Abstract
Fruit plants produce various volatile compounds that emit distinct aroma characteristics and contribute to their flavor qualities. However, some of these substances, especially hydroxyl-group molecules, are in non-volatile glycosylated forms. This study aimed to determine free and glycosidically bound volatile compounds in three Okinawan pineapple cultivars (‘N67-10′, ‘Yugafu’, and ‘Yonekura’). The free volatile components of squashed pineapple juice were analyzed using solid-phase microextraction (SPME)–arrow-gas chromatography–flame ionization detection/mass spectrometry (GC-FID/MS). The glycosides were collected through solid-phase extraction, hydrolyzed by β-glucosidase, and the released volatile compounds were measured. The sugar moieties of the glycosides were confirmed using GC-MS, and their glycoside constituents were analyzed using liquid chromatography (LC)-MS. Okinawan pineapple varied in its content and composition of free volatile components, which were predominantly comprised of esters, followed by alcohols, terpenes, and ketones. Eight hydroxyl-group compounds, including chavicol, eugenol, geraniol, phenylethyl alcohol, benzyl alcohol, 2-ethyl-1-hexanol, 1-hexanol, and 3-methyl-2-butenol, were released from their glycosylated forms via enzymatic hydrolysis, wherein the amounts of most of them were greater in ‘Yonekura’ than in the other cultivars. Moreover, two glycosides, chavicol-O-β-D-glucopyranoside and eugenol-O-β-D-glucopyranoside, were identified in all the cultivars, wherein the aglycones of both glycosides could be potential odor sources of the medicinal-herbal aromas. These results provide important information regarding both volatile-aroma qualities and bounded-aroma resources in Okinawan pineapple for fresh consumption and agroindustrial processing.
1. Introduction
Pineapple (Ananas comosus) is considered one of the important horticultural crops in many tropical and subtropical countries as it produces highly nutritious fruits with unique flavor properties [1,2,3]. Okinawa, the most southern prefecture of Japan, is the only subtropical region that largely farms pineapple in the country, and its produce has economically supported local agroindustry businesses and attracted ecotourism activities in recent years [4,5,6]. Volatile-aroma components greatly contribute to the flavor quality of pineapple fruit by providing distinct sensory properties that are sweet, fruity, pineapple-like, caramel-like, coconut-like, etc. [7,8]. They are mostly highly odorous volatile substances which are derived from the free aglycone molecules of esters, alcohols, aldehydes, terpenes, and lactones [2,3,7].
In addition to producing metabolites in free form, plants, including pineapple, often conjugate various organic compounds with endogenous molecules such as sugars, oligosaccharides, polysaccharides, etc. [9,10]. Volatile precursors are also glycosylated by simple sugars, either monosaccharides or disaccharides, and make them non-volatile and odorless [11,12]. These glycosides may play important roles in plants, for instance, in defense mechanisms wherein stored volatiles are released as chemical defense agents in response to tissue damage due to invading microbes and other exogenous threats [12,13]. On the other hand, glycosylated volatile substances can be considered hidden supplies of bounded-aroma resources in various horticultural crops, and releasing these aglycones can potently alter the aroma profiles of the fruits and their derivatives [10,14].
To our knowledge, there is little information on the composition of the volatile components of Okinawan pineapple, particularly its glycosidically bound volatile compounds. The objective of this study was, thus, to evaluate free and glycosidically bound volatile compounds in Okinawan pineapples of different cultivars. The volatile compounds of squashed pineapple fruit juice were measured using solid-phase microextraction (SPME)–gas chromatography–flame ionization detection/mass spectrometry (GC-FID/MS). The glycosides were extracted via solid-phase extraction and enzymatically hydrolyzed to release their glycosylated volatile compounds. Free and glycosidically bound volatile compounds were compared for three major Okinawan pineapple cultivars, namely ‘N67-10’, ‘Yugafu’, and ‘Yonekura’. Additionally, the composition of glycoside constituents was evaluated using liquid chromatography (LC) with MS detection.
2. Materials and Methods
2.1. Standards and Reagents
The authentic standards for the identification of volatile components were purchased from Sigma-Aldrich (St Louis, MO, USA) and Tokyo Chemical Industry (Tokyo, Japan). 2-Methyl-1-pentanol was purchased from Tokyo Chemical Industry, and D-glucose was obtained from Fujifilm Wako Pure Chemical Industries (Osaka, Japan). Methoxyamine hydrochloride, and p-nitrophenyl-O-β-D-glucopyranoside were purchased from Sigma-Aldrich. N-Methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) was obtained from GL Sciences (Tokyo, Japan). Rapidase® Revelation Aroma was obtained from DSM Food Specialties (Delft, The Netherlands). All the other reagents were of analytical grade.
2.2. Sample Preparation
The fruits of three pineapple cultivars (‘N67-10’, ‘Yugafu’, and ‘Yonekura’) were harvested at the ripe stage from a farm at the Okinawa Prefectural Agricultural Research Center, Nago, Okinawa, Japan from August to October 2019. Upon arrival at the laboratory, the fruits were immediately weighed and peeled; the fruits’ edible parts were then bisected longitudinally, and equally cut into eight pieces. The CIE L*a*b* color traits of the edible fruit flesh were measured using an NF333 colorimeter (Nippon Denshoku Industries, Tokyo, Japan). Afterward, the fruits’ flesh was squashed using a hand-press juicer, and the extracted juice was filtered through a clean muslin cloth. The total soluble solid and titratable acidity of the juice were measured using an NH-2000 Brix/acidity content analyzer (Horiba Advanced Techno, Tokyo, Japan). The juice was stored in sealed vials at −30 °C prior to analysis.
2.3. Volatile Component Analysis
The volatile components of the pineapple were extracted using an SPME Arrow coated with 120 µm of divinylbenzene/polydimethylsiloxane (Restek Corporation, Bellefonte, PA, USA) and analyzed using GC-FID (Agilent J&W, Santa Clara, CA, USA) [15]. Briefly, the pineapple juice (2 mL), internal standard 2-methyl-1-pentanol (3 µg/mL, 20 µL), and NaCl (0.2 g) were placed into a 10 mL glass vial, and sonicated at 25 °C for 10 min. Afterward, the closed vial was heated in a water bath at 40 °C for 7.5 min. The volatile compounds were then absorbed onto an SPME Arrow fiber while heating for 30 min. The SPME Arrow fiber was injected at a split ratio of 1:10 into an Agilent 7890B GC system. The injection temperature was set at 250 °C and the linear velocity of the helium carrier gas flowed at 32 cm/s into a DB-Wax column (60 m × 0.25 mm i.d., film thickness: 0.25 µm, Agilent J&W). The column temperature was initially set at 40 °C for 2 min, and gradually raised to 200 °C at a rate of 2 °C/min (without hold time). The weight intensity of the peak was calibrated to the FID response of internal standard, and the content of volatile compounds was expressed as µg/100 mL. All the assays were performed in triplicate.
Compound identification was performed via comparison of the linear retention indices (RIs) and mass spectra fragmentation patterns with MS data from the National Institute of Standards and Technology (NIST) MS Library, version 2008, and co-injected authentic standards. The mass spectra for compound identification were obtained using a 7890A GC system coupled with 5975C MS (Agilent J&W). SPME Arrow extraction and the GC conditions were set as described above. For MS detection, the electron-impact ion source and interface were both maintained at 230 °C. The ionization energy was 70 eV, and the mass acquisition range was taken at m/z 33–450.
2.4. Glycosides Extraction and Hydrolysis
The glycosidically bound volatile compounds of pineapple were extracted using the solid-phase extraction technique [16]. Briefly, an Oasis® HLB 3cc Vac cartridge containing 60 mg of sorbent (Waters, Milford, MA, USA) was preconditioned with 10 mL of methanol and 10 mL of Milli-Q water on an extraction manifold (Waters). Afterward, 2 mL of pineapple juice was loaded onto the cartridge, and was left to immerse in the sorbent for 1 min. Ten milliliters of Milli-Q water was then eluted, followed by an equal volume of pentane:dichloromethane (2:1, v/v). Subsequently, 2 mL of methanol was applied to the sorbent, held for 1 min, and then, the glycosides were eluted from the cartridge under vacuum conditions. Methanol extraction was repeated using the same volume and elution method. The methanol extract containing glycosides was evaporated using a centrifugal evaporator at 40 °C and dried under a gentle nitrogen stream.
The glycoside extract was dissolved using 500 µL of 0.2 M citric buffer, pH 4.5, and 100 µL of Rapidase® Revelation Aroma solution (20 mg/mL in citric buffer, containing β-glucosidase, enzymatic activity ≥ 4000 u/g). The mixture was then hydrolyzed at 40 °C for 2 h. The reaction without β-glucosidase addition was applied as control. The released volatile compounds were extracted using an SPME Arrow and analyzed using GC-FID/MS under the same conditions as described above. All the assays were performed in triplicate.
2.5. Sugar Moiety Analysis
The sugar moiety of the hydrolyzed glycosides was analyzed using methoxyamine-trimethylsilyl derivatization and analyzed using GC-MS [17]. Briefly, the glycoside extract was dissolved using 1 mL of methanol:chloroform:H2O (3:1:1, v/v/v), and mixed for 10 min. The mixture was centrifuged at 12,000 rpm for 10 min at 4 °C, and the resulting supernatant (100 µL) was dried up using a centrifugal evaporator at 40 °C. The dried extract was then mixed with 30 µL methoxyamine hydrochloride (20 mg/mL in pyridine dehydrate) and incubated for 23 h at room temperature. Afterward, 30 µL of MSTFA was added and incubated for 1 h at 37 °C under shaking. Heptane (60 µL) was added, and the derivatized substances were then analyzed using GC-MS (7890A GC-5975C MS, Agilent J&W). The injection volume was 1 µL with a splitless mode at 250 °C, and the column used was DB-5MS (30 m × 0.25 mm i.d., 0.25 µm, Agilent J&W). The oven was initially maintained at 80 °C for 2 min and raised to 320 °C at a rate of 20 °C/min, and finally, held for 3.5 min. The electron-impact ion source and interface were both maintained at 230 °C. The MS ionization voltage was set at 70 eV, and the scan range was m/z 60–800. Compound identification was performed by comparing the mass spectra fragmentation patterns with MS data from the NIST MS Library, and co-injected derivatized authentic standard (D-glucose). All the assays were performed in triplicate.
2.6. Glycoside Composition Analysis
Glycoside composition was analyzed using LC-MS [12]. Briefly, glycoside extract was dissolved using 480 µL of methanol and 20 µL of 1 mM p-nitrophenyl-O-β-D-glucopyranoside (internal standard), mixed and filtered through a PTFE filter (0.45 µm, Advantec Toyo Kaisha, Tokyo, Japan). The mixture (1 µL) was injected into an LC-20AD XR (Shimadzu, Kyoto, Japan) equipped with an MS Quattro Micro (Waters). The column used was a YMC-Triart C18 (150 × 3.0 mm i.d.) (YMC, Kyoto, Japan) and was set at 40 °C. Mobile phases A and B were 10 mM of ammonium acetate and acetonitrile, respectively. The flow rate was kept at 0.4 mL/min at 40 °C. The eluent of mobile phase B was started under isocratic conditions for 1 min, and then, gradually increased to 60% at a 1–20 min eluent, and finally, held at 10% for 5 min. The MS capillary voltage was 3 kV, and the scan range was m/z 220–800. The content of glycoside compounds was calibrated to the total ion chromatography response of the internal standard and was expressed as µmol/100 mL. All the assays were performed in triplicate.
2.7. Statistical Analysis
Each result was expressed as the mean value and standard deviation, and statistical differences between the parameters were examined using the Tukey–Kramer honestly significant difference (JMP, SAS Institute, Cary, NC, USA).
3. Results
3.1. Physicochemical Properties of Okinawan Pineapple
The fruit weight of three major Okinawan pineapple cultivars ‘N67-10’, ‘Yugafu’, and ‘Yonekura’ ranged from 1172.8 to 1351.0 g, and the color space L* (brightness index) of their edible fruit fleshes ranged from 63.95 to 66.84, but there were no significant differences in these traits among the cultivars (p < 0.05) (Table 1). However, they varied in the other CIE L*a*b* color traits, total soluble solid, and titratable acidity. ‘Yugafu’ had the lowest color space a* (−4.11), followed by ‘N67-10’ and ‘Yonekura’ (−3.42 and −3.19, respectively), wherein a significant difference was found only between ‘Yugafu’ and ‘Yonekura’ for this green color trait. Moreover, significant differences in pineapple cultivars were observed on color space b* (yellow color) in the following order: ‘Yonekura’ > ‘N67-10’ > ‘Yugafu’ (26.34, 19.10, and 10.62, respectively). On the other hand, the total soluble solid and titratable acidity of Okinawan pineapple ranged from 13.60 to 15.70 °Brix and 0.58 to 0.76%, respectively, in which the recorded values of both traits in ‘Yugafu’ were significantly greater than in the other cultivars. In spite of this, the ratio of total soluble solid to titratable acidity in ‘Yugafu’ was lower compared to ‘N67-10′ and ‘Yonekura’ (20.77 vs. 23.80 and 23.29, respectively).

Table 1.
Physicochemical properties of Okinawan pineapple.
3.2. Free Volatile Components of Okinawan Pineapple
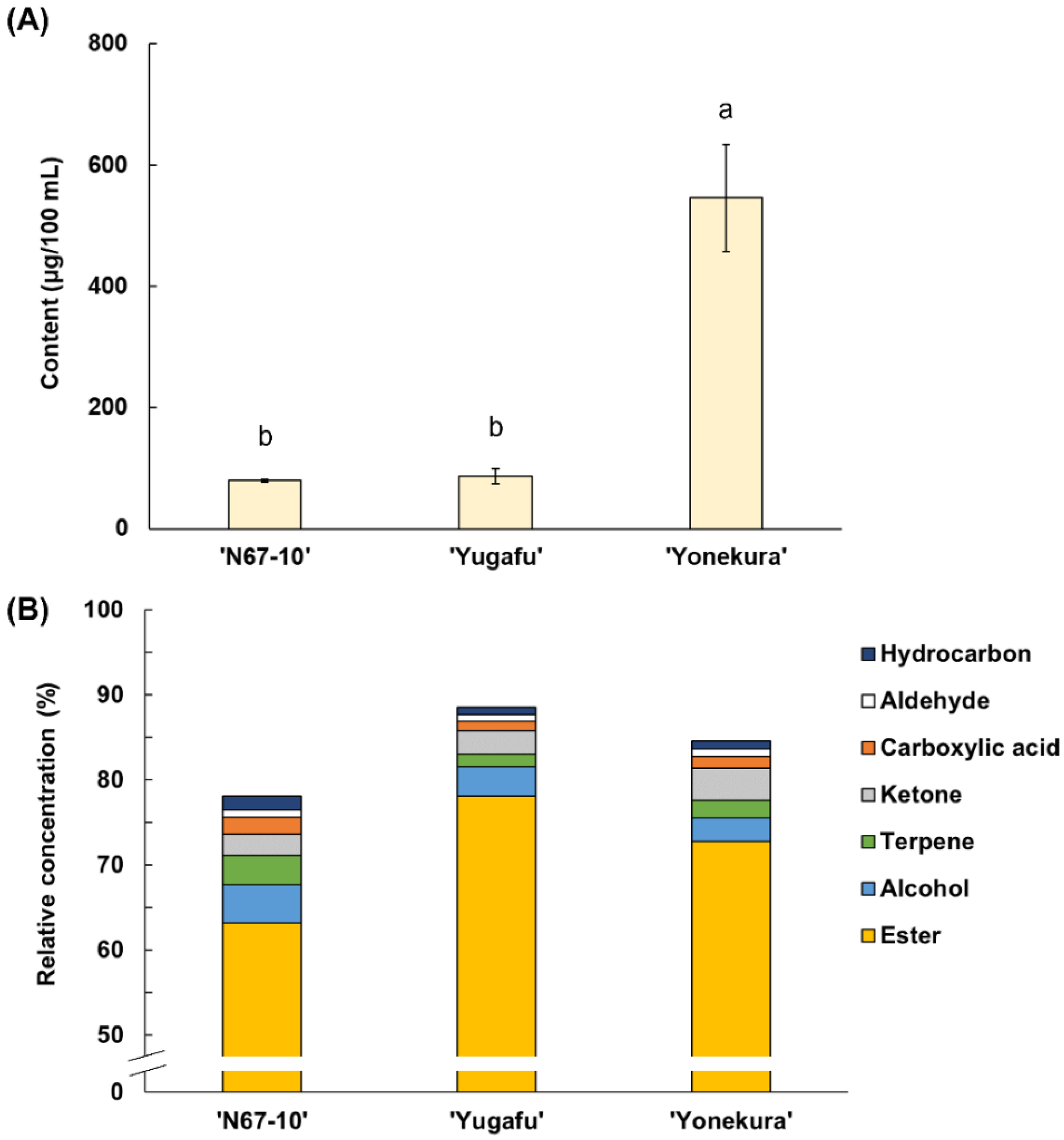
Fifty-eight free volatile compounds were identified in Okinawan pineapples of different cultivars, and were comprised of twenty-nine esters, eight terpenes, seven alcohols, six ketones, three aldehydes, three hydrocarbons, and two carboxylic acids (Table 2). The amount and composition of the identified free volatile compounds varied among the three cultivars (Figure 1). As for the total volatile content, ‘Yonekura’ possessed 505.93 µg/100 mL, which was significantly greater than in ‘N67-10’ and ‘Yugafu’ (62.70 and 74.70 µg/100 mL, respectively) (Figure 1A). The predominant free volatile components in ‘N67-10’ were ester compounds (63.20%, relative concentration), followed by moderate amounts of alcohols, terpenes, and ketones (4.47, 3.41, and 2.57%, respectively), and smaller proportions of carboxylic acids, aldehydes, and hydrocarbons (Figure 1B). On the other hand, ‘Yugafu’ was mainly comprised of 78.11% esters, 3.43% alcohols, and 2.72% ketones, whilst the proportions of these volatiles in ‘Yonekura’ were 72.80, 2.72, and 3.77%, respectively.

Table 2.
Free volatile components of Okinawan pineapple (μg/100 mL).
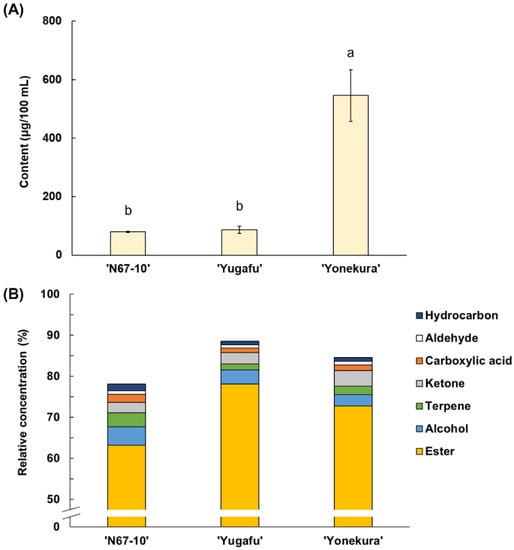
Figure 1.
(A) Content (μg/100 mL) and (B) relative concentration (%) of free volatile components of Okinawan pineapple. Each value is expressed as the mean ± standard deviation of three replicates. Means followed by the same letter are not significantly different at p < 0.05.
The major ester compounds in both ‘N67-10’ and ‘Yonekura’ were methyl hexanoate and methyl 3-(methylthio)propanoate, but their amounts were much greater in ‘Yonekura’ (263.91 and 128.49 μg/100 mL, respectively) (Table 2). The concentrations of these two compounds in ‘N67-10’ were 18.84 and 18.67 μg/100 mL, respectively. Moreover, both cultivars also lacked the same esters, including ethyl propanoate, ethyl 2-methylpropanoate, 3-methylbutyl acetate, and ethyl pentanoate. Conversely, ‘Yugafu’ had a greater number of predominant ester compounds such as ethyl acetate, ethyl 2-methylbutanoate, methyl hexanoate, ethyl hexanoate, and ethyl 3-(methylthio)propanoate (12.58, 11.48, 11.40, 10.13, and 9.45 μg/100 mL, respectively). Except for methyl hexanoate, the concentrations of the other predominant esters of ‘Yugafu’ were significantly higher compared to ‘N67-10’ and ‘Yonekura’. However, methyl (Z)-3-hexenoate, methyl (E)-3-hexenoate, methyl heptanoate, methyl 2-hydroxy-2-methylbutanoate, and dimethyl propanedioate were lacking in ‘Yugafu’.
The second largest proportion of free volatile components were alcohols, which ranged from 3.57 to 5.08 μg/100 mL (Figure 1B; Table 2). 2-Furanmethanol was the main alcohol substance in all the cultivars (0.99–2.10 μg/100 mL), whilst ethanol was detected only in ‘Yugafu’ (1.31 μg/100 mL). The other alcohols were 1-hexanol, 1-heptanol, 2-ethyl-1-hexanol, 1-octanol, and menthol, and except for 1-heptanol, there were significant differences in the concentrations of these alcohol components among cultivars. For instance, both ‘N67-10’ and ‘Yugafu’ contained significantly higher 1-hexanol with the same concentration of 0.47 μg/100 mL, whilst ‘N67-10’ and ‘Yonekura’ had a significantly greater amount of 2-ethyl-1-hexanol than in ‘Yugafu’ (0.95 and 1.14 vs. 0.66 μg/100 mL, respectively).
There were variations in terpene composition wherein ‘N67-10’ was comprised of six of eight identified compounds—namely eucalyptol, α-copaene, β-elemene, terpinen-4-ol, γ-muurolene, and α-muurolene—that accounted for a total amount of 2.68 μg/100 mL. The highest significant amount of eucalyptol was found in ‘Yugafu’ (0.67 μg/100 mL), compared to 0.38 and 0.29 μg/100 mL in ‘N67-10’ and ‘Yonekura’, respectively. Furthermore, eucalyptol, α-copaene, terpinen-4-ol, and α-muurolene, along with solely detected (E)-β-ocimene, and δ-cadinene, were accounted for in ‘Yonekura’, with a greater total concentration (5.76 μg/100 mL). ‘Yonekura’ also had remarkably higher concentrations of ketone components, such as 4-methoxy-2,5-dimethyl-3(2H)-furanone, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, γ-hexalactone, γ-octalactone, δ-octalactone, and methyl heptenone, for a total concentration up to 24.46 μg/100 mL. Additionally, the concentrations of minor components were 1.12–4.61 μg/100 mL for carboxylic acids, 0.71–4.08 μg/100 mL for aldehydes, and 0.93–1.62 μg/100 mL for hydrocarbons.
3.3. Released Volatile Components from the Okinawan Pineapple Glycosides
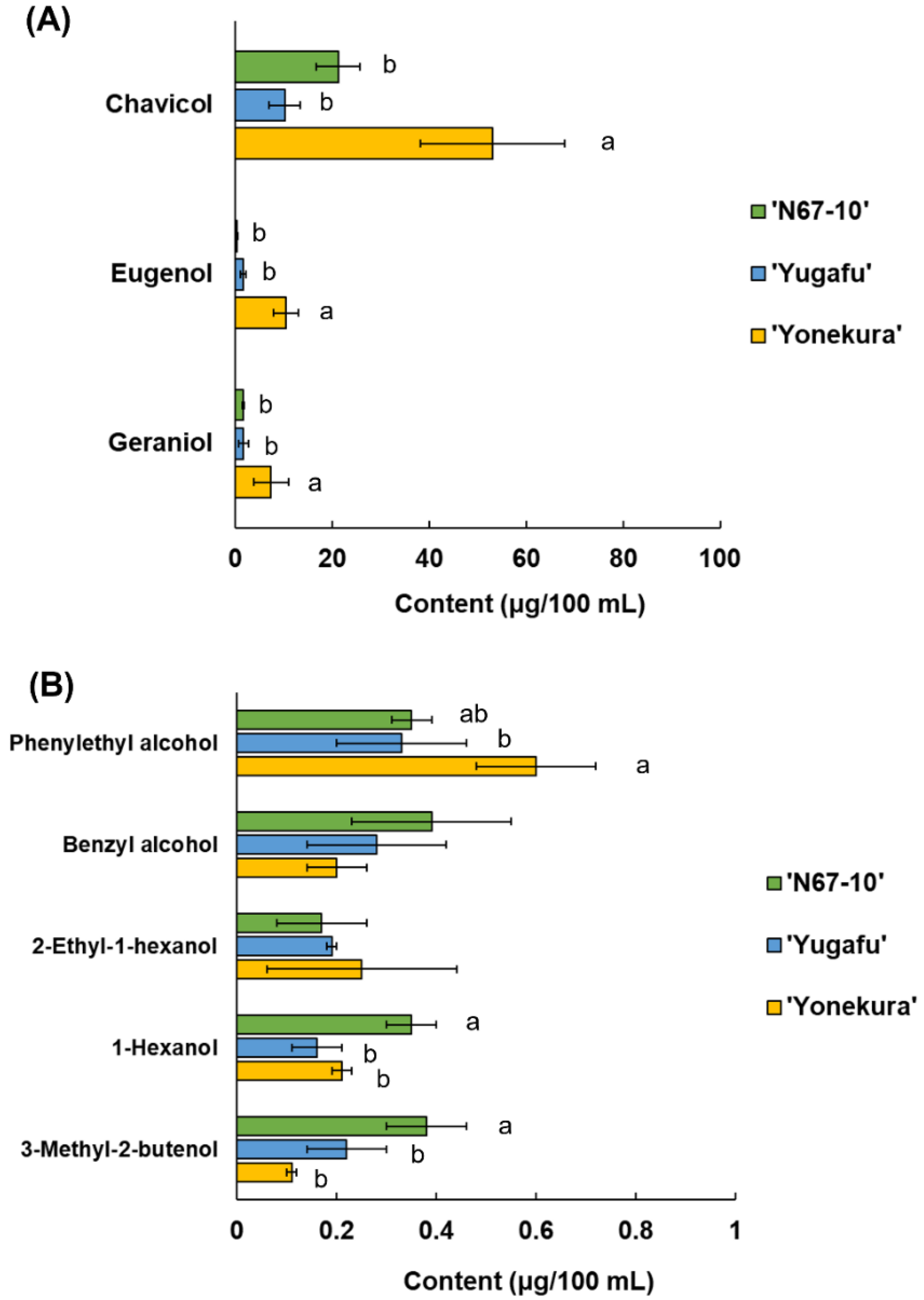
The glycoside extracts from Okinawan pineapple were hydrolyzed by β-glucosidase, and the released volatile components were composed of eight hydroxyl-group compounds. They were chavicol, eugenol, geraniol, phenylethyl alcohol, benzyl alcohol, 2-ethyl-1-hexanol, 1-hexanol, and 3-methyl-2-butenol, and there were significant variances among cultivars (Figure 2). The concentrations of the first three compounds were more than 1 μg/100 mL, wherein the amounts of these released volatile components were significantly higher in ‘Yonekura’ than in the other cultivars (Figure 2A). In detail, the amount of released chavicol in ‘Yonekura’ was 53.03 μg/100 mL compared to 21.18 and 10.19 μg/100 mL in ‘N67-10’ and ‘Yugafu’, respectively. Furthermore, the concentrations of free eugenol and geraniol aglycones in ‘Yonekura’ were 10.44 and 7.37 μg/100 mL, respectively. On the other hand, the minor components with concentrations less than 1 μg/100 mL were comprised of phenylethyl alcohol, benzyl alcohol, 2-ethyl-1-hexanol, 1-hexanol, and 3-methyl-2-butenol (Figure 2B). Hydrolyzed ‘Yonekura’ glycoside also released a significantly higher amount of phenylethyl alcohol (0.60 μg/100 mL), whilst ‘N67-10’ released significantly greater concentrations of 1-hexanol and 3-methyl-2-butenol (0.35 and 0.38 μg/100 mL, respectively). However, there were no significant differences for benzyl alcohol and 2-ethyl-1-hexanol. The average amounts of released 2-ethyl-1-hexanol and 1-hexanol from the glycoside fractions were approximately a quarter and half those of their free forms, respectively (Table 2; Figure 2B).
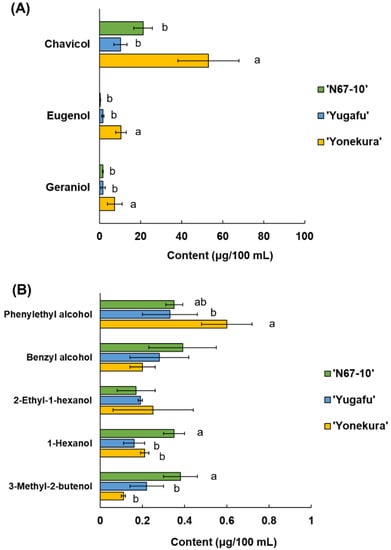
Figure 2.
Released volatile compounds from glycosides of Okinawan pineapple: (A) Major components (content is more than 1 μg/100 mL); (B) minor components (content is less than 1 μg/100 mL). Each value is expressed as the mean ± standard deviation of three replicates. Means in the same group followed by the same letter are not significantly different at p < 0.05.
3.4. Glycosidically Bound Volatile Components of Okinawan Pineapple
The sugar moiety in the hydrolyzed pineapple glycosides was D-glucose, confirmed through GC-MS analysis, specifically as a derivatization product of methoximation and trimethylsilylation, i.e., glucose oxime hexakis(trimethylsilyl). The MS fragment of this sugar was also identical to the MS fragmentation of the corresponding authentic compound of D-glucose, which was also analyzed for comparison. This sugar moiety information was then used for predicting glycosidically bound volatile compounds in LC-MS analysis.
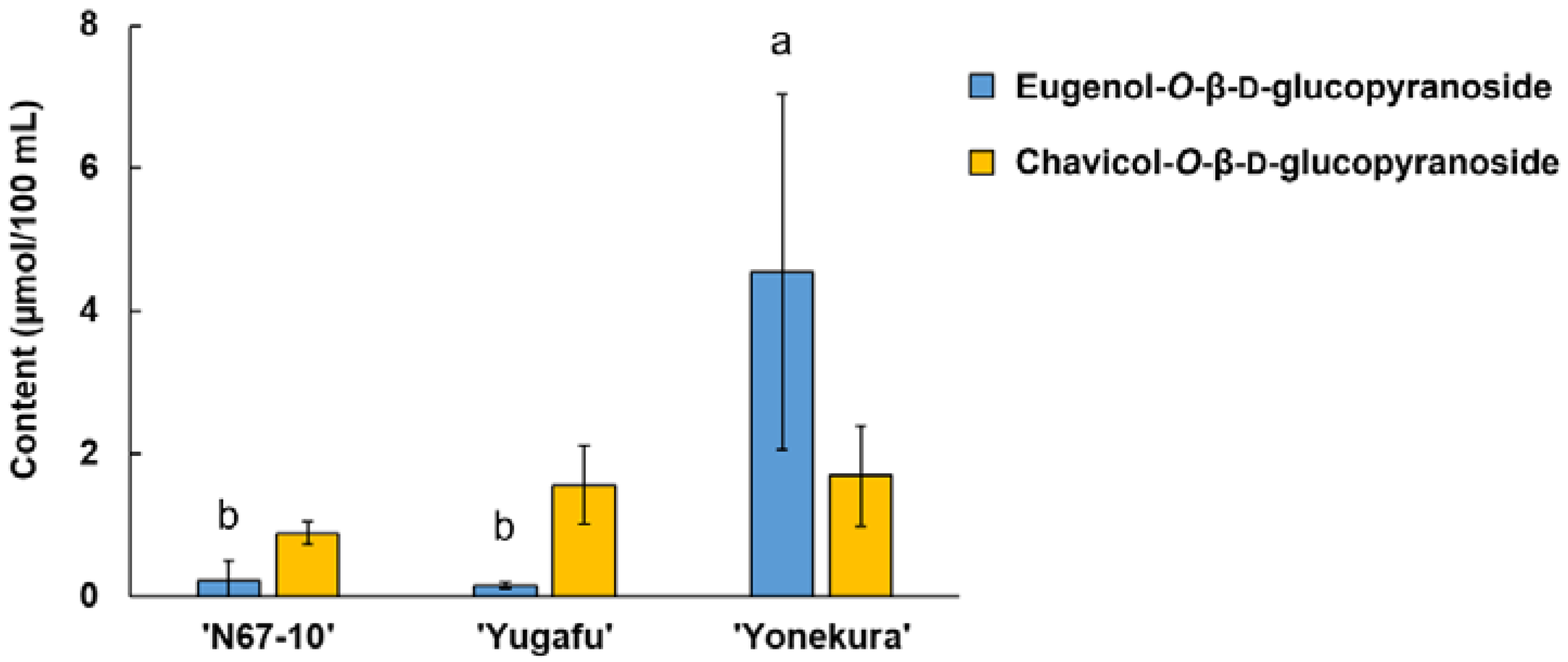
The LC-MS measurement detected two glycosidically bound volatile components: eugenol-O-β-D-glucopyranoside and chavicol-O-β-D-glucopyranoside. The MS fragments of the compounds were identified using negative ion mode with the addition of the acetate ion adduct [M+59.0]−. This MS pattern was also confirmed for p-nitrophenyl-O-β-D-glucopyranoside, which was used as an internal standard in the analysis. The target ions of eugenol-O-β-D-glucopyranoside and chavicol-O-β-D-glucopyranoside were, thus, m/z 385.3 and 355.3, respectively. These two glycosides were discovered in all the cultivars, wherein ‘Yonekura’ possessed a significantly greater amount of eugenol-O-β-D-glucopyranoside than in ‘N67-10’ and ‘Yugafu’ (4.54 vs. 0.22 and 0.15 μg/100 mL, respectively) (Figure 3). On the other hand, although the amounts of chavicol-O-β-D-glucopyranoside varied among cultivars from 0.88 to 1.69 μg/100 mL, there was no significant difference found for this glycoside.
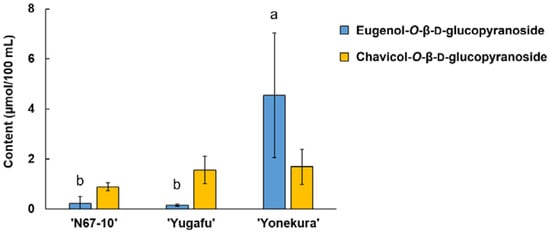
Figure 3.
Glycosidically bound volatile compounds of Okinawan pineapple (μmol/100 mL). Each value is expressed as the mean ± standard deviation of three replicates. Means in the same group followed by the same letter are not significantly different at p < 0.05.
4. Discussion
The three major cultivars of Okinawan pineapple—viz., ‘N67-10′, ‘Yugafu’, and ‘Yonekura’—differed in their green–yellowish color attributes, whilst their fruit size and flesh brightness indexes were comparable (Table 1). ‘Yonekura’ could be distinguished by its presentation of a significantly higher color space b* than the others, in which this yellow color index could be considered one of important physical traits that strongly influence the sensory attributes and acceptance of pineapple [8]. This distinct color quality is derived from phytopigments such as carotenoids, including violaxanthin, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, and β-carotene [5,18]. Furthermore, variations in the total soluble solid and titratable acidity, as well as the ratio of total soluble solid to titratable acidity, could reveal distinctive balances of soluble solids and acidity in the edible pineapple flesh of different cultivars. These traits might also influence the development of perceived aroma, and thus, increase the overall sensorial quality of pineapple [8,19]. Altogether, fruit weight, CIE L*a*b* color spaces, total soluble solid, and titratable acidity descriptions are basic physicochemical information that can be used for databases in future research.
‘Yonekura’ contained about 8.07- and 6.77-times more free volatile components than ‘N67-10’ and ‘Yugafu’, respectively (Figure 1A), indicating its potent superior aroma strength, and thus, greater scent character. The proportions of volatile compounds in Okinawan pineapple (Figure 1 B) are comparable with pineapple cultivars from other countries such as China, Cuba, and Costa Rica, in which the major components in those pineapple were also esters [2,3,19]. Methyl hexanoate and methyl 3-(methylthio)propanoate, which were predominant esters in ‘N67-10’ and ‘Yonekura’, could provide sweet-pineapple odors to both cultivars (Table 2) [2,20]. On the other hand, in addition to comprising methyl hexanoate, which might be responsible for emitting the distinct sweet-pineapple fruity odor, ‘Yugafu’ might be enriched with additional fruity odors from ethyl acetate, ethyl 2-methylbutanoate, ethyl hexanoate, and ethyl 3-(methylthio)propanoate, with distinct augmented scents [7,20]. For instance, ethyl acetate might emit solvent-like notes, whilst ethyl hexanoate could also produce a wine-like scent. In general, short-chain esters are derived from fatty acids, and the biosynthesis of these compounds is catalyzed by the alcohol acyltransferase reaction in acyl-CoA and alcohol precursors [21,22]. For instance, methyl hexanoate might be derived from methanol and hexanoyl-CoA substrates. On the other hand, thioether esters such as methyl 3-(methylthio)propanoate and ethyl 3-(methylthio)propanoate might be synthesized from L-methionine through methionine aminotransferase catalyzation [23].
In the alcohol group, 2-furanmethanol could further supplement pineapple’s fruity odor with caramel-like and cooked-nutty scents [24,25]. Additionally, 1-hexanol and 2-ethyl-1-hexanol might provide floral-oily-green and rose-green aromas, respectively [26,27,28]. Furthermore, terpenes could enhance distinct herbaceous scents in pineapple flavor, including sweet and minty (eucalyptol), spicy and woody (α-copaene), grassy and woody (β-elemene), green and waxy (terpinen-4-ol), sweet and herbal [(E)-β-ocimene], and herbal (δ-cadinene) scents [26,29,30,31] Moreover, it is noteworthy that Okinawan pineapple, particularly ‘Yonekura’, also possessed furanones and lactones. These ketone components could also greatly contribute to the overall aroma characteristics of pineapple fruit [2,7,20]. For instance, there are caramel-like odors from 4-methoxy-2,5-dimethyl-3(2H)-furanone; sweet-pineapple and caramel-like odors from 4-hydroxy-2,5-dimethyl-3(2H)-furanone; fruity and coconut-like odors from γ-octalactone; and coconut-like odors from δ-octalactone. These volatile organic substances, which were found in moderate amounts in Okinawan pineapple, might be derived from various biosynthesis pathways that occur during fruit maturation. For instance, alcohols are synthesized from fatty acids through the lipoxygenase oxidation pathway and from degraded amino acids [32]. On the other hand, the biosynthesis of terpenes is mainly regulated by terpene synthases from universal five-carbon precursors, i.e., isopentenyl diphosphate and dimethylallyl diphosphate [32,33]. Additionally, furanones might be synthesized from the D-fructose-1,6-diphosphate precursor, while lactones are fatty acid-derived molecules [32,34]. Nevertheless, variation in the content and composition of free volatiles revealed the aroma distinctiveness of each cultivar, and thus, the flavor quality of Okinawan pineapple.
Glycosidically bound volatile substances are produced in horticultural plants through various biochemical reactions in the presence of enzymatic catalysts such as UDP-glycosyltransferases that mediate the glycosylation of aglycone acceptors to activated nucleotide sugars [10,12,16]. The releasing of these aglycone volatiles from glycoside extracts revealed that Okinawan pineapples of different cultivars have potent aroma resources which are stored in their fruit tissues. The total content of liberated compounds via enzymatic hydrolysis tended to be associated with total free volatile components in which ‘Yonekura’ had greater concentrations of these two aroma compound forms, whilst ‘N67-10’ and ‘Yugafu’ contained comparable total amounts (Figure 1B and Figure 2). This trend indicates that both the free and glycosylated substances of a pineapple cultivar are produced from equivalent levels of aroma precursors, and their amounts and compositions can be used for differentiating individual pineapple cultivars [11].
The main volatile compounds which were liberated at concentrations of more than 1 μg/100 mL, such as chavicol and eugenol, have strong distinct aromas. These phenylpropenes could emit medicinal-herbal odors such as spicy-floral and spicy-clove scents, respectively [27,35]. Moreover, released geraniol might provide potent sweet-floral, fruity, and citrus aromas [30,31] Additionally, the released minor components from pineapple glycosides have been known to be responsible for producing various characteristic odors, such as rose-honey (phenylethyl alcohol), sweet-floral (benzyl alcohol), rose-green (2-ethyl-1-hexanol), and floral-oily-green (1-hexanol) [27,28,29]. Among these glycosylated volatile molecules, 2-ethyl-1-hexanol and 1-hexanol were also found in their free forms (Table 2; Figure 2B), indicating that complex biochemical reactions had occurred during fruit maturation; thus, more studies are needed to reveal the molecular mechanisms underlying aroma development in pineapple [12].
Higher concentrations of two glycosides of phenylpropenes, chavicol-O-β-D-glucopyranoside and eugenol-O-β-D-glucopyranoside, were detected in ‘Yonekura’, whilst comparable amounts of these non-volatile substances were found in the other cultivars (Figure 3). This trend is also in agreement with the above descriptions on the total content of both free- and released-volatile components (Figure 1B and Figure 2). However, the amount of eugenol-O-β-D-glucopyranoside in ‘Yonekura’ in particular was much higher than that of its released volatile compounds after hydrolysis. Nevertheless, these glycosidically bound volatile components are important potential sources of aroma compounds as strong volatiles in Okinawan pineapple for storing chavicol and eugenol with strong characteristic medicinal-herbal aromas [27,35]. This result provides important information on the potent aroma releases from pineapples of different cultivars in post-harvest treatments, such as fermentation and storage, that may initiate the hydrolysis of glycosidically bound volatile components, and thus, extend further aroma production to the processed pineapple fruits or their derived products [12,36]. On the other hand, careful consideration must be given to the usage of the released aglycones with undesirable strong odors in processed pineapple, which may alter the overall flavor quality of the final products [36].
Taken together, the outcomes of this study could provide systematic knowledge on the free and glycosidically bound volatile components of major pineapple cultivars from Okinawa, Japan. This information could be used as an important basis for further flavor and sensory characterization studies of the possible flavor characteristics of these aroma resources in Okinawan pineapple for practical applications, when they are consumed as fresh fruit or processed into various food and beverage products.
5. Conclusions
Okinawan pineapples of different cultivars varied in the color spaces a* (green index) and b* (yellow index), as well as in their total soluble solid and titratable acidity traits. These cultivars also varied in their content and composition of free volatile compounds, wherein ‘Yonekura’ contained about 8.07- and 6.77-times higher concentrations than ‘N67-10’ and ‘Yugafu’, respectively. They were predominantly comprised of esters (29 compounds), followed by alcohols, terpenes, and ketones. The major ester compounds in ‘Yonekura’ were methyl hexanoate and methyl 3-(methylthio)propionate, respectively. Both esters are known for emitting sweet-pineapple fruity odors that could be strongly related to the overall aroma characteristics of pineapple fruit. Seven alcohols were detected in free form, such as 2-furanmethanol, 2-ethyl-1-hexanol, 1-hexanol, and 1-octanol. Furthermore, glycosidically bound volatiles were hydrolyzed via enzymatic reactions and eight hydroxyl-group compounds were released, including chavicol, eugenol, geraniol, phenylethyl alcohol, benzyl alcohol, 2-ethyl-1-hexanol, 1-hexanol, and 3-methyl-2-butenol. The amounts of glycosidically bound chavicol, eugenol, and geraniol were greater than 1 μg/100 mL and were significantly higher in ‘Yonekura’ than in the other cultivars. Moreover, the amounts of released 2-ethyl-1-hexanol and 1-hexanol from the glycoside fractions were approximately a quarter and half those of their free forms, respectively. The sugar moiety of these glycosides was confirmed to be D-glucose. Additionally, the two glycosides were determined to be chavicol-O-β-D-glucopyranoside and eugenol-O-β-D-glucopyranoside in all three cultivars. The aglycones of these glycoside substances should be further carefully utilized as attractive-aroma resources when processing Okinawan pineapple fruits due to their strong medicinal-floral herb and clove-like scents, respectively. The outcomes of this study, thus, provide important information regarding both free and glycosidically bound volatile compounds in pineapples of Okinawan origin for fresh consumption and processing into various foods and beverages.
Author Contributions
Conceptualization, Y.A., M.T. and K.W.; methodology, Y.A., Y.K., K.T. and K.W; validation, Y.K., K.T. and K.W.; formal analysis, Y.A. and K.S.; investigation, Y.A. and K.T.; resources, M.T. and R.M.; data curation, Y.A. and K.S.; writing—original draft preparation, Y.A. and K.S.; writing—review and editing, Y.A. and K.W.; visualization, Y.A.; supervision, K.W.; project administration, M.T., R.M., and K.W.; funding acquisition, M.T. and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the research program on the development of innovative technology from the project of the Bio-oriented Technology Research Advancement Institution (BRAIN), grant number JPJ007097.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Eriko Arakaki (University of the Ryukyus) for her technical assistance in the volatile component analysis. The glycoside composition analysis was performed at the Center for Research Advancement and Collaboration, University of the Ryukyus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaewtathip, T.; Charoenrein, S. Changes in volatile aroma compounds of pineapple (Ananas comosus) during freezing and thawing. Int. J. Food Sci. Technol. 2012, 47, 985–990. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Sun, G.-M.; Liu, Y.-G.; Lv, L.-L.; Yang, W.-X.; Zhao, W.-F.; Wei, C.-B. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A. Odour-active compounds in pineapple (Ananas comosus [L.] Merril cv. Red Spanish). Int. J. Food Sci. Technol. 2013, 48, 564–570. [Google Scholar] [CrossRef]
- Shoda, M.; Urasaki, N.; Sakiyama, S.; Terakami, S.; Hosaka, F.; Shigeta, N.; Nishitani, C.; Yamamoto, T. DNA profiling of pineapple cultivars in Japan discriminated by SSR markers. Breed. Sci. 2012, 62, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Nishiba, Y.; Takeuchi, M.; Moromizato, C. Carotenoid content in different varieties of pineapple (Ananas comosus L.) cultivated in Okinawa Prefecture. Nippon Shokuhin Kagaku Kogaku Kaishi 2019, 66, 100–107. [Google Scholar] [CrossRef]
- Yao, S. On Asia-Pacific pineapple industry transfer beyond Japanese Empire: Focusing on Hawaii, Taiwan and Okinawa. Hakusan Rev. Anthropol. 2018, 21, 81–104. [Google Scholar]
- Tokitomo, Y.; Steinhaus, M.; Büttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef]
- Romli, S.R.; Easa, A.M.; Murad, M. Influence of post-harvest physiology on sensory perception, physical properties, and chemical compositions of Moris pineapples (Ananas comosus L.). J. Food Sci. 2021, 86, 4159–4171. [Google Scholar] [CrossRef]
- Jana, S.; Mukherjee, S.; Ali, I.; Ray, B.; Ray, S. Isolation, structural features, in vitro antioxidant activity and assessment of complexation ability with β-lactoglobulin of a polysaccharide from Borassus flabellifer fruit. Heliyon 2020, 6, e05499. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Ged, C.; Wang, M.Y.; Matich, A.J.; Tessarotto, L.; Cooney, J.M.; Chervin, C.; Atkinson, R.G. Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J. 2014, 80, 317–330. [Google Scholar] [CrossRef]
- Ghaste, M.; Narduzzi, L.; Carlin, S.; Vrhovsek, U.; Shulaev, V.; Mattivi, F. Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 2015, 188, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, S.; Ono, E.; Horikawa, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; et al. Volatile glycosylation in tea plants: Sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Pankoke, H.; Buschmann, T.; Müller, C. Role of plant β-glucosidases in the dual defense system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major. Phytochemistry 2013, 94, 99–107. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma glycosides in grapes and wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Kamiya, A.; Mizu, M.; Takara, K.; Tamaki, H.; Wada, K. Changes in the physicochemical characteristics, including flavour components and Maillard reaction products, of non-centrifugal cane brown sugar during storage. Food Chem. 2014, 149, 170–177. [Google Scholar] [CrossRef]
- Kamiyoshihara, Y.; Tieman, D.M.; Klee, H.J. Analyses of plant UDP-dependent glycosyltransferases to identify their volatile substrates using recombinant proteins. Methods Mol. Biol. 2016, 1363, 199–207. [Google Scholar] [CrossRef]
- Tamura, Y.; Iwatoh, S.; Miyaura, K.; Asikin, Y.; Kusano, M. Metabolomic profiling reveals the relationship between taste-related metabolites and roasted aroma in aged pork. LWT 2022, 155, 112928. [Google Scholar] [CrossRef]
- Steingass, C.B.; Vollmer, K.; Lux, P.E.; Dell, C.; Carle, R.; Schweiggert, R.M. HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Res. Int. 2020, 127, 108709. [Google Scholar] [CrossRef]
- Montero-Calderón, M.; Rojas-Graü, M.A.; Martín-Belloso, O. Aroma profile and volatiles odor activity along gold cultivar pineapple flesh. J. Food Sci. 2010, 75, S506–S512. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, G. A pineapple flavor imitation by the note method. Food Sci. Technol. 2019, 40, 924–928. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Souleyre, E.J.F.; Matich, A.J.; Chen, X.; Wang, M.Y.; Plunkett, B.; Dare, A.P.; Espley, R.V.; Tomes, S.; Chagné, D.; et al. Alcohol acyl transferase 1 links two distinct volatile pathways that produce esters and phenylpropenes in apple fruit. Plant J. 2017, 91, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kong, W.; Yang, C.; Feng, R.; Xi, W. Alcohol acyltransferase is involved in the biosynthesis of C6 esters in apricot (Prunus armeniaca L.) fruit. Front Plant Sci. 2021, 12, 763139. [Google Scholar] [CrossRef] [PubMed]
- Gonda, I.; Lev, S.; Bar, E.; Sikron, N.; Portnoy, V.; Davidovich-Rikanati, R.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Giovannonni, J.J.; et al. Catabolism of L-methionine in the formation of sulfur and other volatiles in melon (Cucumis melo L.) fruit. Plant J. 2013, 74, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying-solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT-Food Sci Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Lin, L.-Y.; Yang, K.-M.; Chiang, Y.-C.; Chen, M.-H.; Chiang, P.-Y. Effects of roasting sweet potato (Ipomoea batatas L. Lam.): Quality, volatile compound composition and sensory evaluation. Foods 2021, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Nakashima, Y.; Nakahashi, H.; Hara, N.; Nakagawa, H.; Usami, A.; Chavasiri, W. Volatile compounds with characteristic odor of essential oil from Magnolia obovata leaves by hydrodistillation and solvent-assisted flavor evaporation. J. Oleo Sci. 2015, 64, 999–1007. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Aroma potential in early- and late-maturity Pinot noir grapes evaluated by aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 443–450. [Google Scholar] [CrossRef]
- Niu, Y.; Kong, J.; Xiao, Z.; Chen, F.; Ma, N.; Zhu, J. Characterization of odor-active compounds of various Chinese “Wuliangye” liquors by gas chromatography–olfactometry, gas chromatography–mass spectrometry and sensory evaluation. Int. J. Food Prop. 2017, 20, S735–S745. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, C.; Yan, H.; Peng, Y.; Hu, E.; Qi, J.; Lin, Q. Characteristic aroma compound in cinnamon bark extract using soybean oil and/or water. Appl. Sci. 2022, 12, 1284. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Compositional analysis and aroma evaluation of feijoa essential oils from New Zealand grown cultivars. Molecules 2019, 24, 2053. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Koeduka, T.; Aida, M.; Suzuki, H.; Iijima, Y.; Matsui, K. Biosynthesis of volatile terpenes that accumulate in the secretory cavities of young leaves of Japanese pepper (Zanthoxylum piperitum): Isolation and functional characterization of monoterpene and sesquiterpene synthase genes. Plant Biotechnol. 2017, 34, 17–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwab, W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules 2013, 18, 6936–6951. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Honda, T.; Fujita, A.; Kurobayashi, Y.; Kitahara, T. “Aqua-space®”, a new headspace method for isolation of natural floral aromas using humidified air as a carrier gas. Biosci. Biotechnol. Biochem. 2004, 68, 454–457. [Google Scholar] [CrossRef]
- Caffrey, A.; Ebeler, S.E. The occurrence of glycosylated aroma precursors in Vitis vinifera fruit and Humulus lupulus hop cones and their roles in wine and beer volatile aroma production. Foods 2021, 10, 935. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).