Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Microorganism
2.2. Microbial Acclimation
2.3. Strain Isolation and Identification
2.4. Batch Hydrogen Production
2.5. Analytical Methods
2.6. Kinetic Modeling
3. Results and Discussion
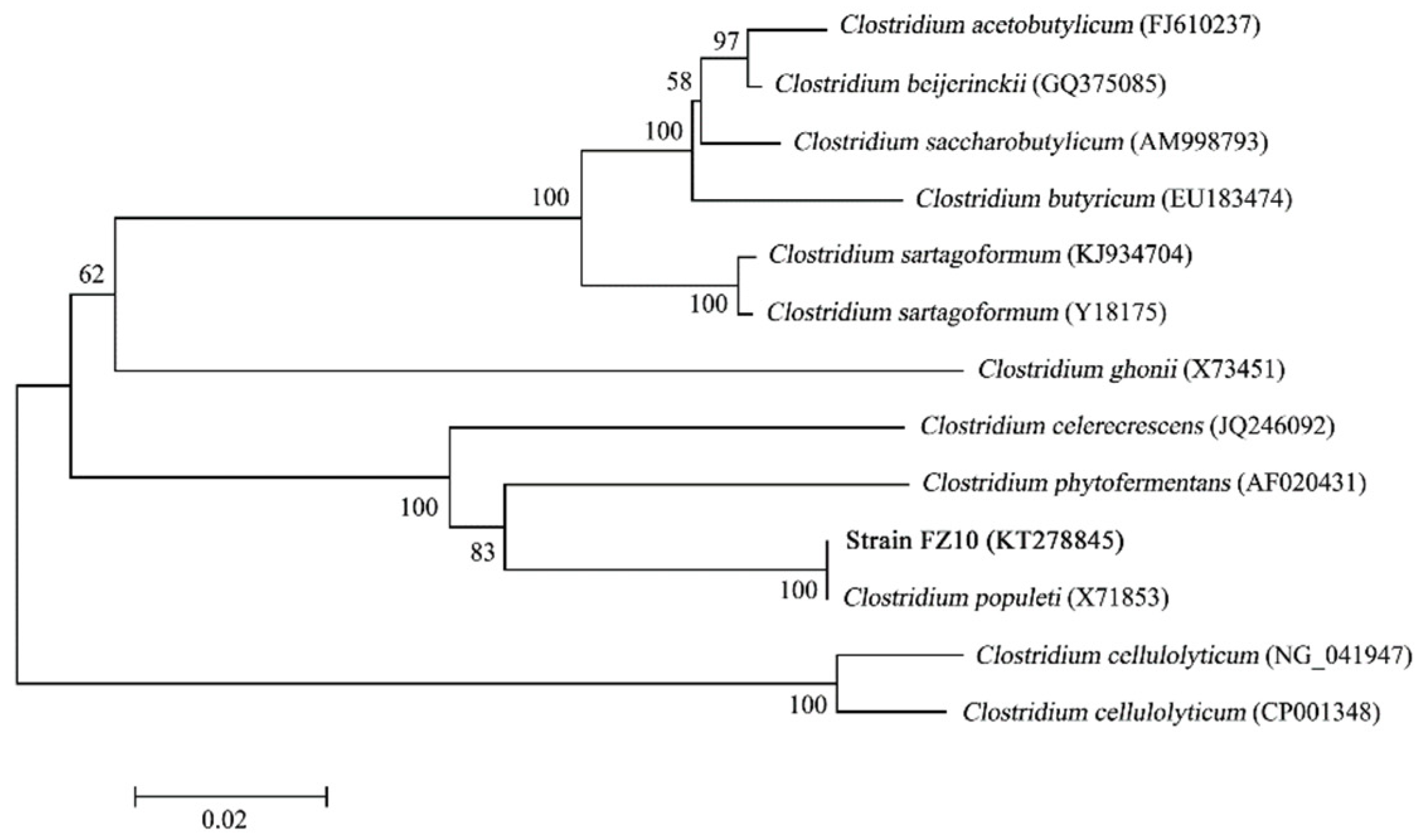
3.1. Isolation and Identification of Bacterial Strain
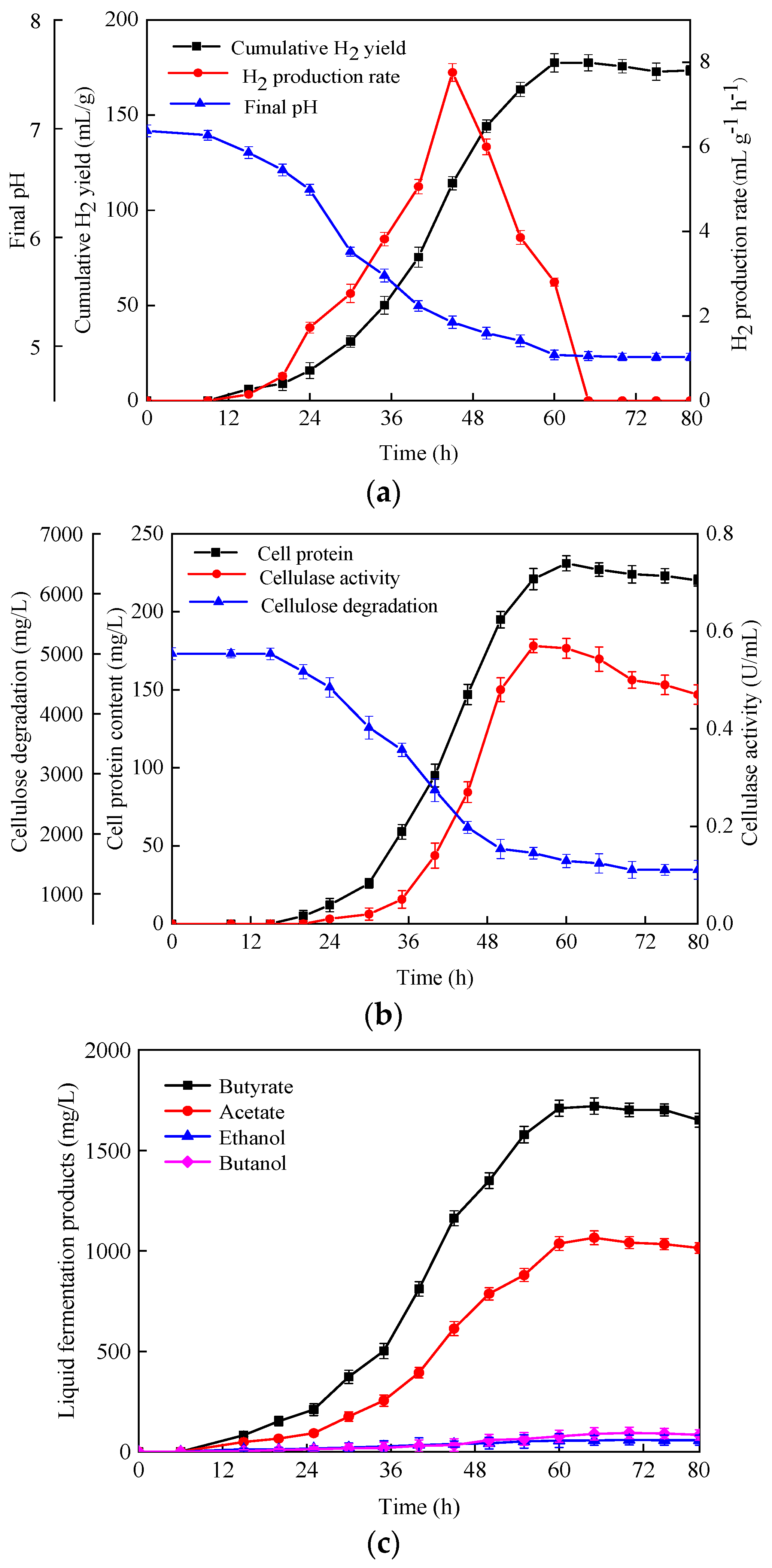
3.2. Hydrogen Production Performance of C. populeti FZ10
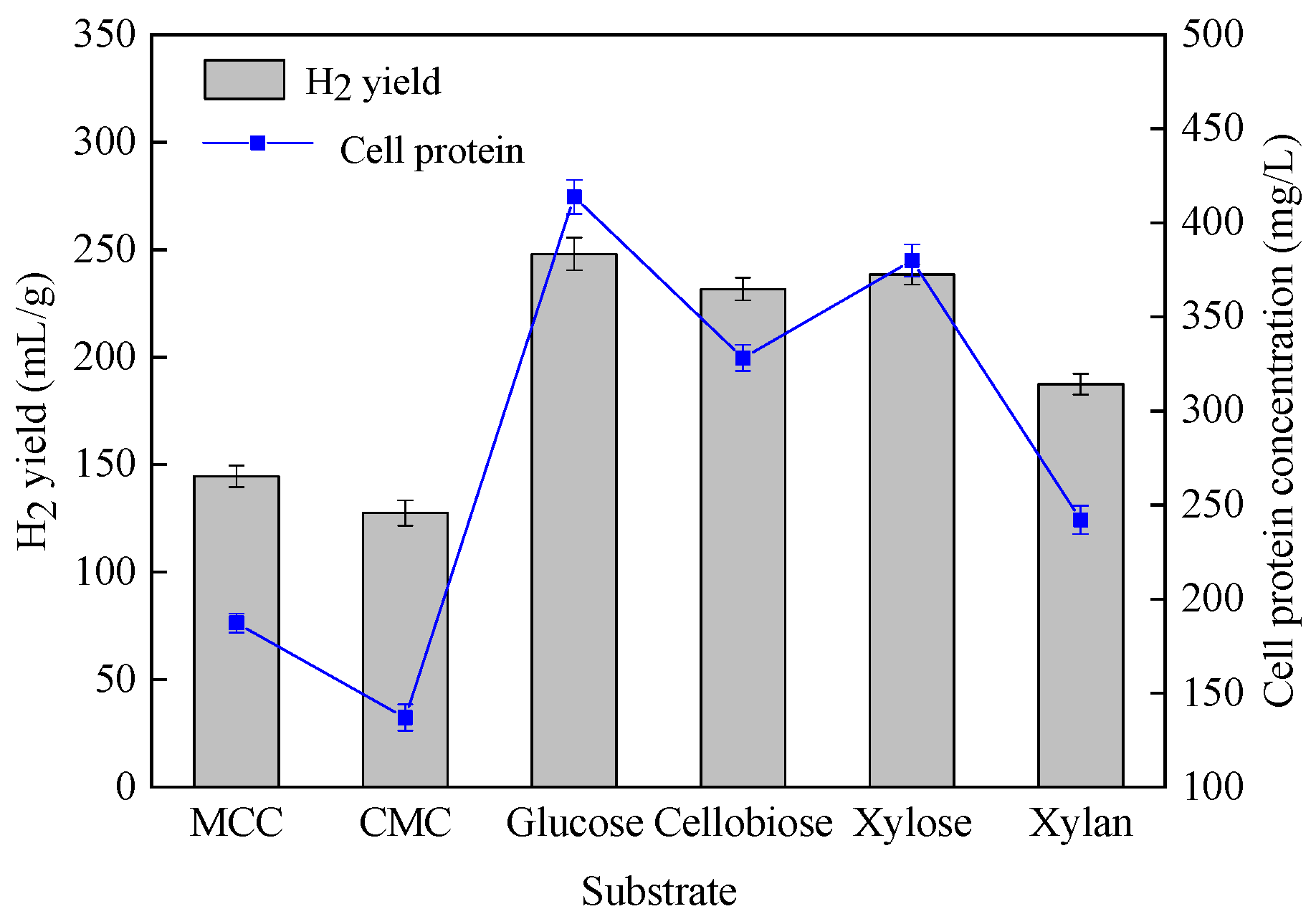
3.2.1. Hydrogen Production Using Defined Carbohydrates
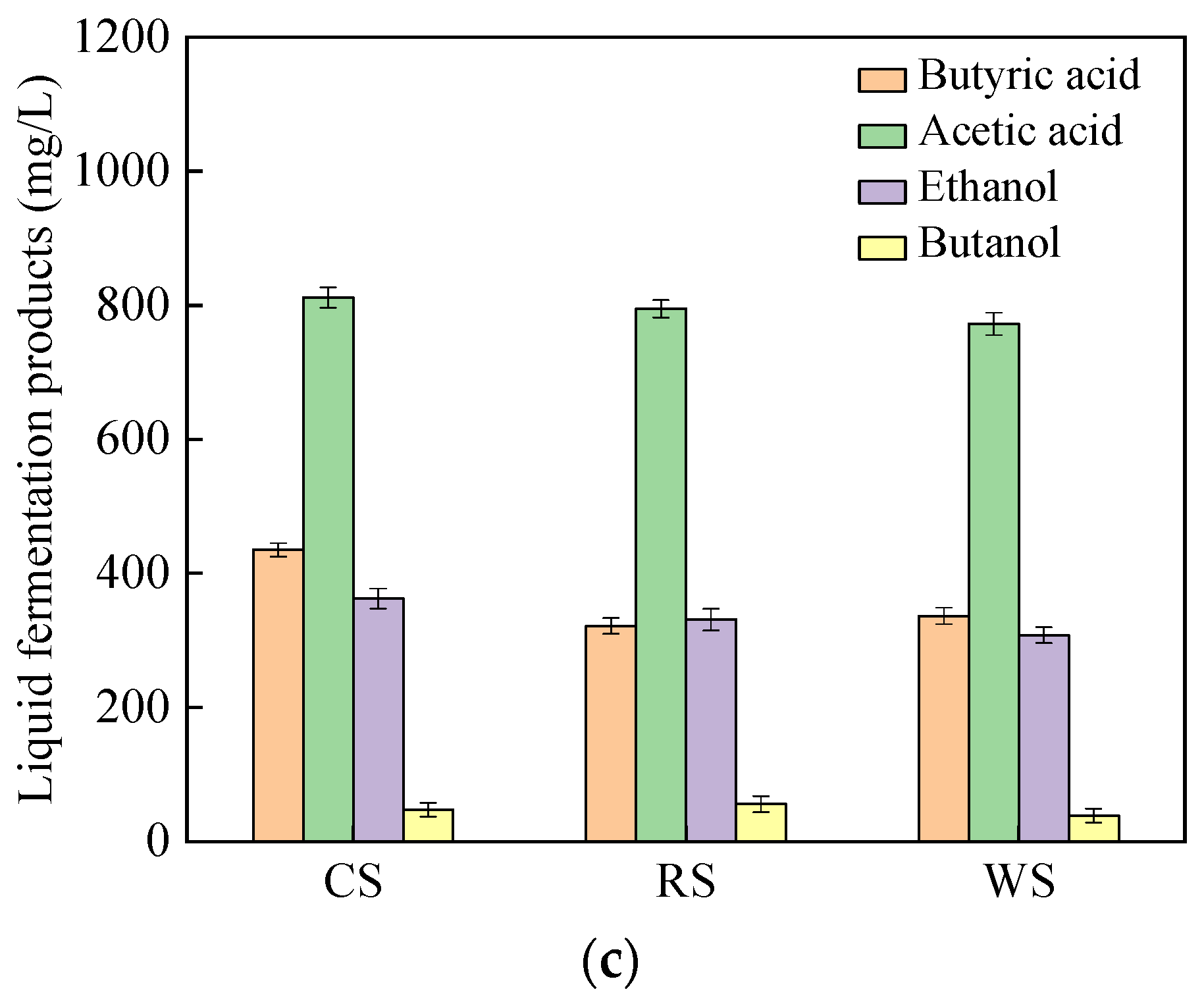
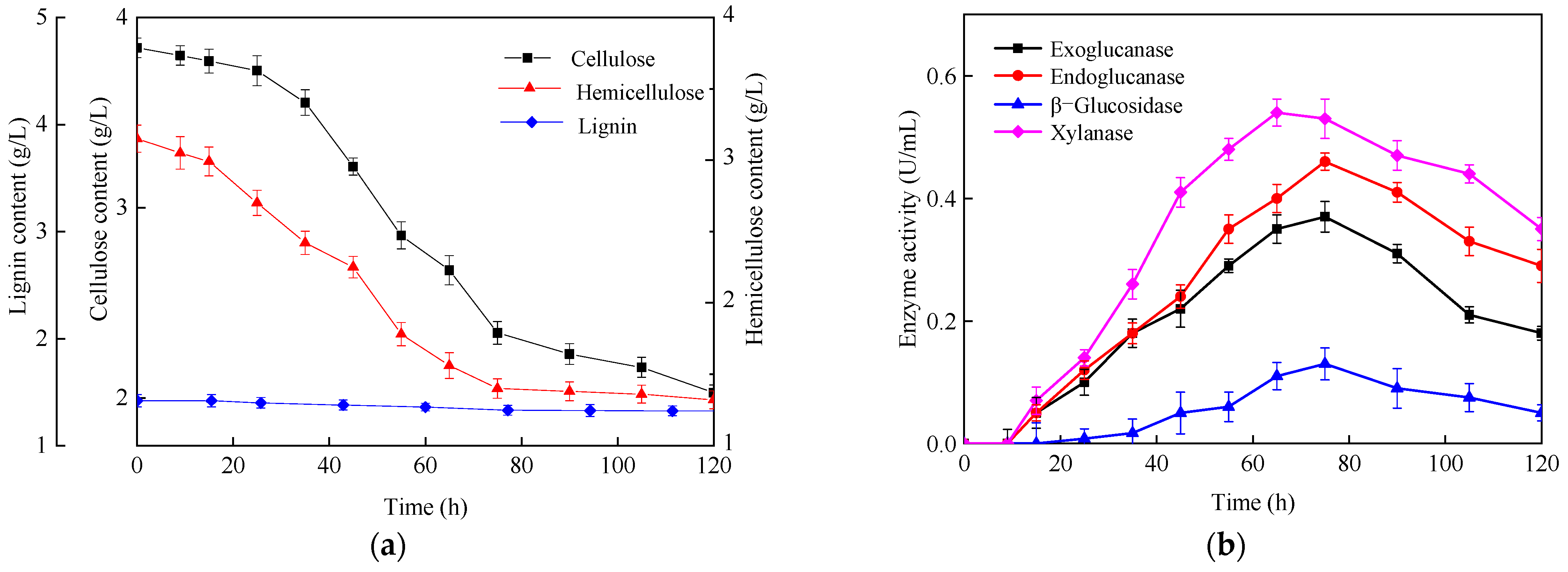
3.2.2. Hydrogen Production Using Natural Lignocellulosic Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenz, O. Hydrogen comes alive. Nat. Energy 2020, 5, 426–427. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; de Souza Candeo, E.; Pedroni Medeiros, A.B.; Cesar de Carvalho, J.; Oliveira de Andrade Tanobe, V.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2021, 286, 124970. [Google Scholar] [CrossRef]
- Wu, X.Y.; Luo, Y.; Hess, F.; Lipinski, W. Sustainable hydrogen for energy, fuel and commodity applications. Front. Energy Res. 2021, 9, 231. [Google Scholar] [CrossRef]
- Abdel-Kader, H.A.A.; Abdel-Basset, R.; Danial, A.W. Yeast and enzymatic hydrolysis in converting Chlorella biomass into hydrogen gas by Rhodobacter sp. and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2022, 47, 1516–1528. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Vo, D.V.N.; Nabgan, W. An overview on the efficiency of biohydrogen production from cellulose. Biomass Convers. Bior. 2020. [CrossRef]
- Baik, J.H.; Jung, J.H.; Sim, Y.B.; Park, J.H.; Kim, S.M.; Yang, J.; Kim, S.H. High-rate biohydrogen production from xylose using a dynamic membrane bioreactor. Bioresour. Technol. 2022, 344, 126205. [Google Scholar] [CrossRef]
- Lu, X.H.; Li, F.; Zhou, X.; Hu, J.R.; Liu, P. Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chem. 2022, 384, 13262. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Das, D.; Lee, J.K.; Kalia, V.C. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J. Clean. Prod. 2021, 287, 125037. [Google Scholar] [CrossRef]
- Usman, M.; Kavitha, S.; Kannah, Y.; Yogalakshmi, K.N.; Sivashanmugam, P.; Bhatnagar, A.; Kumar, G. A critical review on limitations and enhancement strategies associated with biohydrogen production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar]
- Jomnonkhaow, U.; Sittijunda, S.; Reungsang, A. Influences of size reduction, hydration, and thermal-assisted hydration pretreatment to increase the biogas production from Napier grass and Napier silage. Bioresour. Technol. 2021, 331, 125034. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Recent insights into consolidated bioprocessing for lignocellulosic biohydrogen production. Int. J. Hydrogen Energy 2019, 44, 14362–14379. [Google Scholar] [CrossRef]
- Ratti, R.P.; Botta, L.S.; Sakamoto, I.K.; Varesche, M.B.A. Microbial diversity of hydrogen-producing bacteria in batch reactors fed with cellulose using leachate as inoculums. Int. J. Hydrogen Energy 2013, 38, 9707–9717. [Google Scholar] [CrossRef]
- Lo, Y.C.; Su, Y.C.; Chen, C.Y.; Chen, W.M.; Lee, K.S.; Chang, J.S. Biohydrogen production from cellulosic hydrolysate produced via temperature-shift-enhanced bacterial cellulose hydrolysis. Bioresour. Technol. 2009, 100, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Zagrodnik, R.; Duber, A.; Seifert, K. Hydrogen production during direct cellulose fermentation by mixed bacterial culture: The relationship between the key process parameters using response surface methodology. J. Clean. Prod. 2021, 314, 127971. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- RamKumar, N.; Anupama, P.D.; Nayak, T.; Subudhi, S. Scale up of biohydrogen production by a pure strain; Clostridium butyricum TM-9A at regulated pH under decreased partial pressure. Renew. Energy 2021, 170, 1178–1185. [Google Scholar] [CrossRef]
- Koo, J. Enhanced aerobic H2 production by engineering an [FeFe] hydrogenase from Clostridium pasteurianum. Int. J. Hydrogen Energy 2020, 45, 10673–10679. [Google Scholar] [CrossRef]
- Guerrero, K.; Gallardo, R.; Paredes, I.; Quintero, J.; Mau, S.; Conejeros, R.; Gentina, J.C.; Aroca, G. Continuous biohydrogen production by a degenerated strain of Clostridium acetobutylicum ATCC 824. Int. J. Hydrogen Energy 2022, 46, 5100–5111. [Google Scholar] [CrossRef]
- Srimawong, C.; Chulalaksananukul, W. Evaluating biohydrogen production by Clostridium hydrogenum sp. nov. strain CUEA01 isolated from mangrove sediments in Thailand. Int. J. Hydrogen Energy 2022, 47, 9169–9182. [Google Scholar] [CrossRef]
- Constante, F.B.; Giovanni, D.; Cress, G.V.; Sibeli, C.; Parras, M.L.; Latorre, Z.A.L.R.; Melo, F.R.P.; Rita, T.D.; Valeria, R. Use of algae biomass obtained by single-step mild acid hydrolysis in hydrogen production by the beta-glucosidase-producing Clostridium beijerinckii Br21. Waste Biomass Valorization 2020, 11, 1393–1402. [Google Scholar]
- An, Q.; Cheng, J.; Wang, Y.; Zhu, M. Performance and energy recovery of single and two stage biogas production from paper sludge: Clostridium thermocellum augmentation and microbial community analysis. Renew. Energy 2020, 148, 214–222. [Google Scholar] [CrossRef]
- Morales-Martinez, T.K.; Medina-Morales, M.A.; Ortiz-Cruz, A.L.; Rodriguez-De La Garza, J.A.; Moreno-Davila, M.; Lopez-Badillo, C.M.; Rios-Gonzalez, L. Consolidated bioprocessing of hydrogen production from agave biomass by Clostridium acetobutylicum and bovine ruminal fluid. Int. J. Hydrogen Energy 2020, 45, 13707–13716. [Google Scholar] [CrossRef]
- Lo, Y.C.; Chen, W.M.; Hung, C.H.; Chen, S.D.; Chang, J.S. Dark H2 fermentation from sucrose and xylose using H2-producing indigenous bacteria: Feasibility and kinetic studies. Water Res. 2008, 42, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, L.; Islam, R.; Sparling, R.; Levin, D.; Cicek, N. Direct hydrogen production from cellulosic waste materials with a single-step dark fermentation process. Int. J. Hydrogen Energy 2008, 33, 5398–5403. [Google Scholar] [CrossRef]
- Zhang, J.N.; Li, Y.H.; Zheng, H.Q.; Fan, Y.T.; Hou, H.W. Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain Clostridium sartagoforme FZ11. Bioresour. Technol. 2015, 192, 60–67. [Google Scholar] [CrossRef]
- Doetsch, R.N. Determinative methods of light microscopy. In Manual of Methods for General Bacteriology; Gerhardt, P., Ed.; American Society for Microbiology: Washington, DC, USA, 1981; pp. 29–30. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- An, D.; Li, Q.; Wang, X.Q.; Yang, H.H.; Guo, L.J. Characterization on hydrogen production performance of a newly isolated Clostridium beijerinckii YA001 using xylose. Int. J. Hydrogen Energy 2014, 39, 19928–19936. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.X.; Li, X.H.; Li, W.W.; Bai, Y.X.; Fan, Y.T.; Hou, H.W. Direct bioconversion of raw corn stalk to hydrogen by a new strain Clostridium sp. FS3. Bioresour. Technol. 2014, 157, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.L.; Zhao, L.; Wang, A.J.; Wang, Z.Y.; Ren, N.Q. Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol. Biofuels 2014, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.N.; Sun, H.; Pan, C.M.; Fan, Y.T.; Hou, H.W. Optimization of process parameters for directly converting raw corn stalk to biohydrogen by Clostridium sp. FZ11 without substrate pretreatment. Energy Fuels 2016, 30, 311–317. [Google Scholar] [CrossRef]
- Rattanachomsri, U.; Tanapongpipat, S.; Eurwilaichitr, L.; Champreda, V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J. Biosci. Bioeng. 2009, 107, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Li, D.M.; Chen, H.Z. Biological hydrogen production from steam-exploded straw by simultaneous saccharification and fermentation. Int. J. Hydrogen Energy 2007, 32, 1742–1748. [Google Scholar] [CrossRef]
- Lu, Y.; Lai, Q.H.; Zhang, C.; Zhao, H.X.; Ma, K.; Zhao, X.B.; Chen, H.Z.; Liu, D.H.; Xing, X.H. Characteristics of hydrogen and methane production from cornstalks by an augmented two- or three-stage anaerobic fermentation process. Bioresour. Technol. 2009, 100, 2889–2895. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Li, Y.; Liu, X.S.; Ren, N.Q.; Ding, J. Lignocellulosic hydrogen production using dark fermentation by Clostridium lentocellum strain Cel10 newly isolated from Ailuropoda melanoleuca excrement. RSC Adv. 2019, 9, 11179–11185. [Google Scholar] [CrossRef]
- Ren, N.Q.; Wang, A.J.; Gao, L.F.; Liang, X.; Lee, D.J.; Su, A. Bioaugmented hydrogen production from carboxymethyl cellulose and partially delignified corn stalks using isolated cultures. Int. J. Hydrogen Energy 2008, 33, 5250–5255. [Google Scholar] [CrossRef]
- Ramachandran, U.; Wrana, N.; Cicek, N.; Sparling, R.; Levin, D.B. Hydrogen production and end-product synthesis patterns by Clostridium termitidis strain CT1112 in batch fermentation cultures with cellobiose or a-cellulose. Int. J. Hydrogen Energy 2008, 33, 7006–7012. [Google Scholar] [CrossRef]
- Ren, Z.; Ward, T.E.; Logan, B.E.; Regan, J.M. Characterization of the cellulolytic and hydrogen-producing activities of six mesophilic Clostridium species. J. Appl. Microbiol. 2007, 103, 2258–2266. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour. Technol. 2012, 104, 373–379. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Hydrogen production via thermophilic fermentation of cornstalk by Clostridium thermocellum. Energy Fuels 2011, 25, 1714–1720. [Google Scholar] [CrossRef]


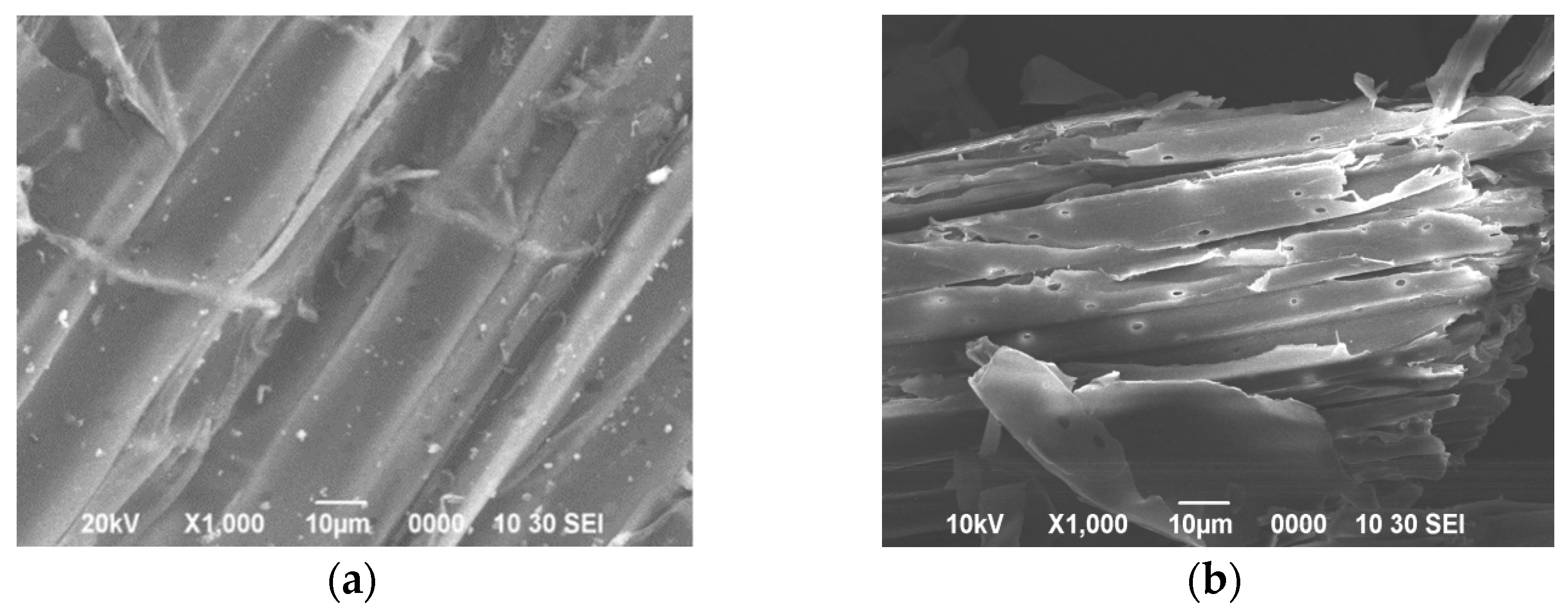
| Chemical Composition | MCC | Peptone | KH2PO4 | K2HPO4 | FeSO4·7H2O | MgCl2 | L-cysteine·HCl·H2O |
| Content (g/L) | 10 | 2 | 1 | 1 | 0.1 | 0.1 | 0.5 |
| Chemical Composition | Carbon source | Peptone | Yeast extract | L-cysteine·HCl·H2O | Nutrient solution |
| Content (g/L) | 10 | 2 | 2 | 0.5 | 10 |
| Chemical Composition | MgSO4·7H2O | FeCl2 | Na2MoO4·2H2O | CaCl2·2H2O | NaCl | MnSO4·7H2O |
| Content (g/L) | 0.1 | 0.00278 | 0.01 | 0.01 | 0.01 | 0.015 |
| Substrate (10 g/L) | Degradation (%) | Fermentation Products (mg/L) | |||
|---|---|---|---|---|---|
| Acetate | Butyrate | Ethanol | Butanol | ||
| MCC | 72 ± 2.4 | 1065.25 ± 34.55 | 1721.11 ± 76.73 | 57.61 ± 3.22 | 93.24 ± 4.11 |
| CMC | 68 ± 2.5 | 907.12 ± 41.23 | 1435.30 ± 70.15 | 75.52 ± 3.13 | 182.64 ± 7.81 |
| Glucose | 100 | 1174.75 ± 56.17 | 1846.89 ± 85.44 | 81.10 ± 2.54 | 56.41 ± 2.23 |
| Xylose | 100 | 1102.43 ± 43.76 | 1701.80 ± 68.52 | 90.71 ± 3.61 | 104.34 ± 4.41 |
| Cellobiose | 100 | 992.51 ± 40.15 | 1517.10 ± 75.31 | 73.14 ± 2.88 | 102.11 ± 3.71 |
| Xylan | 98.2 ± 4.3 | 1091.16 ± 53.61 | 1810.12 ± 68.51 | 66.07 ± 2.67 | 48.55 ± 2.52 |
| Substrate | Ps (mL/g) | λ (h) | Rm (mL·g−1·h−1) | R2 |
|---|---|---|---|---|
| Corn stalk | 92.5 ± 3.7 | 9 | 5.9 ± 0.2 | 0.993 |
| Wheat straw | 86.2 ± 3.5 | 15 | 5.6 ± 0.3 | 0.995 |
| Rice straw | 88.5 ± 4.3 | 16.5 | 5.7 ± 0.2 | 0.997 |
| Microorganism | Substrate | Conditions | T (°C) | H2 Yield (mL/g) | References |
|---|---|---|---|---|---|
| C. lentocellum Cel10 | CMC | Batch, Untreated | 37 | 121.41 | [41] |
| C.sartagoforme FZ11 | MCC | Batch, Untreated | 35 | 77.28 | [26] |
| C. acetobutylicum X9 | MCC | Batch, Untreated | 37 | 67.2 | [42] |
| C. termitidis CT1112 | α-cellulose | Batch, Untreated | 37 | 87.36 | [43] |
| C. cellulolyticum | MCC | Batch, Untreated | 35 | 138.9 | [44] |
| C. populeti FZ10 | MCC | Batch, Untreated | 35 | 177.5 | This study |
| C. lentocellum Cel10 | Cassava residues | Batch, Untreated | 37 | 91.39 | [41] |
| C.sartagoforme FZ11 | Corn stalk | Batch, Untreated | 35 | 87.2 | [26] |
| C. thermocellum 27405 | Dried distillers grain | Batch, Untreated | 60 | 23.97 | [25] |
| C.butyricum FS3 | Corn stalk | Batch, Untreated | 36 | 92.7 | [34] |
| C. thermocellum 7072 | Corn stalk | Batch/Two stage | 55 | 74.4 | [45] |
| C. thermocellum | Corn stalk | Continuous, Untreated | 55 | 61.4 | [46] |
| C. populeti FZ10 | Corn stalk | Batch, Untreated | 35 | 92.5 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, B.; Zhang, H.; Qian, S.; Wei, T.; Zhang, Z.; Song, L.; Yang, X. Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost. Appl. Sci. 2022, 12, 9562. https://doi.org/10.3390/app12199562
Zhang J, Jiang B, Zhang H, Qian S, Wei T, Zhang Z, Song L, Yang X. Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost. Applied Sciences. 2022; 12(19):9562. https://doi.org/10.3390/app12199562
Chicago/Turabian StyleZhang, Jingnan, Baoxuan Jiang, Haokun Zhang, Sitong Qian, Tao Wei, Zhiping Zhang, Lili Song, and Xu Yang. 2022. "Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost" Applied Sciences 12, no. 19: 9562. https://doi.org/10.3390/app12199562





