Therapeutic Potential of Tamarix aphylla in the Prevention of Lung Injury through the Regulation of Inflammation, Oxidative Stress and Cell-Signaling Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Collection and Extraction of T. aphylla Leaves
2.3. Phytochemical Screening
2.4. Total Phenol Content
2.5. Evaluation of the Amount of Total Flavonoids
2.6. In Vitro Study
2.6.1. Antioxidant Activity: H2O2 Free Radical Scavenging Activity
2.6.2. DPPH Method of Free Radical Scavenging Activity
2.6.3. An Evaluation of In Vitro Anti-Inflammatory Activity by Protein Denaturation Inhibition
2.6.4. Anti-Proteinase Action
2.6.5. Cell Culture
2.6.6. Cell Viability Assay
2.6.7. Measurement of Inflammatory Cytokines
2.7. In Vivo Study
2.7.1. Animal Model
2.7.2. Grouping of Animals and Treatment Plan
2.7.3. Measurement of Wet-to-Dry Weight (W/D) Ratio of Lung
2.7.4. Measurement of Inflammatory Cytokines in BALF
2.7.5. Measurement of Lipid Peroxidation and Superoxide Dismutase
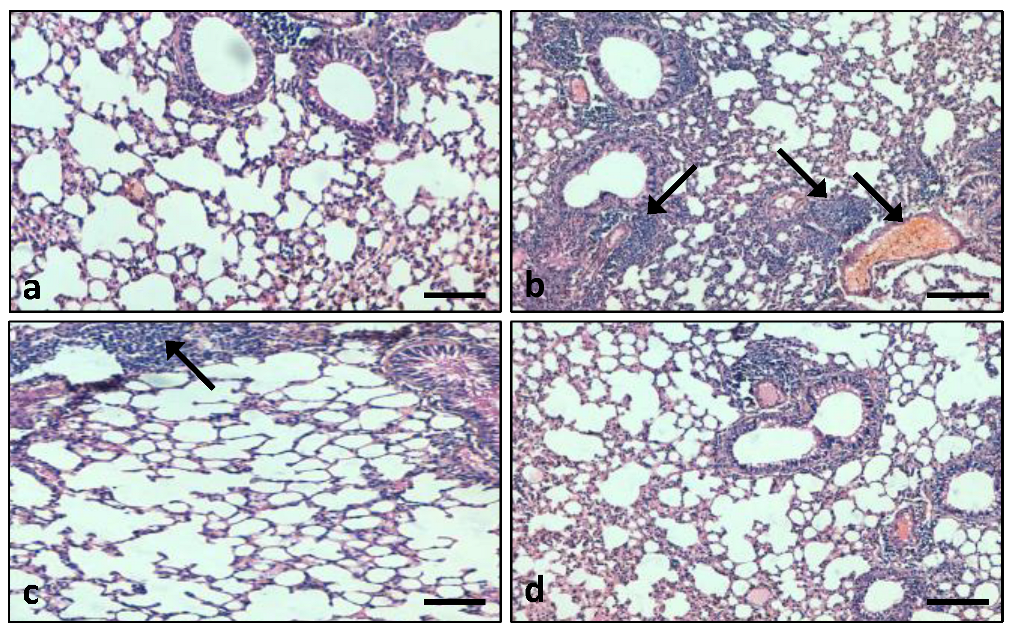
2.7.6. Histopathological Evaluation
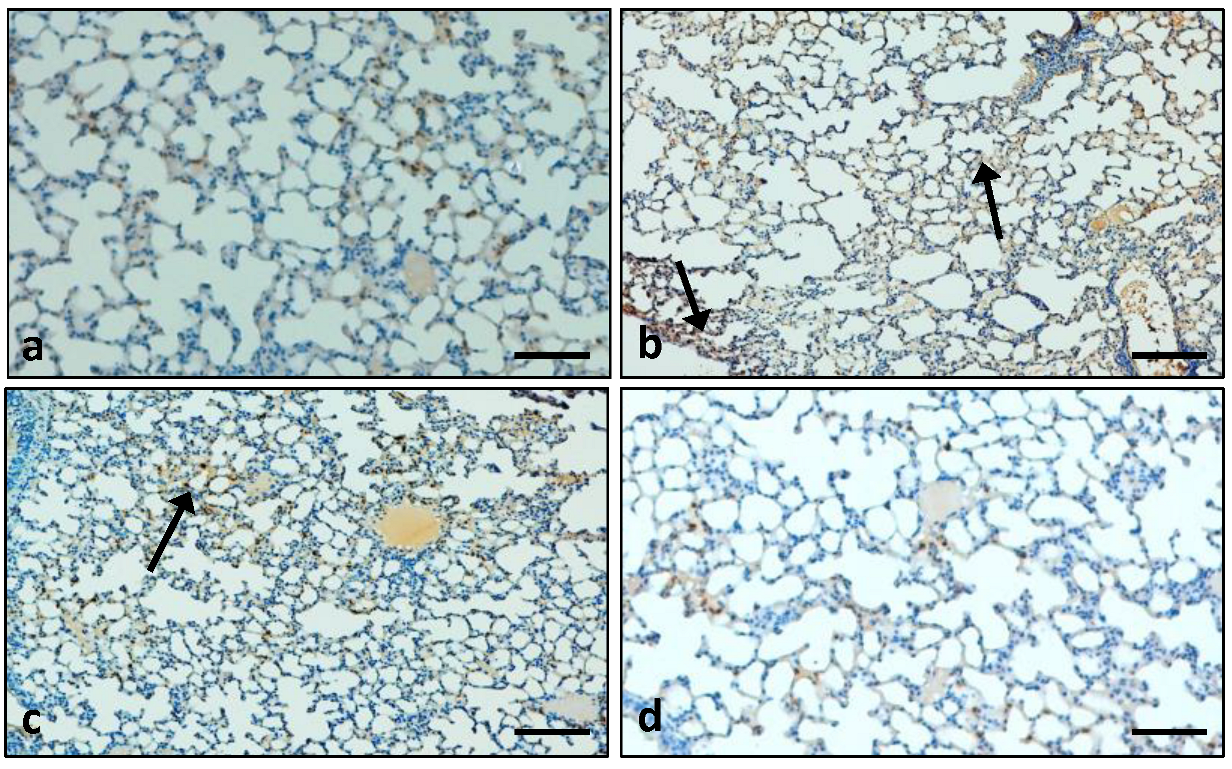
2.7.7. Immunohistochemical Analysis of Protein Expressions
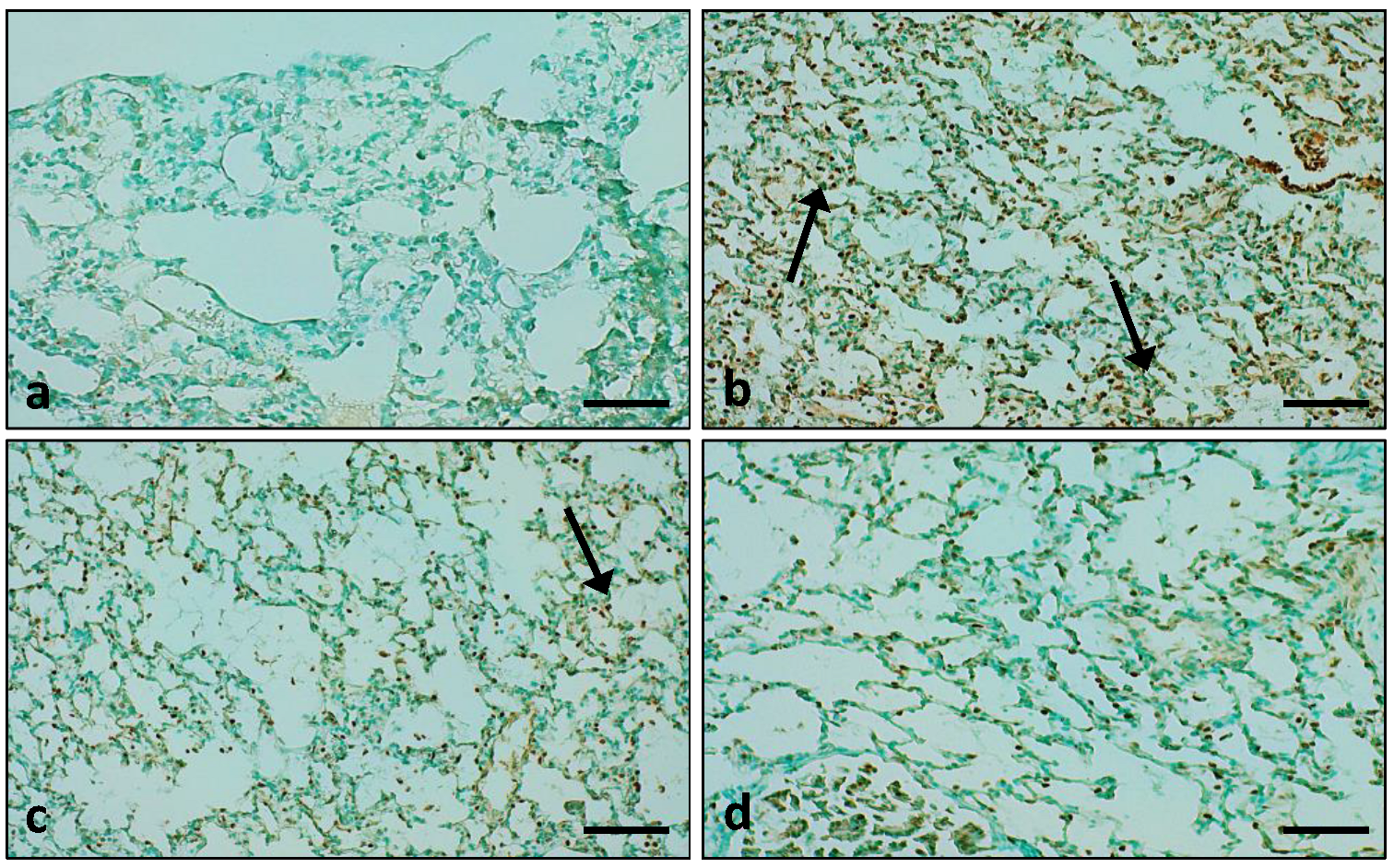
2.7.8. TUNEL Assay
2.8. Statistical Analysis
3. Results
3.1. Flavonoid, Phenolic and Preliminary Screening
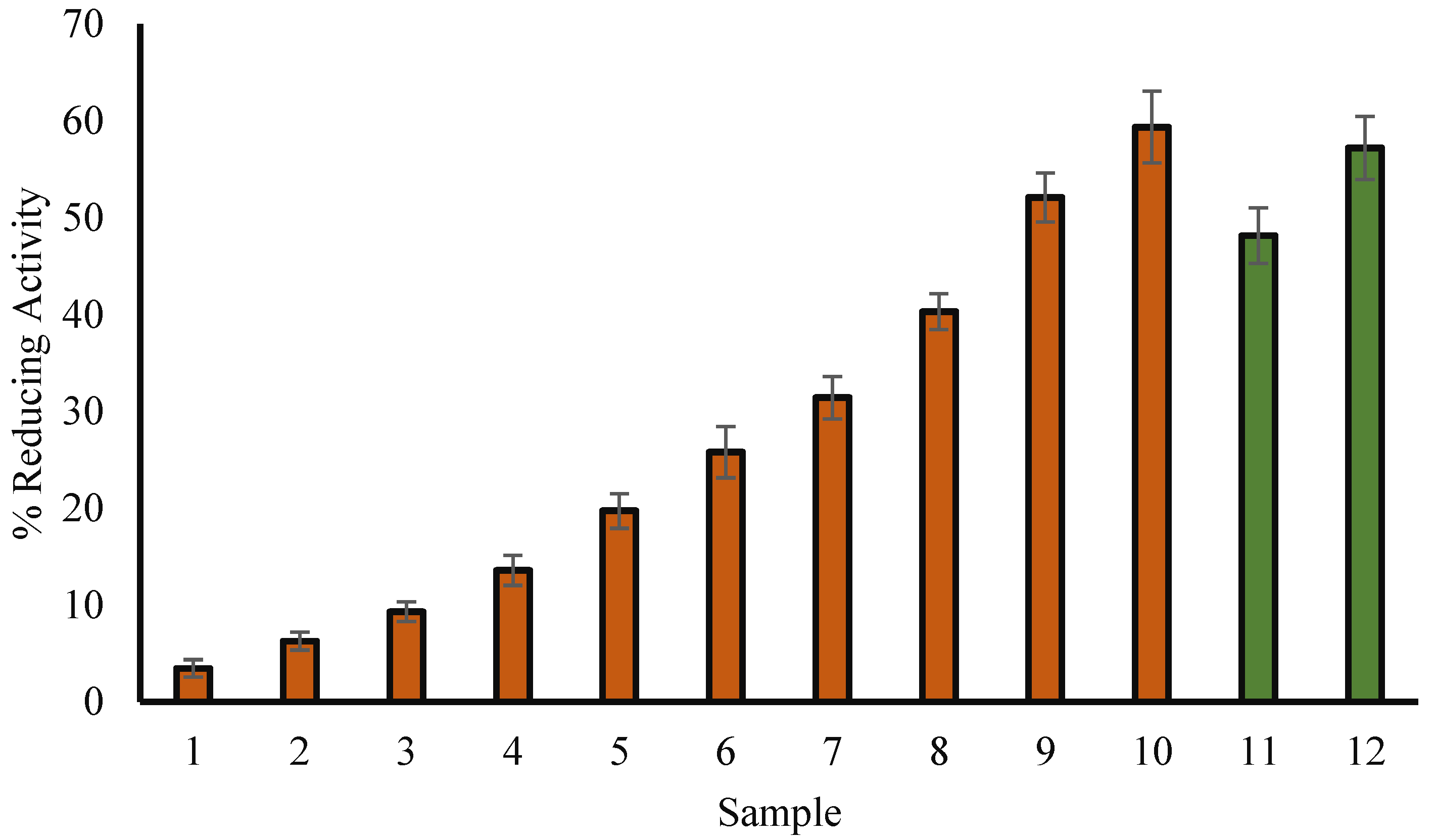
3.2. H2O2 Reducing Ability
3.3. Scavenging Assay for DPPH Radicals
3.4. Protein Denaturation Inhibition: An Evaluation of Anti-Inflammatory Activity
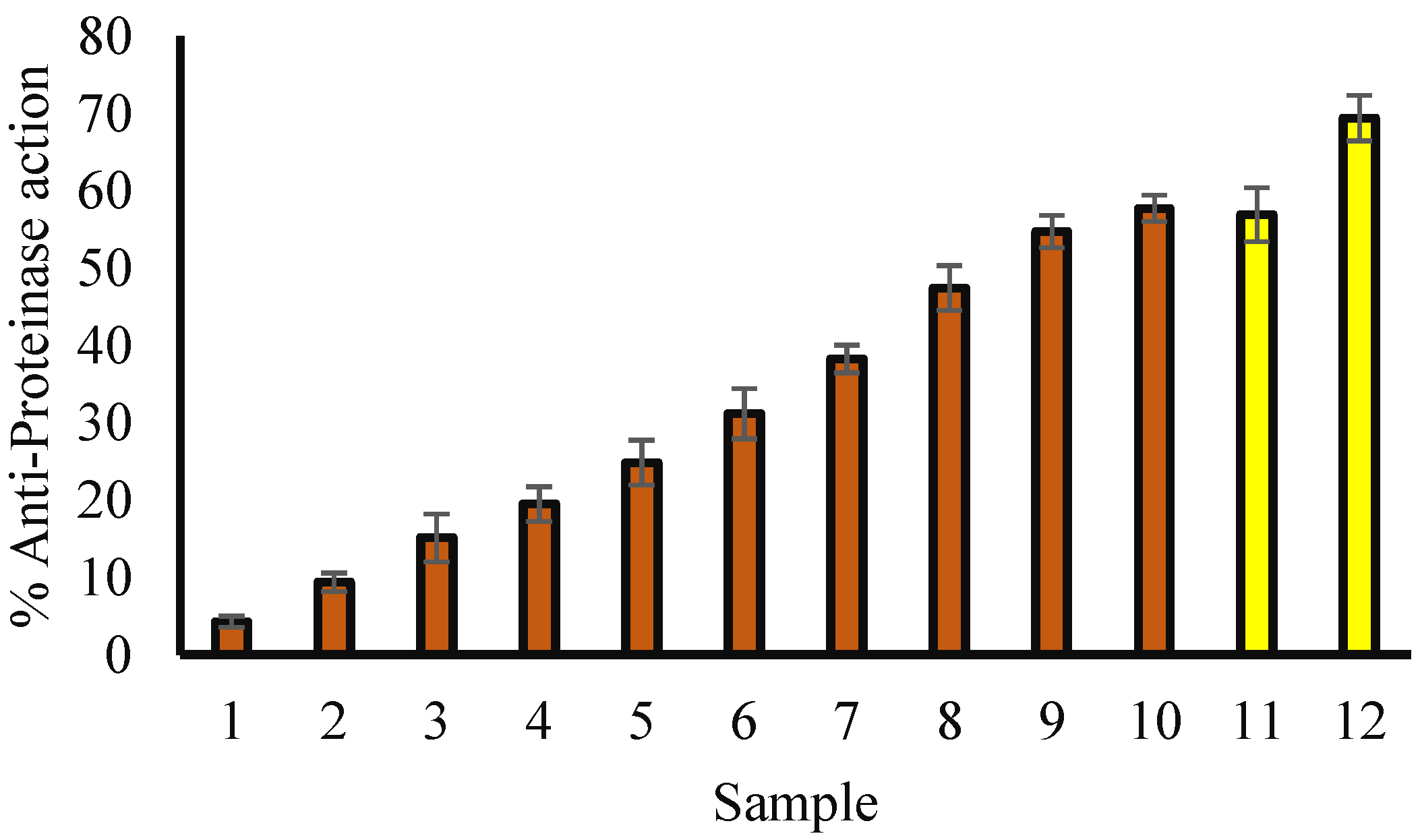
3.5. Anti-Proteinase Potential
3.6. Effect of Extract on Cell Viability
3.6.1. Effects of Leaf Extract on Inflammatory Markers in RAW 264.7 Cells Stimulated by LPS
3.6.2. Effects of the Extract on in the Total Number of Cells, Neutrophils and Macrophage Cell Counts
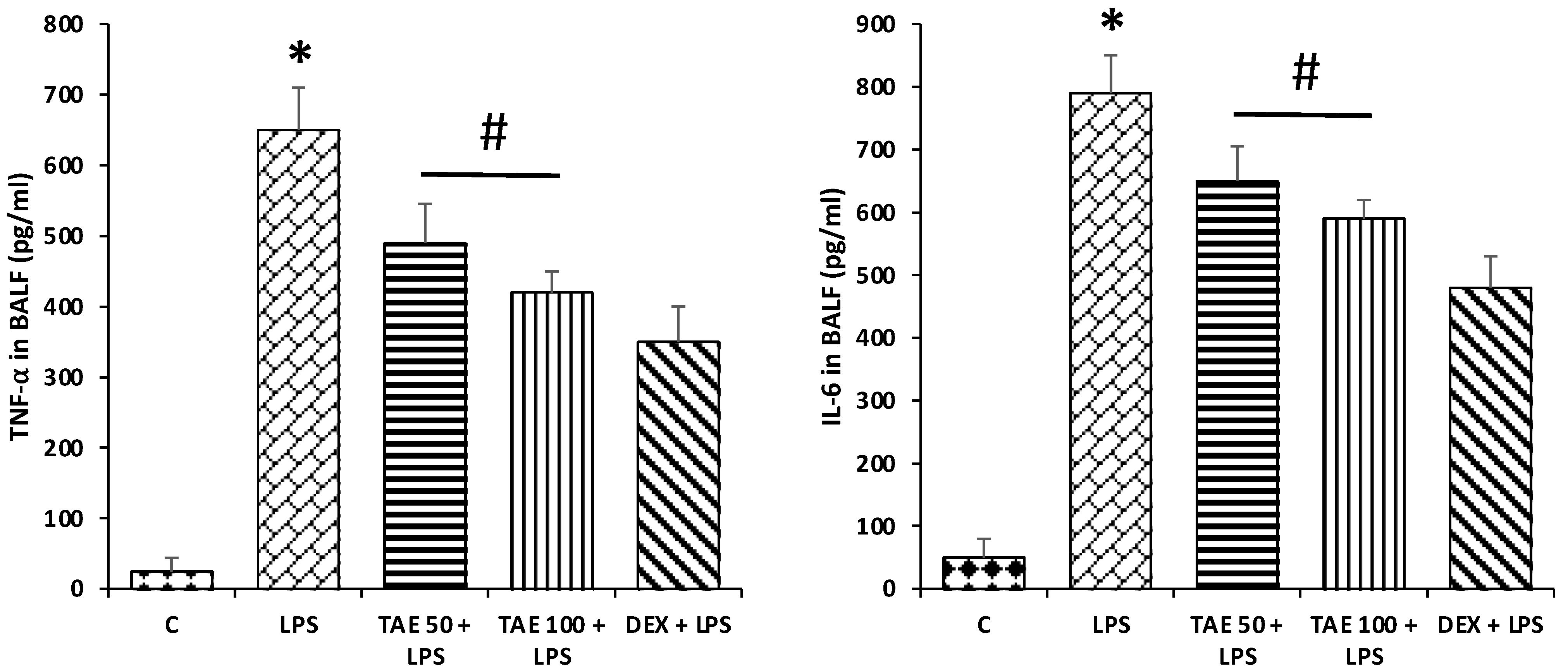
3.6.3. Effects of the Extract on TNF-α and IL-6 Level in LPS-Induced ALI
3.6.4. The Extract Attenuates Lung Oxidative Stress in LPS-Induced Lung Injury in Mice
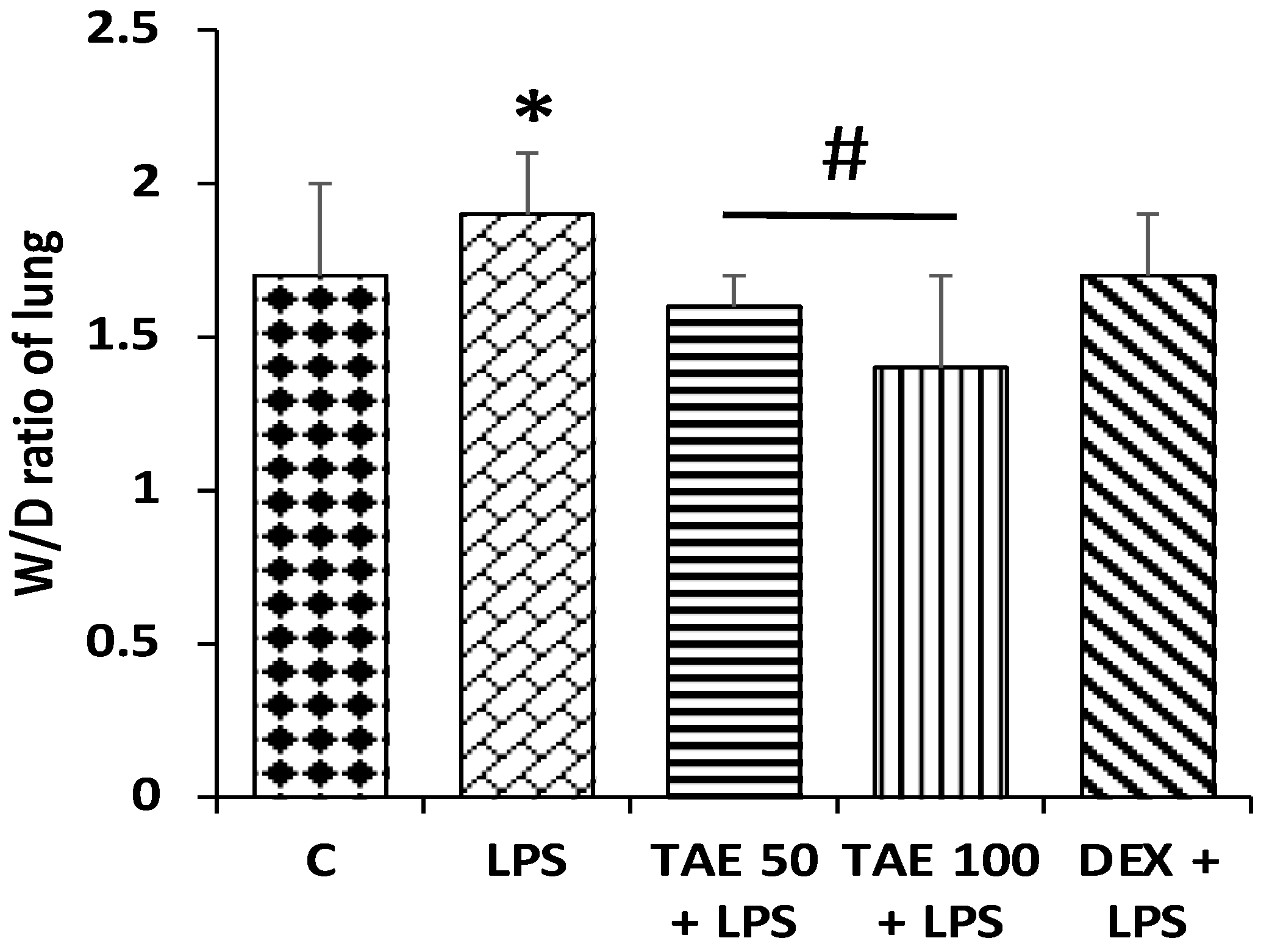
3.7. The Role of the Extract on Wet/Dry Lung Ratios
3.7.1. Role of the Extract on Lung Tissue Architectures
3.7.2. Effects of the Extract on the Expression Pattern of the Cox-2 Protein in Lung Tissue
3.7.3. Role of the Extract on LPS-Administered Lung Tissue Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarwar, S.; Lin, Z.S.; Ku, C.F.; Guan, Y.F.; Xiao, H.T.; Shi, X.K.; Wang, H.Q.; Bian, Z.X.; Tsang, S.W.; Zhang, H.J. Protective effect of dihydro-resveratrol against lung injury in rats with cerulein-induced acute pancreatitis. Planta Med. 2016, 82, P959. [Google Scholar] [CrossRef]
- Tanaka, R.; Ishima, Y.; Enoki, Y.; Kimachi, K.; Shirai, T.; Watanabe, H.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Therapeutic impact of human serum albumin–thioredoxin fusion protein on influenza virus-induced lung injury mice. Front. Immunol. 2014, 5, 561. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Goligher, E.C.; Brower, R.G. Fifty years of research in ARDS. Setting positive end-expiratory pressure in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Deng, J.S.; Pan, H.P.; Liao, J.C.; Huang, S.S.; Huang, G.J. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int. Immunopharmacol. 2017, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kolomaznik, M.; Nova, Z.; Calkovska, A. Pulmonary surfactant and bacterial lipopolysaccharide: The interaction and its functional consequences. Physiol. Res. 2017, 66, S147–S157. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Wan, Z.; Liang, J.; Betti, M.; Hrynets, Y.; Xue, X.; Wu, L.; Wang, K. Bee pollen extracts modulate serum metabolism in lipopolysaccharide-induced acute lung injury mice with anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 7855–7868. [Google Scholar] [CrossRef]
- Ding, H.; Ci, X.; Cheng, H.; Yu, Q.; Li, D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019, 66, 169–176. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective effects of thymoquinone, an active compound of nigella sativa, on rats with Benzo (a) pyrene-Induced Lung injury through regulation of oxidative stress and inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef]
- Alzohairy, M.A.; Khan, A.A.; Ansari, M.A.; Babiker, A.Y.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective Effect of Quercetin, a Flavonol against Benzo (a) pyrene-Induced Lung Injury via Inflammation, Oxidative Stress, Angiogenesis and Cyclooxygenase-2 Signalling Molecule. Appl. Sci. 2021, 11, 8675. [Google Scholar] [CrossRef]
- Park, S.M.; Jung, C.J.; Lee, D.G.; Choi, B.R.; Ku, T.H.; La, I.J.; Cho, I.J.; Ku, S.K. Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice. Antioxidants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Li, J.; Bi, A.; Jia, H.; Li, Q.; Liu, Y.; Jiang, X.; Huang, D.; Xia, S. Thymoquinone ameliorates the PM2.5-induced lung injury in rats. Exp. Lung Res. 2020, 46, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Wang, F.; Huang, D.; Shi, S.; Wang, X.; Wang, Y.; Li, S.; Wu, Y.; Wang, Z. Astragaloside IV protects from PM2.5-induced lung injury by regulating autophagy via inhibition of PI3K/Akt/mTOR signaling in vivo and in vitro. J. Inflamm. Res. 2021, 14, 4707. [Google Scholar] [CrossRef]
- Panhwar, A.Q.; Abro, H. Ethnobotanical studies of Mahal Kohistan (Khirthar national park). Pak. J. Bot. 2007, 39, 2301–2315. [Google Scholar]
- Ahmad, M.; Zafar, M.; Sultana, S. Salvadora persica, Tamarix aphylla and Zizyphus mauritiana-Three woody plant species mentioned in Holy Quran and Ahadith and their ethnobotanical uses in north western part (DI Khan) of Pakistan. Pak. J. Nutr. 2009, 8, 542–547. [Google Scholar]
- Orabi, M.A.; Yoshimura, M.; Amakura, Y.; Hatano, T. Ellagitannins, gallotannins, and gallo-ellagitannins from the galls of Tamarix aphylla. Fitoterapia 2015, 104, 55–63. [Google Scholar] [CrossRef]
- El-Aarag, B.; Khairy, A.; Khalifa, S.A.M.; El-Seedi, H.R. Protective Effects of Flavone from Tamarix aphylla against CCl4-Induced Liver Injury in Mice Mediated by Suppression of Oxidative Stress, Apoptosis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 5215. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Butt, Z.A.; Hamayun, M.; Ahmad, A.; Huang, D.; Hussain, A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral Biol. 2017, 81, 175–185. [Google Scholar] [CrossRef]
- Qadir, M.I.; Abbas, K.; Hamayun, R.; Ali, M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L. (Saltcedar) in mice. Pak. J. Pharm. Sci. 2014, 27, 1985–1988. [Google Scholar] [PubMed]
- Soliman Yu, H.; Ibrahim Al, S. Anti-inflammatory and Wound Healing Activities of Herbal Gel Containing an Antioxidant Tamarix aphylla Leaf Extract. Int. J. Pharmacol. 2011, 7, 829–835. [Google Scholar] [CrossRef]
- Hamed, A.I.; Said, R.B.; Kontek, B.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Stochmal, A.; Olas, B. LC–ESI-MS/MS profile of phenolic and glucosinolate compounds in samh flour (Mesembryanthemum forsskalei Hochst. ex Boiss) and the inhibition of oxidative stress by these compounds in human plasma. Food Res. Int. 2016, 85, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Sakat, S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Gao, H.; Sun, W.; Zhao, W.; Hao, W.; Leung, C.H.; Lu, J.; Chen, X. Total tanshinones-induced apoptosis and autophagy via reactive oxygen species in lung cancer 95D cells. Am. J. Chin. Med. 2015, 43, 1265–1279. [Google Scholar] [CrossRef]
- Masocha, W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J. Neuroimmunol. 2009, 214, 78–82. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Ali, H.; Khan, A.; Ali, J.; Ullah, H.; Khan, A.; Ali, H.; Irshad, N.; Khan, S. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol. 2020, 21, 81. [Google Scholar] [CrossRef]
- Peng, L.Y.; Shi, H.T.; Yuan, M.; Li, J.H.; Song, K.; Huang, J.N.; Yi, P.F.; Shen, H.Q.; Fu, B.D. Madecassoside protects against LPS-induced acute lung injury via inhibiting TLR4/NF-κB activation and blood-air barrier permeability. Front. Pharmacol. 2020, 11, 807. [Google Scholar] [CrossRef]
- Ali, J.; Khan, A.U.; Shah, F.A.; Ali, H.; Islam, S.U.; Kim, Y.S.; Khan, S. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. 2019, 239, 116888. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.U. Evaluation of in vitro and in vivo therapeutic efficacy of Ribes alpestre Decne in Rheumatoid arthritis. Braz. J. Pharm. Sci. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef]
- Salomao, R.; Brunialti, M.K.; Rapozo, M.M.; Baggio-Zappia, G.L.; Galanos, C.; Freudenberg, M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock 2012, 38, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, A.; Grosso, C.; Gonçalves, R.F.; Khelifi, E.; Hammami, S.; Achour, S.; Trabelsi-Ayadi, M.; Valentão, P.; Andrade, P.B.; Mighri, Z. Evaluation of antioxidant, anticholinesterase, and antidiabetic potential of dry leaves and stems in Tamarix aphylla growing wild in Tunisia. Chem. Biodivers. 2016, 13, 1747–1755. [Google Scholar] [CrossRef]
- Suleiman, M.H. Ethnobotanical, phytochemical, and biological study of Tamarix aphylla and Aerva javanica medicinal plants growing in the Asir region, Saudi Arabia. Trop. Conserv. Sci. 2019, 12, 1940082919869480. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute lung injury: A clinical and molecular review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Frutos-Vivar, F.; Nin, N.; Esteban, A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr. Opin. Crit. Care 2004, 10, 1–6. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 19686699. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lu, Q.; Wang, K.; Lu, J.; Gu, X.; Zhu, D.; Liu, F.; Guo, Z. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J. Cell. Physiol. 2018, 233, 6615–6631. [Google Scholar] [CrossRef] [PubMed]
- Wyns, H.; Plessers, E.; De Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunopathol. 2015, 166, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Gonzalez-Rivera, B.L.; Redus, L.; Parrott, J.M.; O’Connor, J.C. Indoleamine 2, 3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 2012, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Ali, M.; Alhazmi, H.A.; Ansari, S.H.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and bioactive profile compilations of less discussed but effective naturally growing Saudi plant. In Plant and Human Health; Springer: Cham, Switzerland, 2019; Volume 3, pp. 343–352. [Google Scholar]
- Gadallah, A.S.; Yousuf, S.; Jabeen, A.; Swilam, M.M.; Khalifa, S.A.; El-Seedi, H.R.; Choudhary, M.I. Anti-inflammatory principles from Tamarix aphylla L.: A bioassay-guided fractionation study. Molecules 2020, 25, 2994. [Google Scholar] [CrossRef]
- Al Sobeai, S.M. Anticancer, cytotoxic effect of Tamarix aphylla, and antibacterial screening efficiency against multidrug-resistant human pathogens. Asian J. Pharm. Clin. Res. 2018, 11, 241–246. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-inflammatory and anti-oxidant activity of Portulaca oleracea extract on LPS-induced rat lung injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef]
- Shelby, B.D.; Nelson, A.; Morris, C. γ-Herpesvirus neoplasia: A growing role for COX-2. Microsc. Res. Tech. 2005, 68, 120–129. [Google Scholar] [CrossRef]
- Huang, X.L.; Wei, X.C.; Guo, L.Q.; Zhao, L.; Chen, X.H.; Cui, Y.D.; Yuan, J.; Chen, D.F.; Zhang, J. The therapeutic effects of Jaceosidin on lipopolysaccharide-induced acute lung injury in mice. J. Pharmacol. Sci. 2019, 140, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Am Lee, S.; Lee, S.H.; Kim, J.Y.; Lee, W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J. Thorac. Dis. 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Wang, B.; Wu, D.; Li, H.; Lu, H.; Wu, H.; Chai, Y. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp. Biol. Med. 2014, 239, 1653–1662. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Gong, S.; Tang, J.; Zhang, J.; Li, F.; Yu, B.; Zhang, Y.; Kou, J. Ruscogenin alleviates LPS-induced pulmonary endothelial cell apoptosis by suppressing TLR4 signaling. Biomed. Pharmacother. 2020, 125, 109868. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, N.; Zhan, X.; Zheng, T.; Huang, X.; Chen, Q.; Song, Z.; Yang, F.; Nie, H.; Zhang, Y.; et al. Capsaicin Protects against Lipopolysaccharide-Induced Acute Lung Injury Through the HMGB1/NF-κB and PI3K/AKT/mTOR Pathways. J. Inflamm. Res. 2021, 14, 5291. [Google Scholar] [CrossRef] [PubMed]






| Preliminary Screening | T. aphylla Extract |
|---|---|
| Dry weight of leaves | 50 g |
| Yield | 5.98% |
| Extract | Methanol |
| Color | Greyish Green |
| Odor | Not definite |
| Texture | Sticky |
| Phytochemical Constituents | Leaves |
|---|---|
| Tannins | + |
| Alkaloids | − |
| Saponins | − |
| Glycosides | + |
| Flavonoids | + |
| Phenolic compounds | + |
| Terpenoids | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Syed, M.A.; Rahmani, A.H. Therapeutic Potential of Tamarix aphylla in the Prevention of Lung Injury through the Regulation of Inflammation, Oxidative Stress and Cell-Signaling Molecules. Appl. Sci. 2022, 12, 9925. https://doi.org/10.3390/app12199925
Almatroodi SA, Khan AA, Aloliqi AA, Syed MA, Rahmani AH. Therapeutic Potential of Tamarix aphylla in the Prevention of Lung Injury through the Regulation of Inflammation, Oxidative Stress and Cell-Signaling Molecules. Applied Sciences. 2022; 12(19):9925. https://doi.org/10.3390/app12199925
Chicago/Turabian StyleAlmatroodi, Saleh A., Amjad Ali Khan, Abdulaziz A. Aloliqi, Mansoor Ali Syed, and Arshad Husain Rahmani. 2022. "Therapeutic Potential of Tamarix aphylla in the Prevention of Lung Injury through the Regulation of Inflammation, Oxidative Stress and Cell-Signaling Molecules" Applied Sciences 12, no. 19: 9925. https://doi.org/10.3390/app12199925
APA StyleAlmatroodi, S. A., Khan, A. A., Aloliqi, A. A., Syed, M. A., & Rahmani, A. H. (2022). Therapeutic Potential of Tamarix aphylla in the Prevention of Lung Injury through the Regulation of Inflammation, Oxidative Stress and Cell-Signaling Molecules. Applied Sciences, 12(19), 9925. https://doi.org/10.3390/app12199925









