Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gas Chromatography–Mass Spectrometry Analysis
2.2. Antioxidant Activity
2.3. Antibacterial and Antibiofilm Activity
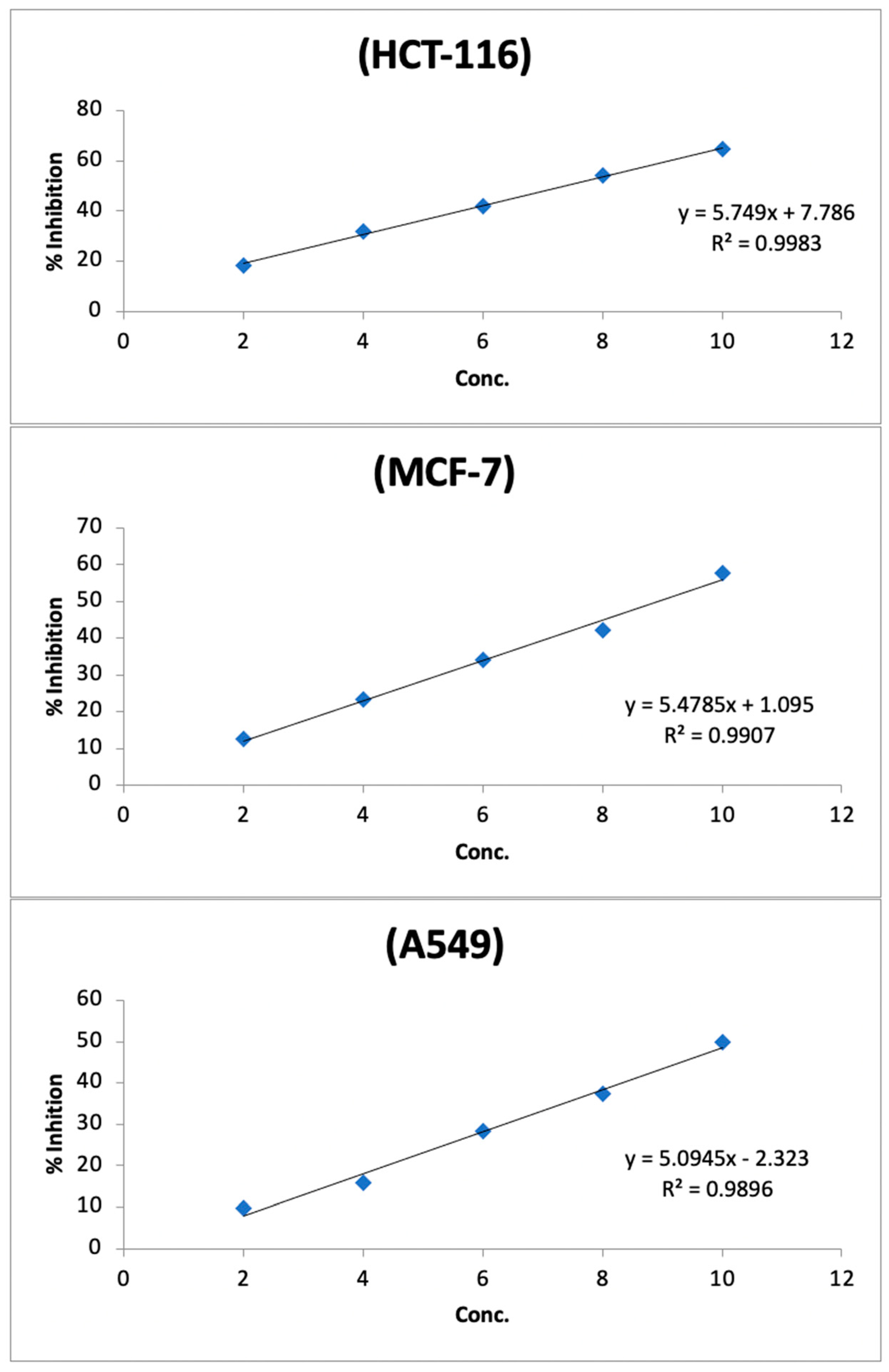
2.4. Anticancer Potential
3. Materials and Methods
3.1. Honey Sample
3.2. Gas Chromatography–Mass Spectrometry Analysis
3.3. Antioxidant Assays
3.3.1. DPPH Scavenging Activity
3.3.2. ABTS Scavenging Assay
3.3.3. Beta-Carotene Assays
3.4. Antibacterial Activity
3.5. Antibiofilm Assay
3.6. Anticancer Assay (MTT Assay)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duru, C.E.; Duru, I.A. Phytochemical evaluation and health risk assessment of honey from an Apiary in Amizi, Ikuano local government area, Abia State, Nigeria. Sci. Afr. 2021, 13, e00885. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Aldarhami, A.; Gattan, H.; Aljuhani, B. Saudi Honey: A Promising Therapeutic Agent for Treating Wound Infections. Cureus 2021, 13, e18882. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.T. The Greek Herbal of Dioscorides; The University of Chicago Press: Chicago, IL, USA, 1959. [Google Scholar]
- Al Somal, N.; Coley, K.; Molan, P.; Hancock, B. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 1994, 87, 9. [Google Scholar] [CrossRef]
- Topham, J. Why do some cavity wounds treated with honey or sugar paste heal without scarring? J. Wound Care 2002, 11, 53–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markelov, V.; Trushin, M. Bee venom therapy and low dose naltrexone for treatment of multiple sclerosis. Nepal J. Neurosci. 2006, 3, 71–77. [Google Scholar]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef] [PubMed]
- Aljadi, A.; Kamaruddin, M. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Arawwawala, M.; Hewageegana, S. Health benefits and traditional uses of honey: A review. J. Apither. 2017, 2, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: Comparison with dextrose and sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Hussain, M.B.; Saeed, F.; Waheed, M.; Tufail, T. Phytochemistry, metabolism, and ethnomedical scenario of honey: A concurrent review. Int. J. Food Prop. 2017, 20, S254–S269. [Google Scholar] [CrossRef] [Green Version]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.M.; Schneider, K.R.; Cisneros, K.V.; Gu, L. Determination of antioxidant capacities, α-dicarbonyls, and phenolic phytochemicals in Florida varietal honeys using HPLC-DAD-ESI-MS n. J. Agric. Food Chem. 2014, 62, 8623–8631. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abde-llah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakman, P.H.; Te Velde, A.A.; De Boer, L.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef] [Green Version]
- Bazaid, A.S.; Barnawi, H.; Qanash, H.; Alsaif, G.; Aldarhami, A.; Gattan, H.; Alharbi, B.; Alrashidi, A.; Al-Soud, W.A.; Moussa, S.; et al. Bacterial Coinfection and Antibiotic Resistance Profiles among Hospitalised COVID-19 Patients. Microorganisms 2022, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Aldarhami, A.; Gattan, H.; Barnawi, H.; Qanash, H.; Alsaif, G.; Alharbi, B.; Alrashidi, A.; Eldrehmy, E.H. Antibiogram of Urinary Tract Infections and Sepsis among Infants in Neonatal Intensive Care Unit. Children 2022, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Tomislav, J.; Sieber, R. PhD and Gallmann, P.J. Am. College Nut. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Schneider, M.; Coyle, S.; Warnock, M.; Gow, I.; Fyfe, L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013, 27, 1162–1168. [Google Scholar] [CrossRef]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef]
- Saleem, H.; Zengin, G.; Khan, K.-U.-R.; Ahmad, I.; Waqas, M.; Mahomoodally, F.M.; Rengasamy, K.R.; Zainol, N.; Abidin, S.A.Z.; Ahemad, N. New insights into the phytochemical composition, enzyme inhibition and antioxidant properties of desert cotton (Aerva javanica (Bum. f) Shult.-Amaranthaceae). Nat. Prod. Res. 2021, 35, 664–668. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kirillova, K.; Lehto, X. Travelers’ food experience sharing on social network sites. J. Travel Tour. Mark. 2017, 34, 680–693. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Miao, Y.-H.; Hu, Y.-H.; Yang, J.; Liu, T.; Sun, J.; Wang, X.-J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [Green Version]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New series of thiazole derivatives: Synthesis, structural elucidation, antimicrobial activity, molecular modeling and MOE docking. Molecules 2019, 24, 1741. [Google Scholar] [CrossRef] [Green Version]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the volatile composition in thyme honeys from several origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef]
- Awasum, C.A.; Fotzo, S.L.M.; Ndukum, J.A.; Genesis, C.D.; Zoli, A. Gas chromatography-mass spectroscopy analysis and chemical composition of Ngaoundere, Cameroon honey. Am. J. Biosci. Bioeng. 2015, 3, 33–36. [Google Scholar]
- Liu, Y.E.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? Geroscience 2022, 44, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J. Agric. Food Chem. 2018, 67, 688–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurshid, U.; Ahmad, S.; Saleem, H.; Nawaz, H.A.; Zengin, G.; Locatelli, M.; Mahomoodally, M.F.; Abidin, S.A.Z.; Tousif, M.I.; Ahemad, N. Phytochemical composition and in vitro pharmacological investigations of Neurada procumbens L.(Neuradaceae): A multidirectional approach for industrial products. Ind. Crops Prod. 2019, 142, 111861. [Google Scholar] [CrossRef]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M. A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk.: An edible halophyte medicinal plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef]
- Patel, S.; Cichello, S. Manuka honey: An emerging natural food with medicinal use. Nat. Prod. Bioprospect. 2013, 3, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, H.A.; Boukraâ, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B.A.; Kolayli, S.; Sahin, H. Evaluation of the antioxidant activity of three varieties of honey from different botanical and geographical origins. Glob. J. Health Sci. 2012, 4, 191. [Google Scholar] [CrossRef] [Green Version]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Jubri, Z.; Rahim, N.B.A.; Aan, G.J. Manuka honey protects middle-aged rats from oxidative damage. Clinics 2013, 68, 1446–1454. [Google Scholar] [CrossRef]
- Henriques, A.; Jackson, S.; Cooper, R.; Burton, N. Free radical production and quenching in honeys with wound healing potential. J. Antimicrob. Chemother. 2006, 58, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Murayama, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J. Sci. Food Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Altern. Med. 2011, 2011, 908743. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on Manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Pinto, M.; Langer, T.M.; Hüffer, T.; Hofmann, T.; Herndl, G.J. The composition of bacterial communities associated with plastic biofilms differs between different polymers and stages of biofilm succession. PLoS ONE 2019, 14, e0217165. [Google Scholar]
- Zacchino, S.A.; Butassi, E.; Di Liberto, M.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Aryappalli, P.; Al-Qubaisi, S.S.; Attoub, S.; George, J.A.; Arafat, K.; Ramadi, K.B.; Mohamed, Y.A.; Al-Dhaheri, M.M.; Al-Sbiei, A.; Fernandez-Cabezudo, M.J. The IL-6/STAT3 signaling pathway is an early target of manuka honey-induced suppression of human breast cancer cells. Front. Oncol. 2017, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; Al-Ramadi, B.K. Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS ONE 2013, 8, e55993. [Google Scholar] [CrossRef] [Green Version]
- Al-Nemari, R.; Al-Senaidy, A.; Semlali, A.; Ismael, M.; Badjah-Hadj-Ahmed, A.Y.; Ben Bacha, A. GC-MS profiling and assessment of antioxidant, antibacterial, and anticancer properties of extracts of Annona squamosa L. leaves. BMC Complement. Med. Ther. 2020, 20, 296. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Reddy, M.N.; Alshammari, E. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep. 2018, 8, 1740. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Aslan, M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J. Ethnopharmacol. 2009, 122, 327–332. [Google Scholar] [CrossRef]
- Soulef, S.; Seddik, K.; Nozha, M.; Smain, A.; Saliha, D.; Hosni, K. Phytochemical screening and in vivo and in vitro evaluation antioxidant capacity of Fargaria ananassa, Prunus armeniaca and Prunus persica fruits growing in Algeria. Prog. Nutr. 2020, 22, 236–252. [Google Scholar]
- Bazaid, A.S.; Forbes, S.; Humphreys, G.J.; Ledder, R.G.; O’Cualain, R.; McBain, A.J. Fatty Acid Supplementation Reverses the Small Colony Variant Phenotype in Triclosan-Adapted Staphylococcus aureus: Genetic, Proteomic and Phenotypic Analyses. Sci. Rep. 2018, 8, 3876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, A.S.; Campos, L.M.; Melo, L.; Guedes, M.C.; Oliveira, L.G.; Silva, T.P.; Melo, R.C.; Rocha, V.N.; Aguiar, J.A.; Apolônio, A.C. Antibacterial and antibiofilm activities of psychorubrin, a pyranonaphthoquinone isolated from Mitracarpus frigidus (Rubiaceae). Front. Microbiol. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elasbali, A.M.; Al-Soud, W.A.; Al-Oanzi, Z.H.; Qanash, H.; Alharbi, B.; Binsaleh, N.K.; Alreshidi, M.; Patel, M.; Adnan, M. Cytotoxic Activity, Cell Cycle Inhibition, and Apoptosis-Inducing Potential of Athyrium hohenackerianum (Lady Fern) with Its Phytochemical Profiling. Evid. Based Complement. Altern. Med. 2022, 2022, 2055773. [Google Scholar] [CrossRef]
- Lombardi, V.R.; Carrera, I.; Cacabelos, R. In vitro screening for cytotoxic activity of herbal extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 2675631. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; TM, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Reddy, M.N.; Adnan, M.; Alreshidi, M.M.; Saeed, M.; Patel, M. Evaluation of anticancer, antibacterial and antioxidant properties of a medicinally treasured fern Tectaria coadunata with its phytoconstituents analysis by HR-LCMS. Anti Cancer Agents Med. Chem. Former. Curr. Med. Chem. Anti Cancer Agents 2020, 20, 1845–1856. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
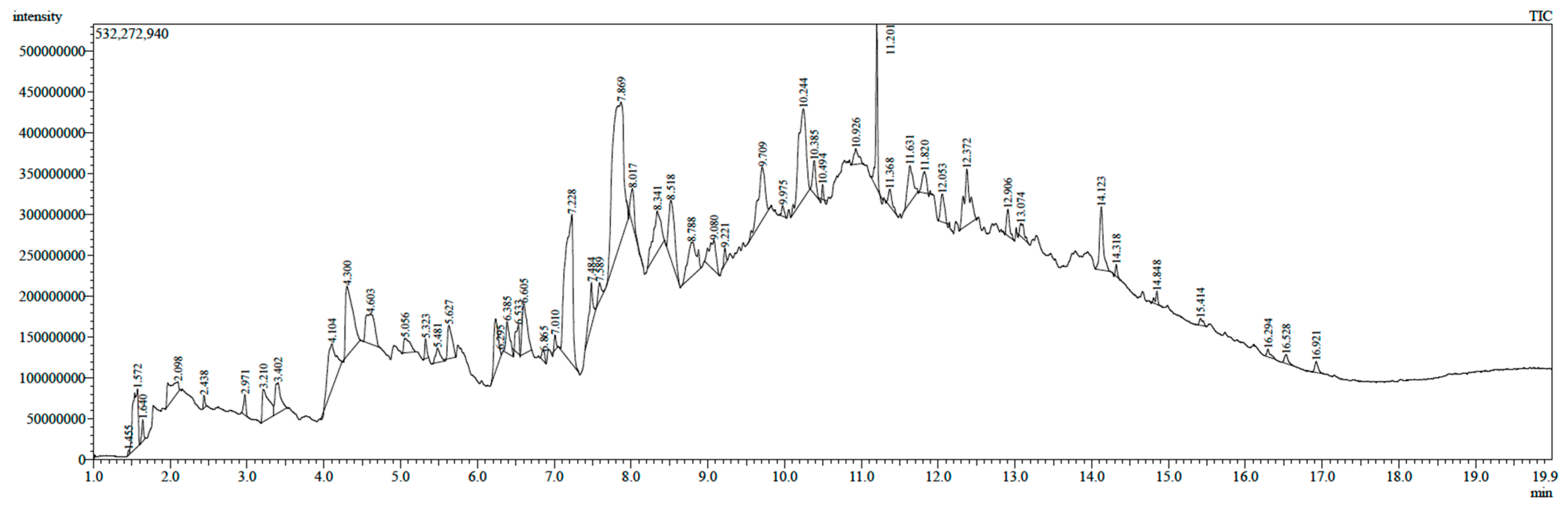
| No. | Retention Time (min) | Area (%) | Tentative Compounds Identification |
|---|---|---|---|
| 1 | 7.869 | 16.11 | 2(3H)-Benzofuranone, hexahydro-3-methylene- |
| 2 | 7.228 | 10.61 | 5-Methyl-2-ethylamino-2-thiazoline |
| 3 | 9.709 | 3.62 | 2-Propenal, 3-(2-nitrophenyl)- |
| 4 | 8.518 | 3.37 | Heptyl caprylate |
| 5 | 12.372 | 3.23 | 2-(Furan-2-yl)ethan-1-amine |
| 6 | 1.572 | 2.97 | 2-Chloroethyl methyl sulfoxide |
| 7 | 8.788 | 2.95 | Silane, [3-(2,3-epoxypropoxy)propyl]cethoxydim |
| 8 | 11.201 | 2.66 | Benzoic acid, 4-hydroxy-3,5-dimethoxy-, methyl |
| 9 | 4.603 | 2.54 | Thiophene, tetrahydro-2-methyl- |
| 10 | 14.123 | 2.24 | 4-[3-(4-Fluorobenzyloxy)propyl]-1H-imidazole |
| 11 | 3.21 | 2.07 | 1-Methylcyclopropanemethanol |
| 12 | 11.631 | 1.95 | 3,4,5-Trihydroxybenzyl methyl ether |
| 13 | 3.402 | 1.69 | Propanedioic acid, oxo-, diethyl ester |
| 14 | 7.484 | 1.62 | 4H-Pyran-4-one, 5-hydroxy-2-(hydroxymethyl |
| 15 | 2.098 | 1.57 | Propane, 1,2-bis(difluoroamino)-2-methyl- |
| 16 | 5.627 | 1.41 | L-Valine, N-ethoxycarbonyl- |
| 17 | 10.385 | 1.0 | 2,4-Dimethoxy-5-pyrimidine carboxaldehyde |
| 18 | 12.053 | 0.99 | 3,5-Methano-2H-cyclopenta[b]furan-2-one, 6-b |
| 19 | 5.056 | 0.88 | 3H-Pyrazol-3-one, 2,4-dihydro-5-methyl- |
| 20 | 11.82 | 0.87 | Butyl dimethyl phosphorothioate |
| 21 | 12.906 | 0.77 | 1,4-Methanonaphthalene, 6,7-diethyldecahydro-, c |
| 22 | 10.926 | 0.72 | p-Menthan-3-one, semicarbazone |
| 23 | 13.074 | 0.51 | 4,5-Imidazoledimethanol |
| 24 | 2.971 | 0.42 | S-Methyl-L-cysteine, N-(n-propyloxycarbonyl) |
| 25 | 1.64 | 0.39 | Oxirane, (fluoromethyl)- |
| 26 | 5.323 | 0.37 | 2-Furanmethanol, 5-methyl- |
| 27 | 16.921 | 0.33 | Cirsiumaldehyde |
| 28 | 16.528 | 0.32 | Succinic acid, dodec-9-yn-1-yl pentyl ester |
| 29 | 9.221 | 0.3 | 2-Aminoresorcinol |
| 30 | 6.865 | 0.27 | 2H-Pyran-2-one, 5,6-dihydro- |
| 31 | 9.975 | 0.26 | (6R,7aR)-3,6-Dimethyl-5,6,7,7a-tetrahydrobenz |
| 32 | 14.848 | 0.26 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymeth |
| 33 | 16.294 | 0.25 | Octadecanoic acid, 2,3-dihydroxypropyl ester |
| 34 | 15.414 | 0.22 | 2-Furaldehyde azine |
| 35 | 2.438 | 0.21 | 2,3-Dimethylpentanoic acid |
| 36 | 14.318 | 0.17 | (8S)-Eremophila-7(11)-en-12,8-lactam |
| 37 | 10.949 | 0.08 | 1,7-Dimethylxanthine |
| Antioxidant Assays | IC50 Values | |
|---|---|---|
| Radical Scavenging activity | DPPH | 7.36 ± 0.03 |
| ABTS | 4.49 ± 0.1 | |
| Bleaching assay | Beta-carotene | 37.51 ± 0.64 |
| Tested Bacteria | Tested Honey Sample (10%) Zone of Inhibition (mm) | |
|---|---|---|
| Gram-positive | B. subtilis | 9.54 ± 0.3 |
| S. aureus | 6.55 ± 0.24 | |
| Gram-negative | E. coli | 4.6 ± 0.25 |
| P. aeruginosa | 3.7 ± 0.15 | |
| Tested Bacteria | Mean | % Inhibition | |
|---|---|---|---|
| B. subtilis | Control | 0.76 ± 0.01 | 67.13 |
| 1/2 MIC | 0.25 ± 0.01 | ||
| S. aureus | Control | 0.84 ± 0.01 | 56.52 |
| 1/2 MIC | 0.36 ± 0.05 | ||
| E. coli | Control | 0.72 ± 0.03 | 48.28 |
| 1/2 MIC | 0.37 ± 0.01 | ||
| P. aeruginosa | Control | 0.87 ± 0.01 | 42.78 |
| 1/2 MIC | 0.50 ± 0.04 | ||
| Tested Cell Lines | Percentage of Inhibition and Cell Viability | Honey Concentrations (%) | IC50 | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |||
| HCT-116 | Inhibition rate (%) | 18.52 | 31.78 | 42.03 | 54.34 | 64.73 | 48.65 |
| Cell viability (%) | 81.47 | 68.21 | 57.96 | 45.65 | 35.26 | ||
| MCF-7 | Inhibition rate (%) | 12.53 | 23.22 | 34.04 | 42.23 | 57.81 | 9.05 |
| Cell viability (%) | 87.46 | 76.77 | 65.95 | 57.76 | 42.18 | ||
| A549 | Inhibition rate (%) | 9.73 | 15.86 | 28.31 | 37.43 | 49.89 | 9.37 |
| Cell viability (%) | 90.26 | 84.13 | 71.68 | 62.56 | 50.10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaid, A.S.; Alamri, A.; Almashjary, M.N.; Qanash, H.; Almishaal, A.A.; Amin, J.; Binsaleh, N.K.; Kraiem, J.; Aldarhami, A.; Alafnan, A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Appl. Sci. 2022, 12, 9928. https://doi.org/10.3390/app12199928
Bazaid AS, Alamri A, Almashjary MN, Qanash H, Almishaal AA, Amin J, Binsaleh NK, Kraiem J, Aldarhami A, Alafnan A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Applied Sciences. 2022; 12(19):9928. https://doi.org/10.3390/app12199928
Chicago/Turabian StyleBazaid, Abdulrahman S., Abdulwahab Alamri, Majed N. Almashjary, Husam Qanash, Ali A. Almishaal, Junaid Amin, Naif K. Binsaleh, Jamil Kraiem, Abdu Aldarhami, and Ahmed Alafnan. 2022. "Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey" Applied Sciences 12, no. 19: 9928. https://doi.org/10.3390/app12199928
APA StyleBazaid, A. S., Alamri, A., Almashjary, M. N., Qanash, H., Almishaal, A. A., Amin, J., Binsaleh, N. K., Kraiem, J., Aldarhami, A., & Alafnan, A. (2022). Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Applied Sciences, 12(19), 9928. https://doi.org/10.3390/app12199928








