Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity
Abstract
:1. Introduction
| Disease, Associated with | Model | Treatment | Results | References |
|---|---|---|---|---|
| Obesity | Rats | 1000 mg/kg/day for 30 days | Improvement of the measured parameters | [52] |
| Human | 2.8 g of Arthrospira/Spirulina thrice a day over a period of 4 weeks | Statistically significant reduction of body weight in obese outpatients | [53] | |
| High cholesterol | Human | Arthrospira/Spirulina 2 g daily for 2 months | Reduces total cholesterol and triglycerides, free fatty acid levels | [54] |
| Cerebral ischemia injury | Rats | Arthrospira/Spirulina at a dose of 180 mg/kg once a day, for 7 days. | Improvement of neurological deficit score, reduction of oxidative stress biomarkers | [55] |
| Diabetes | Human | 2 g water soluble fraction of Arthrospira/Spirulina for 21 days | Reduce blood glucose | [56] |
| Oral leukoplakia | Human | 1 g/day for 1 year | Complete regression of lesions in 45% of the intervention group | [57] |
2. Materials and Methods
2.1. Samples for Research
2.1.1. Conditions for Production of Biomass into a Bioreactor
- ○
- Creating sowing;
- ○
- Changing the habitat of the samples from the laboratory into the production conditions;
- ○
- Growing the samples into a large volume;
2.1.2. Creating Sowing
2.1.3. Changing the Habitat of the Samples from the Laboratory into the Production Conditions
2.1.4. Growing the Samples into a Large Volume
2.2. Methods
2.2.1. Methods for Conservation, Storage, Preparation, Analysis, and Observation of Algal Samples
Freezing and Refrigerator Storage
Convective Drying
Lyophilization
Microscopic Studies
2.2.2. Methods for Evaluating the Antioxidant Activity, Total, and Individual Polyphenolic Compounds
Samples Preparation
Oxygen Radical Absorbance Capacity (ORAC) Method
Hydroxyl Radical Averting Capacity (HORAC) Method
Determination of Total Polyphenolic Content
Determination of Pigment Content
HPLC Determination of Phenolic Acids and Flavonoids
Protein Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Characteristics of Wild Seaweed and Freshwater Algae
3.1.1. Identification of Plant Material
3.1.2. Marine Algae
- ✓
- Brown algae (Phaeophyceae),
- ○
- E. crinita
- ✓
- Green algae (Chlorophyta),
- ○
- U. intestinalis
- ○
- C. linum
3.1.3. Freshwater Algae and Cyanobacteria, Cultivated in a Bioreactor
- ○
- A. platensis (Cyanobacteria)
- ○
- Chlorella spp. (Chlorophyta)
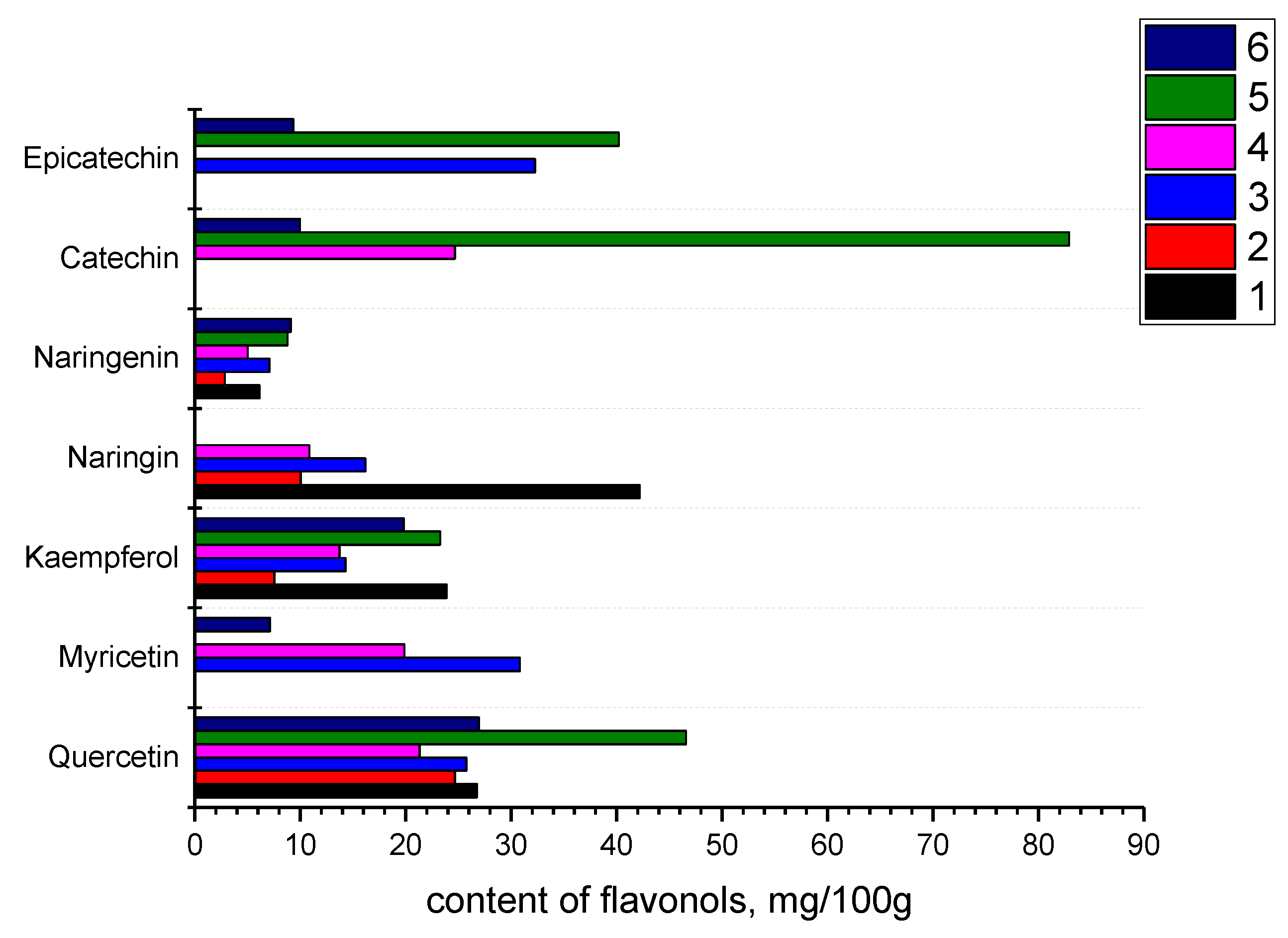
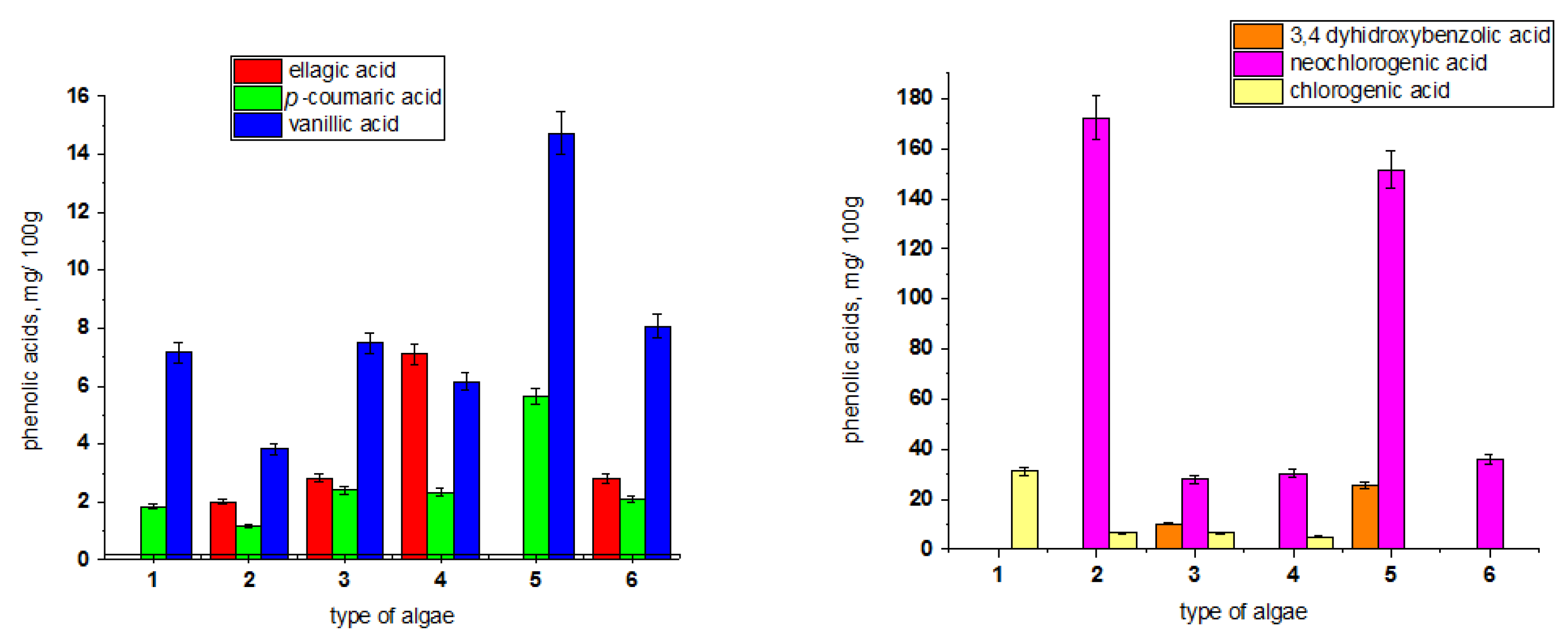
3.2. Some Chemical and Phytochemical Components
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical Composition of Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 79, pp. 124–136. [Google Scholar]
- Stirk, W.A.; Reinecke, D.L.; Van Staden, J. Seasonal variation in antifungal, antibacterial and acetylcholineesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, V. Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. ECronicon Nutr. 2019, 2, 126–141. [Google Scholar]
- del Morales-Amaral, M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sol. Algal Res. 2015, 12, 99–108. [Google Scholar] [CrossRef]
- Guinea, M.; Franco, V.; Araujo-Bazán, L.; Rodríguez-Martín, I.; González, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef]
- Hudson, J.B.; Kim, J.H.; Lee, M.K.; DeWreede, R.E.; Hong, Y.K. Antiviral compounds in extracts of Korean seaweeds: Evidence for multiple activities. J. Appl. Phycol. 1999, 10, 427–434. [Google Scholar] [CrossRef]
- Yang, Y.J.; Nam, S.J.; Kong, G.; Kim, M.K. A case control study on seaweed consumption and the risk of breast cancer. Br. J. Nutr. 2010, 103, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef]
- Mou, H.; Jiang, X.; Guan, H. A k-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J. Appl. Phycol. 2003, 15, 297–303. [Google Scholar]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Enhanced Antitumoral Activity of Extracts Derived from Cultured Udotea flabellum (Chlorophyta). Evid. Based. Complement. Alternat. Med. 2011, 21, 969275–969282. [Google Scholar]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P.; Sundarapandiyan, R. Renal peroxidative changes mediated by oxalate: The protective role of fucoidan. Life Sci. 2006, 79, 1789–1795. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan pro-tects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef]
- Sugawara, T.; Matsubara, K.; Akagi, R.; Mori, M.; Hirata, T. Antiangiogenic activity of brown algae fucoxan thin and its deacetylated product, fucoxanthinol. J. Agri. Food Chem. 2006, 54, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol defciency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffthsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Carrington, M.F.; Walsh, N.A. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food. Chem.Toxicol. 2005, 43, 1073–1081. [Google Scholar] [CrossRef]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and anti-infammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef]
- Ahn, S.M.; Hong, Y.K.; Kwon, G.S.; Sohn, H.Y. Evaluation of antioxidant and nitrite scavenging activity of seaweed extracts. J. Life Sci. 2011, 21, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Wata-nabe, F. Antioxidant activity of the phycoeryth- robilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food. Sci. Technol. Res. 2010, 16, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.T.; O’Connell, S.; Lyons, H.; Bradley, B.; Hall, M. Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chem. Pap. 2010, 64, 434–442. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa Sinha, R.P.; Singh, S.P.; Hader, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [PubMed]
- Lohrmann, N.L.; Logan, B.A.; Johnson, A.S. Seasonal acclimatization of antioxidants and photosynthesis in Chondrus crispus and Mastocarpus stellatus, two co-occurring red algae with differing stress tolerances. Biol. Bull. 2004, 207, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Nahas, R.; Abatis, D.; Anagnostopoulou, M.A.; Kefalas, P.; Vagias, C.; Roussis, V. Radical-scavenging activity of Aegean Sea marine algae. Food Chem. 2007, 102, 577–581. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Amico, V.; Ruberto, G. Antioxidant activity of phenolic meroditerpenoids from marine algae. J. Photochem. Photobiol. B Biol. 1994, 26, 159–164. [Google Scholar] [CrossRef]
- Romay, C.H.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of diabetic complications by C-phycoerythrin in rats: Antioxidant activity of C-phycoerythrin including copper-induced lipoprotein and serum oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38–42. [Google Scholar] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Rocha, H.A.O.; Franco, C.R.C.; Trindade, E.S.; Carvalho, L.C.M.; Veiga, S.S.; Leite, E.L.; Dietrich, C.P.; Nader, H.B. A fucan from the brown seaweed Spatoglossum schröederi inhibits Bilan, M.I.; Usov, A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008, 3, 1639–1648. [Google Scholar]
- Barahona, T.; Chandía, N.P.; Encinas, M.V.; Matsuhiro, B.; Zúñiga, E.A. Antioxidant capacity of sulfated polysaccharides from seaweed: A kinetic approach. Food Hydrocoll. 2011, 25, 529–535. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Josephine, A.; Nithya, K.; Amudha, G.; Veena, C.K.; Preetha, S.P.; Varalakshmi, P. Role of sulphated polysaccharides from Sargassum Wightii in Cyclosporine A-induced oxidative liver injury in rats. BMC Pharmacol. 2008, 8, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.M.A.; Costa, L.S.; Medeiros, V.P.; Cordeiro, S.L.; Costa, M.S.S.P.; Franco, C.R.C.; Nader, H.B.; Leite, E.L. A non-anticoagulant heterofucan has antithrombotic activity in vivo. Planta Med. 2008, 74, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.A.O.; Bezerra, L.C.; Albuquerque, I.R.L.; Costa, L.S.; Guerra, C.M.; Abreu, L.D.; Nader, H.B.; Leite, E.L. Axylogalactofucan from the brown seaweed Spatoglossum schroederi stimulates the synthesis of an antithrombotic heparan sulfate from endothelial. Planta Med. 2005, 71, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.; Sundaram, C.; Singh, S.; Kuppusamy, P.; Kutala, V.K. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 1030–1037. [Google Scholar] [CrossRef]
- Khan, M.; Varadharaj, S.; Shobba, J.C.; Naidu, M.U.; Parinandi, N.L.; Kutala, V.K.; Kuppusamy, P. C-Phycocyanin ameliorates doxorubicin-induced oxidative stress nd apoptosis in adult rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2006, 47, 9–20. [Google Scholar] [CrossRef]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipidemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: A preliminary report. Lipids Health Dis. 2007, 26, 6–33. [Google Scholar]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A randomized double-blind, placebo-controlled study to establish the effects of Spirulina in elderly. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A. A review of recent clinical trials of the nutritional supplement Chlorella spp. pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern. Ther. Health Med. 2001, 7, 79–91. [Google Scholar]
- Madhavadas, S.; Subramanian, S. Combination of Spirulina with glycyrrhizin prevents cognitive dysfunction in aged obese rats. Indian. J. Pharmacol. 2015, 47, 39–43. [Google Scholar]
- Becker, E.W.; Jakover, B.; Luft, D.; Schmuelling, R.M. Clinical and biochemical evaluations of the alga Spirulina with regard to its application in the treatment of obesity: A double-blind cross-over study. Nutr. Rep. Int. 1986, 33, 565–574. [Google Scholar]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the long-term effect of Spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J. Nutraceut. Funct. Med. Foods 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Thaakur, S.; Sravanthi, R. Neuroprotective effect of Spirulina in cerebral ischemia–reperfusion injury in rats. J. Neural Transm. 2010, 117, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ghaeni, M.; Roomiani, L. Review for Application and Medicine Effects of Spirulina Microalgae. J. Adv. Agric. Technol. 2016, 3, 114–117. [Google Scholar]
- Mathew, B.; Sankaranarayanan, R.; Nair, P.; Varghese, C.; Somanathan, T.; Amma, P.; Amma, N.; Nair, M. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr. Cancer 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Berber, I.; Avşar, C.; Koyuncu, H. Antimicrobial and antioxidant activities of Cystoseira crinita Duby and Ulva intestinalis Linnaeus from the coastal region of Sinop, Turkey. J. Coast. Life Med. 2015, 3, 441–445. [Google Scholar]
- Sirbu, R.; Negreanu-Pirjol, T.; Mirea, M.; Negreanu-Pirjol, B.S. Bioactive compounds from three green algae species along Romanian Black sea coast with therapeutically properties. Eur. J. Nat. Sci. Med. 2020, 3, 87–106. [Google Scholar] [CrossRef]
- Sava, C.; Sîrbu, R. Analytical study of the determination of flavonoids in Black Sea algae. Ovidius Univ. Ann. Chem. 2010, 21, 29–34. [Google Scholar]
- Panayotova, V.; Stancheva, M.; Dobreva, D. Alpha-tocopherol and ergocalciferol contents of some macroalgae from Bulgarian Black Sea coast. Ovidius Univ. Ann. Chem. 2013, 24, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, V.; Stancheva, M.; Petrova, D. Fatty acid composition of black sea Ulva rigida and Cystoseira crinite. Bulg. J. Agric. Sci. 2013, 19, 42–47. [Google Scholar]
- Manev, I.; Kirov, V.; Neshovska, H. Heavy metals accumulation in Black sea ecosystems: Seawater, sediment, algae, benthic organisms. Tradit. Mod. Vet. Med. 2020, 5, 88–99. [Google Scholar]
- Panayotova, V.; Stancheva, M. Mineral composition of marine macroalgae from the Bulgarian Black Sea coast. Scr. Sci. Med. 2013, 45, 42–45. [Google Scholar]
- Denev, P. Comparison of Different Methods for Determining Antioxidant Activity in Food and Biological Samples—Postgraduate Course “Of Efficiency and Safety of Intake of Biologically Active Substances”; Report; Bulgarian Academy of Sciences: Sofia, Bulgaria, 10–12 November 2010. [Google Scholar]
- Ou, B.; Hampsh-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ou, B.; Hampsh Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolibdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Transact. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Determination of Protein in Foods. National Food Safety Standard (NFSS) of the People’s Republic of China; China National Center for Food Safety Risk Assessment: Beijing, China, 2016. [Google Scholar]
- Jiménez-Escrig, A.; Sánchez-Muniz, F. Dietary fibre from edible seaweeds: Chemical structure, physiochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant Activity. Total Phenolics and Flavonoid Contents of some Edible Green Seaweeds from Northern Coasts of the Persian Gulf. Iran. J. Pharm. Res. 2014, 13, 163–170. [Google Scholar]
- Cristina de Medeiros, D.; Mizokami, S.S.; Sfeir, N.; Georgetti, S.R.; Urbano, A.; Casagrande, R.; Verri, W.A.; Baracat, M.M. Preclinical Evaluation of Rutin-Loaded Microparticles with an Enhanced Analgesic Effect. ACS Omega 2019, 4, 1221–1227. [Google Scholar] [CrossRef]
- Yongmoon, H. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211. [Google Scholar]
- Zaragoza, C.; Monserrat, J.; Mantecon, C.; Villaescusa, L.; Alvarez-Mon, M.A.; Zaragoza, F.; Alvarez-Mon, M. Binding and antiplatelet activity of quercetin, rutin, diosmetin, and diosmin flavonoids. Biomed. Pharmacother. 2021, 141, 111867. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Fazel Nabavi, S.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Liu, M.C.; Lin, C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother. 2003, 52, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Abdu-llah Al-Saif, S.S.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Yoshie-Stark, Y.; Hsieh, Y.-P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Kut, B.Y.; Roy, G.; Cürük, P.; Roy, D.; Sanver, F.; Tekünay, A.A. Effects of Ulva rigida and Cystoseira barbata Meals as a Feed Additive on Growth Performance, Feed Utilization, and Body Composition of Nile Tilapia, Oreochromis niloticus. Turk. J. Vet. Anim. Sci. 2007, 31, 91–97. [Google Scholar]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutra Foods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Krachanova, M.; Krachanov, H.; Denev, P.; Nikolova, M.; Karagiozov, V.; Slavov, A.; Yanakieva, I.; Richnev, A. Functional foods from fruits and vegetables rich in antioxidants. In Proceedings of the Third International Symposium “Environmental Approaches to Safe Food Production”, Plovdiv, Bilgaria, 6 October 2010; Academic Press Agricultural University: Plovdiv, Bulgaria, 2010; pp. 41–48. [Google Scholar]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; Paepe, D.D.; Baart, G.J.E.; Cooma, L.D. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernaldo de Quirós, A.; Lage-Yusty, M.A.; López-Hernández, J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010, 121, 634–638. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M. Two Classes of pigments, carotenoids and c-phycocyanin, in Spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.Y.; Lee, H.Y. Enhancement of Chlorophyll a Production from marine Spirulina maxima by an optimized ultrasonic extraction process. Appl. Sci. 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitan, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Manev, Z.; Petkova, N. Component composition and antioxidant potential of Cystoseira barbata from the black sea. Series ii: Forestry wood industry. Agric. Food Eng. 2021, 14, 163–172. [Google Scholar]
- Durmaz, Y.; Duyar, H.A.; Gökpinar, Ş.; Taskaya, L.; Öğretmen, Y.; Bandarra, N.M.; Nunes, M.L. Fatty Acids, α-tocopherol and Total Pigment Contents of E. crinita, Ulva spp. and Zostera spp. from Sinop Bay (Turkey). Int. J. Nat. Eng. Sci. 2019, 2, 111–114. [Google Scholar]
- Danesi, E.D.G.; Rangel-Yagui, C.O.; Carvalho, J.C.M.; Sato, S. Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenergy 2004, 26, 329–335. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Abdul-Adel, E.; Saleh, M.M.; Salman, J.M. Production of phyotosynthesis pigments by Spirulina platensis under different NaCl concentrations. Plant Arch. 2019, 19, 3254–3258. [Google Scholar]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Inhibitory action of natural carrageenans on Herpes simplex virus infection of mouse astrocytes. Chemotherapy 1999, 45, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef] [PubMed]

| Antioxidant | Health Benefits | References |
|---|---|---|
| β-carotene, lutein | Protection against breast cancer | [11] |
| Bromophenol Carrageenan Oligosaccharides Fucoidan | Inhibition of α-glucosidase | [12] |
| Antitumor effect | [13] | |
| Anti-HIV-effect | [14] | |
| Improves hyperoxaluria | [15] | |
| Protection against | [16] | |
| neurodegenerative disorder | ||
| Fucoflorethols | Chemopreventive effect | [17] |
| Fucoxanthin | Antiangiogenic effect | [18] |
| Protective effect against retinol deficiency | [19] | |
| Galactan sulfate | Antiviral effect | [20] |
| Phenolic functional groups and mycosporine (as amino acids) | Anticancer effect | [21] |
| Phlorotannins | Anti-inflammatory and bactericidal effect | [21] |
| Inhibition of H2O2—mediator of DNA damage | [22] | |
| Photochemopreventive effect | [22] | |
| Phycoerythrin | Improvement of diabetic complications | [23] |
| Polyphenols | Vascular chemoprotection | [24] |
| Antimicrobial effect | ||
| Inhibition of α-glucosidase | ||
| Porphyran, shinorine | Anti-aging effect | [25] |
| Components | Algal Source | References |
|---|---|---|
| β-carotene | Chondrus crispus Mastocarpus stellatus | [26] |
| Fucoxanthin | Brown algae | [27] |
| Antheraxanthin Lutein Violaxanthin Xanthophylls Zeaxanthin | Red algae | [28] |
| Stypodiol Isoepitaondiol Taondiol | Taonia atomaria | [29] |
| Terpenoids | Ericaria crinita | [30] |
| Phycoerythrin Phycocyanin | Red algae | [31] [32] [33] [34] |
| Catechin Epicatechin gallate | Halimeda spp. | [35] |
| Flavonoids Phlorotannins | Palmaria palmata | [20] |
| Sargassum pallidum | [36] | |
| Fucus vesiculos | [37] | |
| Ascorbate | Chondrus crispus Mastocarpus stellatus Sargassum spp. | [25] [37] |
| Vitamin A | Kappaphycus alvarezii | [6] |
| Fucoidan Alginic acid Laminaran | Turbinaria conoides | [38] |
| Fucoidan | Saccharina japonica | [16] |
| Sulfated galactans | (formerly Laminaria japonica) | [39,40] |
| (Lambda-carrageenan) | Some red seaweeds | |
| Galactans | Most of the red algae | [41] |
| Sulfated glycosaminoglycan | Sargassum wightii | [42] |
| Porphyran | Porphyra spp. | [43] |
| U. intestinalis | C. linum | E. crinita (Formerly Cystoseira crinita) | |
|---|---|---|---|
| Phylum | Chlorophyta | Chlorophyta | Phaeophyta |
| Class | Ulvophyceae | Cladophorophyceae | Phaeophyceae |
| Order | Ulvales | Cladophorales | Fucales |
| Family | Ulvaceae | Cladophoraceae | Cystoseiraceae |
| Genus | Ulva | Chaetomorpha | E. crinita |
| Locality | Asparuhovo, Varna | Asparuhovo, Varna | Pomorie |
| Geographical Coordinates | 43.173645, 27.916596 | 43.189680, 27.884336 | 42.560546, 27.633244 |
| Number of samples | 3 | 3 | 3 |
| Algae | TPC, mg GAE/100 g | ORAC, µmol TE/g | HORAC, µmol GAE/g |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| A. platensis (convection dried) | |||
| A. platensis (lyophilized) | |||
| U. intestinalis (lyophilized) | |||
| C. linum (lyophilized) | |||
| E. crinita (lyophilized) | |||
| Chlorella spp. (lyophilized) |
| Regression Model | Correlation Coefficient |
|---|---|
| ORAC = 0.1651.TPC + 24.357 | 0.993 |
| HORAC = 0.0422.TPC + 0.7389 | 0.990 |
| Algae | Rutin Content, mg/100 g | Protein Content, % |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| A. platensis (convection dried) | ||
| A. platensis (lyophilized) | ||
| U. intestinalis (lyophilized) | ||
| C. linum (lyophilized) | ||
| E.crinitea (lyophilized) | ||
| Chlorella spp. (lyophilized) |
| Algae | Chlorophyll a, µg/g | Chlorophyll b, µg/g |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Chlorella spp. (lyophilized) | ||
| E. crinita (lyophilized) | ||
| U. intestinalis (lyophilized) | ||
| A. platensis (lyophilized) | ||
| A. platensis (convection dried) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. https://doi.org/10.3390/app12199935
Gentscheva G, Milkova-Tomova I, Pehlivanov I, Gugleva V, Nikolova K, Petkova N, Andonova V, Buhalova D, Pisanova E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Applied Sciences. 2022; 12(19):9935. https://doi.org/10.3390/app12199935
Chicago/Turabian StyleGentscheva, Galia, Iliana Milkova-Tomova, Ivaylo Pehlivanov, Viliana Gugleva, Krastena Nikolova, Nadezhda Petkova, Velichka Andonova, Dragomira Buhalova, and Ekaterina Pisanova. 2022. "Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity" Applied Sciences 12, no. 19: 9935. https://doi.org/10.3390/app12199935









