In vitro—HT-29 | [52] |
| v6 Fab-PLGA-PEG | Double emulsion solvent evaporation | ~345 | Bevacizumab | 25 mg/mL | In vitro—MKN74-CD44std and CD44v6+ | [53] |
| CS-Chitosan | Single emulsion solvent evaporation | ~289 | Camptothecin | 6 mg | In vitro—CT-26 In vivo—BALB/c mice | [25,54] |
| PMMA | Single emulsion solvent evaporation | ~154 | Benznidazole | 0.0125 mg/25 mL | In vitro—HT-29 | [55] |
| PEG | Single emulsion solvent evaporation | ~114 | PTX and DHA | 6 µg/mL | In vitro—HT-29 | [56] |
| PCL-PEG-PCL | Double emulsion solvent evaporation | ~95 | Cur and MTX | 4 mg and 2 mg | In vitro—CL-40, SW1417 | [57] |
| d,l-PLGA | Modified salting-out | ~200 | Meloxicam | n/d | In vitro—HT-29 | [28] |
| PEG-PLGA | Double emulsion solvent evaporation | ~147 | PTX | 1 mg | In vitro—S174T, COLO205, HCT116 | [58] |
| PEG-PLGA | Double emulsion solvent evaporation | ~289 | Sorafenib and PEDF | 2 mg and 25 µg | In vitro—C26 In vivo—BALB/c mice | [59,60] |
| PNPs | Formulation Method | Size (nm) | Drug | Dose | In Vitro/In Vivo | Cancer | Ref. |
|---|---|---|---|---|---|---|---|
| d,l-PLGA | Modified nanoprecipitation | ~70 | Cur | 1 mg | In vitro—A2780, A2780CP | Ovarian | [73] |
| PLGA-PEG-HA | Single emulsion solvent evaporation | ~268 | SN-38 | 3 mg/mL | In vitro—SKOV-3, CHO | Ovarian | [74] |
| PEG-b-PLA | Single emulsion solvent evaporation | ~112 | Bortezomib | 500 µg | In vitro—MDA-MB-468, HCC1937 In vivo—NOD/SCID and ICR mice | TNBC | [75] |
| Chitosan- EGFRvIII | Ionotropic gelation technique | ~146 | Gemcitabine | 5 mg | In vitro—OVAR-8 | Ovarian | [76] |
| PEG-PLA | Single emulsion solvent evaporation | ~88 | DOX | 10 mg | In vitro—MDA-MB-231 In vivo—NOD/SCI mice | Breast | [77] |
| PEG-PLA | Double emulsion solvent evaporation | ~79 | DAC | 5 mg | In vitro—MDA-MB-231 In vivo—NOD/SCI mice | Breast | [77] |
| mPEG-PLGA | Nanoprecipitation | ~101 | Nos | 5 mg | In vitro—4T1 In vivo—BALB/c mice | Breast | [78] |
| PCEC | Double emulsion solvent evaporation | ~28 | PTX and Cur | 3 mg/mL | In vitro—MCF-7 In vivo—BALB/c mice | Breast | [79] |
| mPEG-PLGA | Single emulsion solvent evaporation | ~165 | Piperine | 8.5 mg | In vitro—MDA-MB-488, BT-549 | TNBC | [80] |
| PCL | Single emulsion solvent evaporation | ~154 | PTX and IR780 | 140 µg and 148 µg | In vitro—SKOV-3, ST30 In vivo—BALB/c mice | Ovarian | [81] |
| Chitosan-PLGA | Ionic gelation | ~156 | Carboplatin | n/d | In vitro—PEO1 | Ovarian | [82] |
| PEG-PLA-FA | Nanoprecipitation | ~192 | PTX | 50 mg | In vitro—SKOV-3, HO-89110, A2780 In vivo—BALB/c mice | Ovarian | [83] |
| PLGA-PEG-maleimide | Single emulsion solvent evaporation | ~209 | PTX | 5 mg | In vitro—LM2 In vivo—BALB/c mice | TNBC | [84] |
| PLGA with anti-CD133 mAb | Single emulsion solvent evaporation | ~320 | PTX | 6 mg | In vitro—MCF-7, MDA-MB-231-luc In vivo—BALB/c mice | Breast | [85] |
| Identifier | Drug Delivery System | Title | Cancer | Phase | Status |
|---|---|---|---|---|---|
| NCT03774680 | PNPs | Targeted Polymeric Nanoparticle Loaded With Cetuximab and Decorated With Somatostatin Analogue to Colon Cancer | Colon Cancer | I | Unknown |
| NCT02010567 | PNPs | Neoadjuvant Chemoradiotherpay With CRLX-101 and Capecitabine for Rectal Cancer | Rectal Cancer | II | Terminated |
| NCT03505528 | Nab–paclitaxel | An Early Phase Study of Abraxane Combined With Phenelzine Sulfate in Paient With Metastatic or Adcanced Breast Cancer (Epi-PRIMED) | Breast Cancer | I | Completed |
| NCT02788981 | Nab–paclitaxel | Abraxane® With or Without Mifepristone for Advanced, Glucocorticoid Receptor-Positive, Triple-Negative Breast Cancer | TNBC | I | Active |
| NCT04249167 | Nab–paclitaxel | Cryoablation, Atezolizumab/Nab-paclitaxel for Locally Advanced or Metastatic Triple Negative Breast Cancer | TNBC | I | Withdrawn |
| NCT00499252 | Nab-paclitaxel | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Recurrent or Persistent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | Ovarian Cancer | II | Completed |
| NCT03942068 | Nab–paclitaxel | Apatinib With Albumin-bound Paclitaxel in Patients With Platinum-resistant Recurrent Ovarian Cancer | Ovarian Cancer | II | Unknown |
| NCT01652079 | PNPs | CRLX101 in Combination With Bevacizumab for Recurrent Ovarian/Tubal/Peritoneal Cancer | Ovarian Canacer | II | Completed |
| NCT00313599 | Nab–paclitaxel | Lapatinib and Paclitaxel in Treating Patients With Advanced Solid Tumors | Ovarian Cancer | I | Completed |
| NCT03719326 | Nab–paclitaxel | A Study to Evaluate Safety/Tolerability of Immunotherapy Combinations in Participants With Triple-Negative Breast Cancer or Gynecologic Malignancies | TNBC, Ovarian Cancer | I | Completed |
| NCT00989131 | PNPs | Study of Paclitaxel in Patients With Ovarian Cancer | Ovarian Cancer | III | Completed |
| PNPs | Formulation Method | Size (nm) | Drug | Dose | In Vitro/In Vivo | Ref. |
|---|---|---|---|---|---|---|
| d,l-PLGA | Nanoprecipitation | ~250 | Cur | 1 mg | In vitro—DKMG/EGFRvIII, DK-MGlow | [94] |
| mPEG-PLGA | Nanoprecipitation | <150 | PTX and etoposide | 5 mg | In vitro—U87, C6 In vivo—Wistar rats | [95] |
| d,l-PLGA | Nanoprecipitation | ~212 | PTX and MTX | 2.5 mg | In vitro—U87MG, B65 | [96] |
| mPEG–PTMC | Single emulsion solvent evaporation | ~49 | PTX | 10 mg | In vitro—U87MG In vivo—Sprague Dawley rats | [97] |
| mPEG-PLGA | Double emulsion solvent evaporation | ~206 | PTX and TMZ | 0.2 mg/mL and 4.4 mg/mL | In vitro—U87, C6 In vivo—BALB/c mice | [98] |
| d,l-PLGA | Single emulsion solvent evaporation | ~135 | PTX | 5 mg | In vitro—C6 In vivo—Sprague Dawley rats | [99] |
| d,l-PLGA | Double emulsion solvent evaporation | ~110 | DOX | 23 mg/mL | In vivo—Wistar rats | [100] |
| mPEG-(LA)-(TBPC) | Nanoprecipitation | ~68 | DOX | 2 mg/mL | In vitro—U87, GIN-8, GIN-28, GIN-31 | [101] |
| Receptor -mediated d,l-PLGA | Modified single emulsion solvent evaporation | ~187 | TMZ | 1 mg | In vitro—U87, U215, NHA | [102] |
| d,l-PLGA | Emulsion solvent evaporation techniques | ~200 | TMZ | 3.3 mg/mL | In vitro—U87 | [103] |
| PNPs | Formulation Method | Size (nm) | Molecule | Dose | In Vitro/In Vivo | Cancer | Ref. |
|---|---|---|---|---|---|---|---|
| d,l-PLGA | Double emulsion solvent evaporation | ~197 | BLC2 siRNA | 50 µg | In vitro—SKOV3-TR. A2780-CP20 | Ovarian | [112] |
| HA-PLGA | Double emulsion solvent evaporation | ~232 | PTX; FAK siRNA | 900 µg and 125 µg | In vitro—SKOV3, TR, HeyA8, MDR In vivo—BALB/c mice | Ovarian | [113] |
| Chitosan | Solvent evaporation method | ~135 | NEAT siRNA | 1:1 | In vitro—LoVo, SW480, HCT116 | CRC | [114] |
| Chitosan | Polyelectrolyte complexation | ~172 | DOX,CMD and siRNA | 2.5 µg/mL, 1 mg/mL and 5 µL | In vitro—HCT-116 | CRC | [115] |
| d,l-PLGA | Double emulsion solvent evaporation | ~159 | AFP siRNA | 100 µL | In vitro—HepG2, HeLa, MDA-MB-231 | HCC, cervical breast | [116] |
| PBAE-PEI-HA | Solvent evaporation technique | ~182 | EMB and pTRAIL | 1.33 mg | In vitro—MCF-7, MDA-MB-231 | TNBC | [117] |
| d,l-PLGA | Double emulsion solvent evaporation | ~145 | PNA targeting miRNA-155 | 1 µM | In vitro—HeLa, SUDHL-5, | Cervical, lymphoma | [118] |
| PLGA/PLA-PEG-FA | Single emulsion solvent evaporation | ~232 | miRNA-204-5p | n/d | In vitro—HT-29, HCT-116 In vivo—BALB/c mice | CRC | [119] |
| PLGA-chitosan with 5TR1 | Double emulsion solvent evaporation | ~222 | Epirubicin | 2 mg/mL | In vitro—MCF7, CHO In vivo—BALB/c mice | Breast | [120] |
| AS1411 aptamer PLGA-PEG | Double emulsion solvent evaporation | ~113 | Cisplatin; miR-21 | 8.4 mg/mL; 10 mg/mL | In vitro—A2780 S/R | Ovarian | [121] |
| PCL-AC | Nanoprecipitation | ~194 | LCS-1 | n/d | In vitro—HCT116 | CRC | [122] |
| AS1411 aptamer PLGA | Single emulsion solvent evaporation | ~200 | Paclitaxel | 10 µg/mL | In vitro—GI-1 | Glioblastoma | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. https://doi.org/10.3390/app12199479
Madej M, Kurowska N, Strzalka-Mrozik B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Applied Sciences. 2022; 12(19):9479. https://doi.org/10.3390/app12199479
Chicago/Turabian StyleMadej, Marcel, Natalia Kurowska, and Barbara Strzalka-Mrozik. 2022. "Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies" Applied Sciences 12, no. 19: 9479. https://doi.org/10.3390/app12199479
APA StyleMadej, M., Kurowska, N., & Strzalka-Mrozik, B. (2022). Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Applied Sciences, 12(19), 9479. https://doi.org/10.3390/app12199479






