The Accumulation Characteristics of Different Heavy Metals in Sea Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Design and Treatments
2.2.1. Seedling Preparation
2.2.2. Soil Preparation with Different Heavy Metal Levels
2.3. Plant Harvest and Plant Digestion
2.3.1. Biological Yield of Sea Rice at the Mature Stage
2.3.2. Cd, Zn, and Cu Contents in Sea Rice
2.4. Statistical Analysis
3. Results and Analysis
3.1. Cd Accumulation Characteristics of HH12
3.1.1. Biomass of Different HH12 Parts
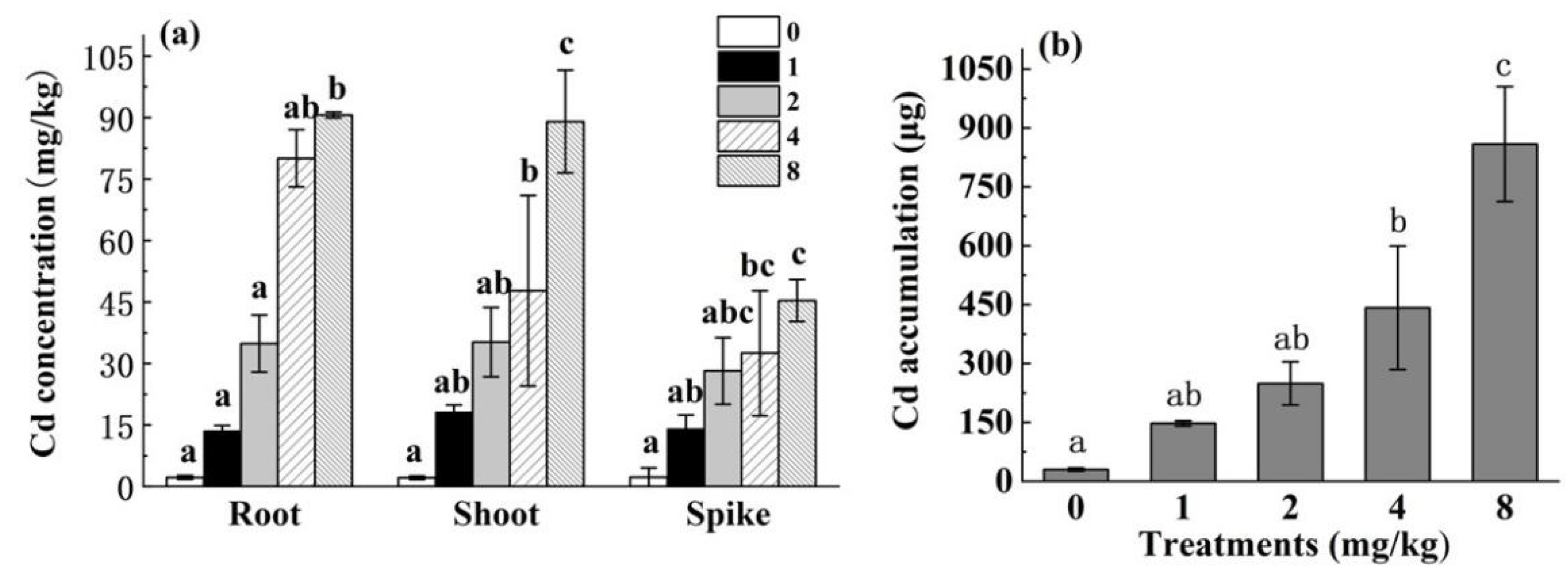
3.1.2. Cd Content and Cd Accumulation in Different HH12 Parts
3.1.3. Cd Transport Coefficients of Different HH12 Parts
3.2. Cu Accumulation Characteristics of HH12
3.2.1. Biomass of Different HH12 Parts
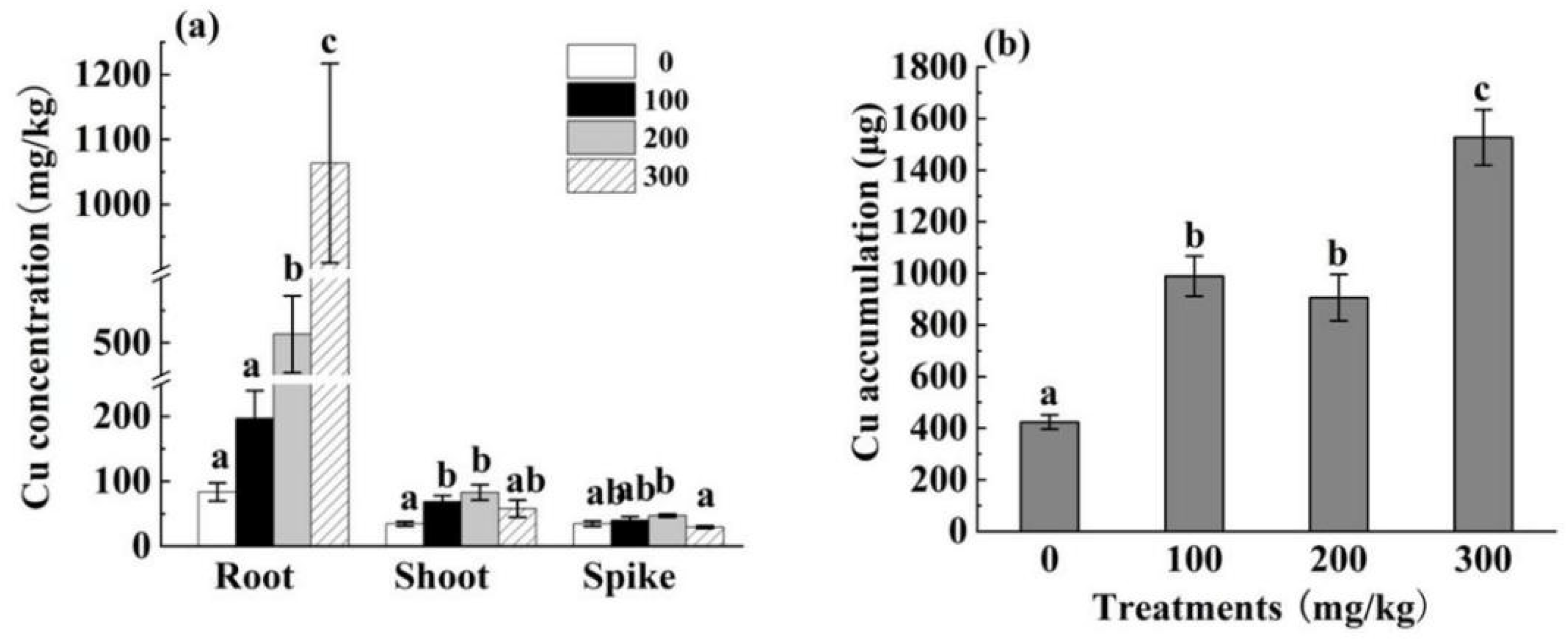
3.2.2. Cu Content and Cu Accumulation in Different HH12 Parts
3.2.3. Cu Transport Coefficient of Different HH12 Parts
3.3. Zn Accumulation Characteristics of HH12
3.3.1. Biomass of Different HH12 Parts
3.3.2. Zn Content and Accumulation in Different HH12 Parts
3.3.3. Zn Transport Coefficients in Different HH12 Parts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhou, Y. Reflections on China’s food security and land use policy under rapid urbanization. Land Use Policy 2021, 109, 105699. [Google Scholar] [CrossRef]
- Guo, F. Research on the Development Countermeasures of Sea Rice Industry in Zhanjiang. Master Thesis, Guangdong Ocean University, Zhanjiang, China, 2019. (In Chinese). [Google Scholar]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2020, 406, 124325. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, Q.; Liu, S.; Zhao, Y.; Long, Y.; Pan, Y. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef]
- Ji, P.; Sun, T.; Song, Y.; Ackland, M.L.; Liu, Y. Strategies for enhancing the phytoremediation of cadmium-contaminated agricultural soils by Solanum nigrum L. Environ. Pollut. 2011, 159, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, J.; Li, H. How intercropping and mixed systems reduce cadmium concentration in rice grains and improve grain yields. J. Hazard. Mater. 2021, 402, 123762. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shao, T.; Lv, Z.; Yue, Y.; Liu, A.; Long, X.; Zhou, Z.; Gao, X.; Rengel, Z. The mechanisms of improving coastal saline soils by planting rice. Sci. Total Environ. 2020, 703, 135529. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Wang, Q.; Ma, Y.; Su, D.; Qiao, Y.; Jiang, R.; Li, H. Ecological risk of copper and zinc and their different bioavailability change in soil-rice system as affected by biowaste application. Ecotoxicol. Environ. Saf. 2020, 192, 110301. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, L.; Yang, L.; Yu, J.; Lv, J.; Meng, M.; Li, G. Heavy metal pollution and the risk from tidal flat reclamation in coastal areas of Jiangsu, China. Mar. Pollut. Bull. 2020, 158, 111427. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Chai, M.; Shen, X.; Xu, H.; Qiu, G. Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay, South China. Mar. Pollut. Bull. 2015, 101, 448–456. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, S.; Feng, J.; Wang, W.; Wang, Y.; Yu, Q.; Liao, Y.; Mo, Y.; Xu, Z.; Li, L.; et al. Overexpression of a stress response membrane protein gene OsSMP1 enhances rice tolerance to salt, cold and heavy metal stress. Environ. Exp. Bot. 2021, 182, 104327. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chi, J.; Liu, L.; Li, Q.; Liu, Z. Effect of Seawater Stress on Physiological and Biochemical Responses of Five Jerusalem Artichoke Ecotypes. Pedosphere 2009, 19, 208–216. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kalembasa, S.; Malinowska, E.; Kalembasa, D.; Pakula, K.; Symanowicz, B.; Becher, M.; Jaremko, D. Impact of foliar application of Tytanit on Zn, Li, Ni, Cr, Pb, and Cd contents in celery leaves. Pol. J. Environ. Stud. 2015, 24, 1621–1631. [Google Scholar]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Song, Z. A Dual Role of Se on Cd Toxicity: Evidences from the Uptake of Cd and Some Essential Elements and the Growth Responses in Paddy Rice. Biol. Trace Elem. Res. 2013, 151, 113–121. [Google Scholar] [CrossRef]
- Liao, G.; Wu, Q.; Feng, R.; Guo, J.; Wang, R.; Xu, Y.; Ding, Y.; Fan, Z.; Mo, L. Efficiency evaluation for remediating paddy soil contaminated with cadmium and arsenic using water management, variety screening and foliage dressing technologies. J. Environ. Manag. 2016, 170, 116–122. [Google Scholar] [CrossRef]
- Shao, G.; Chen, M.; Wang, D.; Xu, C.; Mou, R.; Cao, Z.; Zhang, X. Using iron fertilizer to control Cd accumulation in rice plants: A new promising technology. Sci. China Life Sci. 2008, 51, 245–253. [Google Scholar] [CrossRef]
- Yoshida, S.; Fomo, D.A.; Cock, J.H.; Gomez, K.A. Routine procedures for growing rice plants in culture solution. Lab. Man. Physiol. Stud. Rice 1976, 61–66. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Yang, X.; Lin, R.; Zhang, W.; Xu, Y.; Wei, X.; Zhuo, C.; Qin, J.; Li, H. Comparison of Cd subcellular distribution and Cd detoxification between low/high Cd-accumulative rice cultivars and sea rice. Ecotoxicol. Environ. Saf. 2019, 185, 109698.1–109698.8. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Pan, X.; Yan, Y.; Cheng, Z.; Jiang, D. Salicylic acid alleviates the toxicity effect of cadmium on germination, seedling growth, and amylase activity of rice. J. Plant Nutr. Soil Sci. 2010, 173, 300–305. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, W.; Yang, W.; Gu, J.; Gao, Z.; Chen, L.; Du, W.; Zhang, P.; Peng, P.; Liao, B. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages. Ecotoxicol. Environ. Saf. 2018, 152, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Peng, O. Effects of Cadmium Stress on Rice Growth and Development and Study on the Key Period of Cadmium Accumulation in Rice Grain. Master Thesis, Hunan Agriculture University, Changsha, China, 2017. (In Chinese). [Google Scholar]
- Chen, Y. Study on the Toxic Effects of Soil Zinc on Crops and the Toxicity Thresholds of Zn for the Soil. Master Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2011. (In Chinese). [Google Scholar]
- Zhao, J. Effect of Soil Copper Contamination on Growth and Development of Rice. Master Thesis, Yangzhou University, Yangzhou, China, 2008. (In Chinese). [Google Scholar]
- Xu, J.; Yang, L.; Wang, Z.; Dong, G.; Huang, J.; Wang, Y. Effects of Soil Copper Concentration on Growth, Development and Yield Formation of Rice (Oryza sativa). Rice Sci. 2005, 12, 125–132. [Google Scholar]
- Krzeslowska, The cell wall in plant cell response to trace metals:polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 2011, 33, 35–51. [CrossRef]
- Mu, S.; Yamaji, N.; Sasaki, A.; Luo, L.; Du, B.; Che, J.; Shi, H.; Zhao, H.; Huang, S.; Deng, F. A transporter for delivering zinc to the developing tiller bud and panicle in rice. Plant J. 2020, 105, 786–799. [Google Scholar] [CrossRef]


| Treatments (mg/kg) | Root-Shoot | Shoot-Spike |
|---|---|---|
| 0 | 1.098 ± 0.348 a | 1.103 ± 0.806 a |
| 1 | 1.361 ± 0.161 a | 0.8 ± 0.206 a |
| 2 | 1.109 ± 0.361 a | 0.773 ± 0.088 a |
| 4 | 0.643 ± 0.345 a | 0.835 ± 0.048 a |
| 8 | 0.981 ± 0.109 a | 0.519 ± 0.153 a |
| Treatments (mg/kg) | Root-Shoot | Shoot-Spike |
|---|---|---|
| 0 | 0.43 ± 0.065 b | 0.994 ± 0.026 b |
| 100 | 0.368 ± 0.041 b | 0.579 ± 0.046 a |
| 200 | 0.169 ± 0.035 a | 0.603 ± 0.129 a |
| 300 | 0.057 ± 0.014 a | 0.562 ± 0.116 a |
| Treatments (mg/kg) | Root-Shoot | Shoot-Spike |
|---|---|---|
| 0 | 1.070 ± 0.007 a | 0.492 ± 0.002 b |
| 100 | 0.835 ± 0.195 a | 0.374 ± 0.051 b |
| 200 | 0.853 ± 0.133 a | 0.228 ± 0.041 a |
| 300 | 0.815 ± 0.192a | 0.170 ± 0.035 a |
| 400 | 0.953 ± 0.210 a | 0.420 ± 0.061 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, X.; Zheng, Y.; Tang, L.; Lai, Z.; Liu, N.; Li, H. The Accumulation Characteristics of Different Heavy Metals in Sea Rice. Appl. Sci. 2022, 12, 9718. https://doi.org/10.3390/app12199718
Li J, Yang X, Zheng Y, Tang L, Lai Z, Liu N, Li H. The Accumulation Characteristics of Different Heavy Metals in Sea Rice. Applied Sciences. 2022; 12(19):9718. https://doi.org/10.3390/app12199718
Chicago/Turabian StyleLi, Jiachun, Xu Yang, Yuze Zheng, Lele Tang, Zhenai Lai, Na Liu, and Huashou Li. 2022. "The Accumulation Characteristics of Different Heavy Metals in Sea Rice" Applied Sciences 12, no. 19: 9718. https://doi.org/10.3390/app12199718






