Study on the Tribological Properties of F-T DS/ZnFe-LDH Composite Lubricating Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment, Raw Materials, and Reagents
2.2. Preparation of Lubricating Materials
2.3. Preparation of the Oil Sample for the Friction Test
2.4. Tribological Experiments
2.5. Analysis Method
3. Results and Discussion
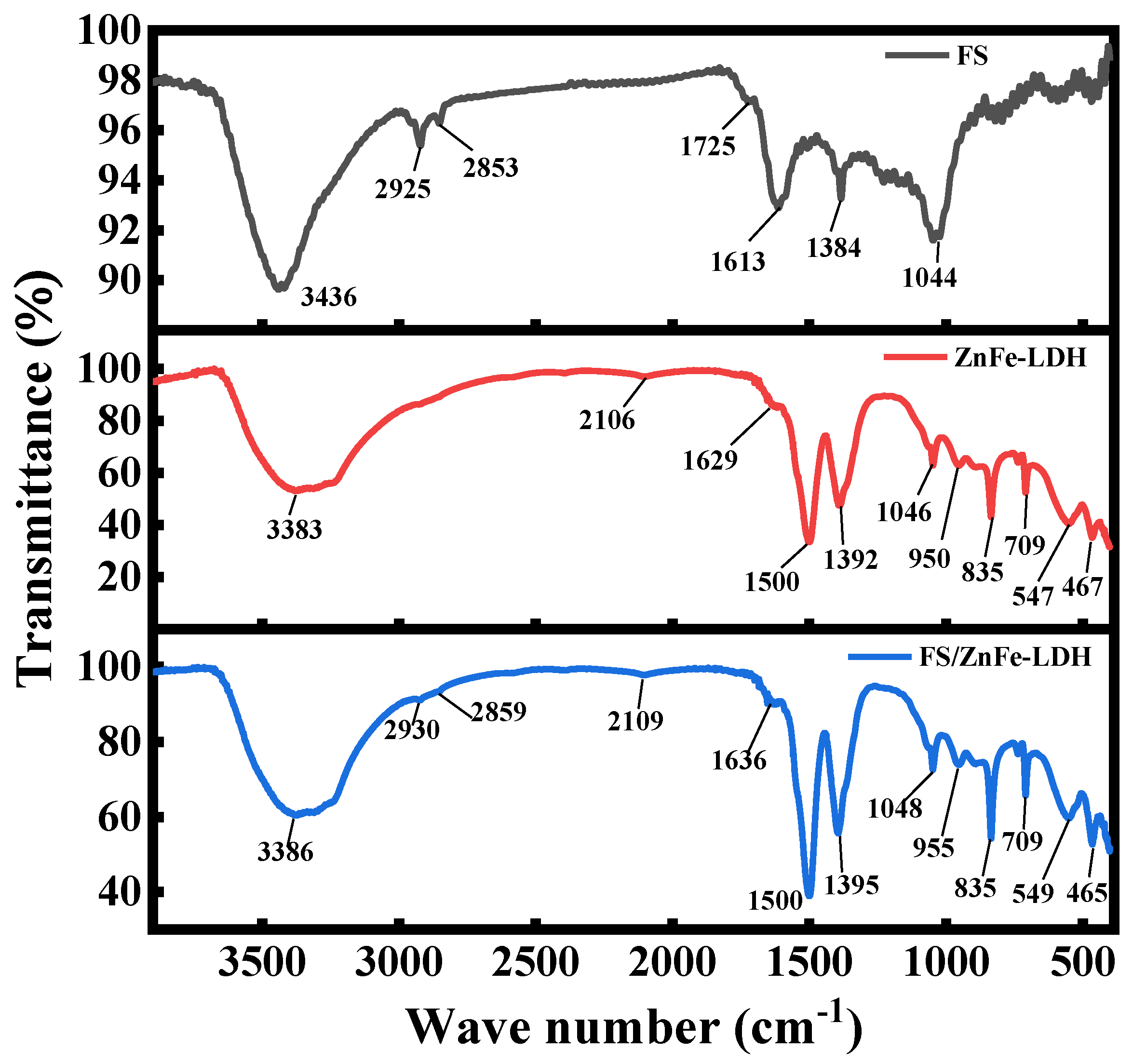
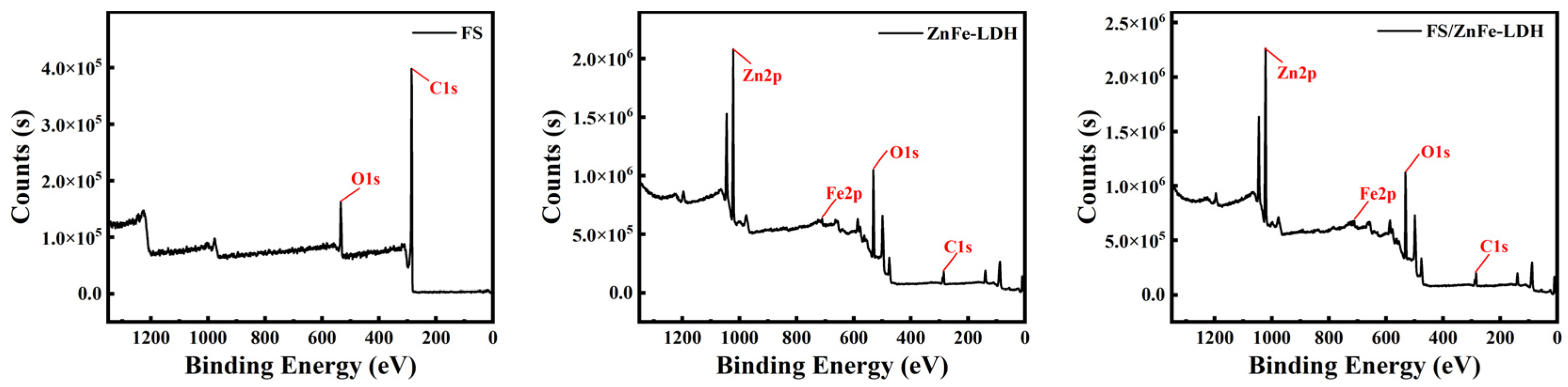
3.1. FTIR and XPS Analysis
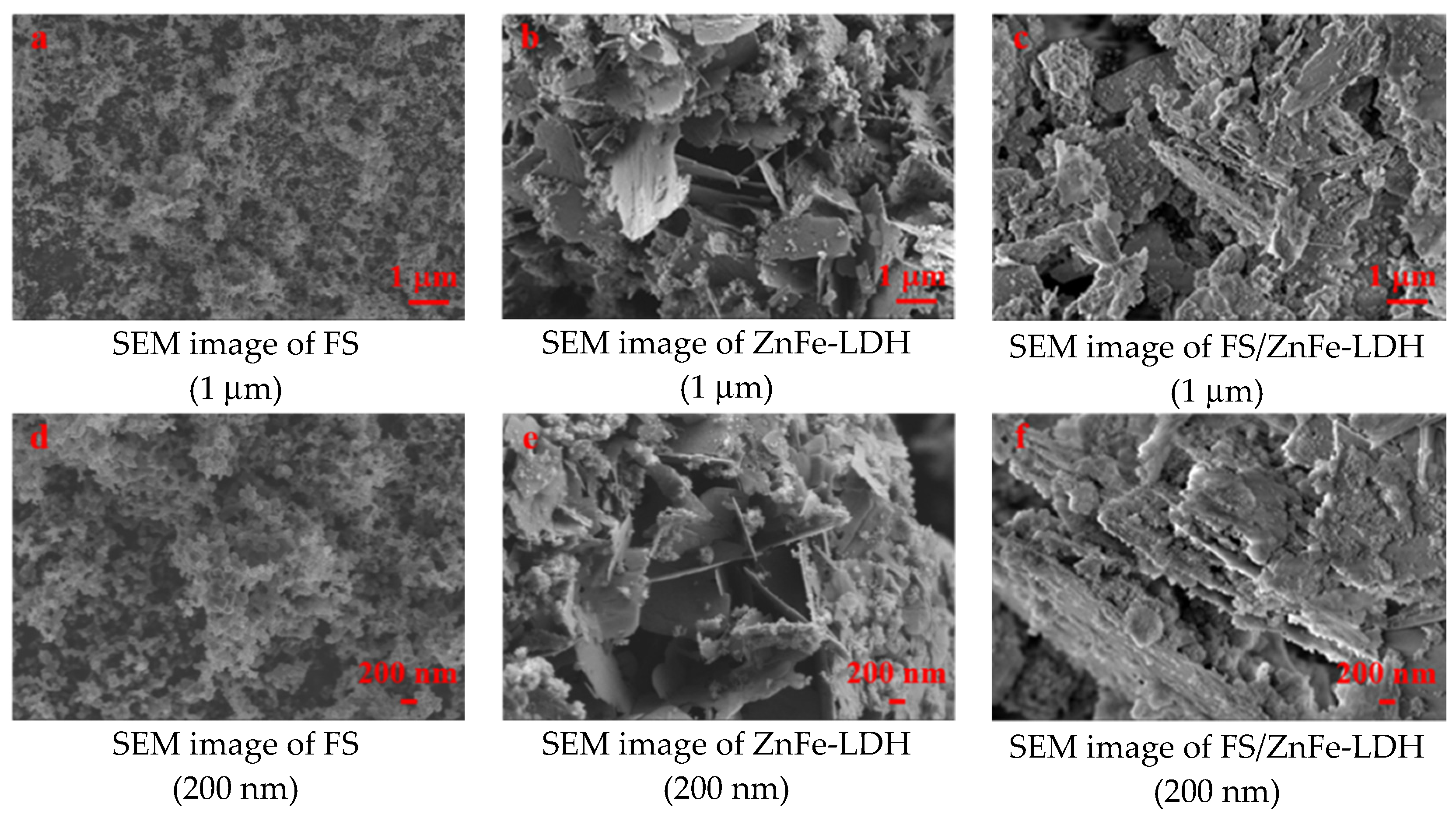
3.2. Structure Analysis and Surface Topography Analysis
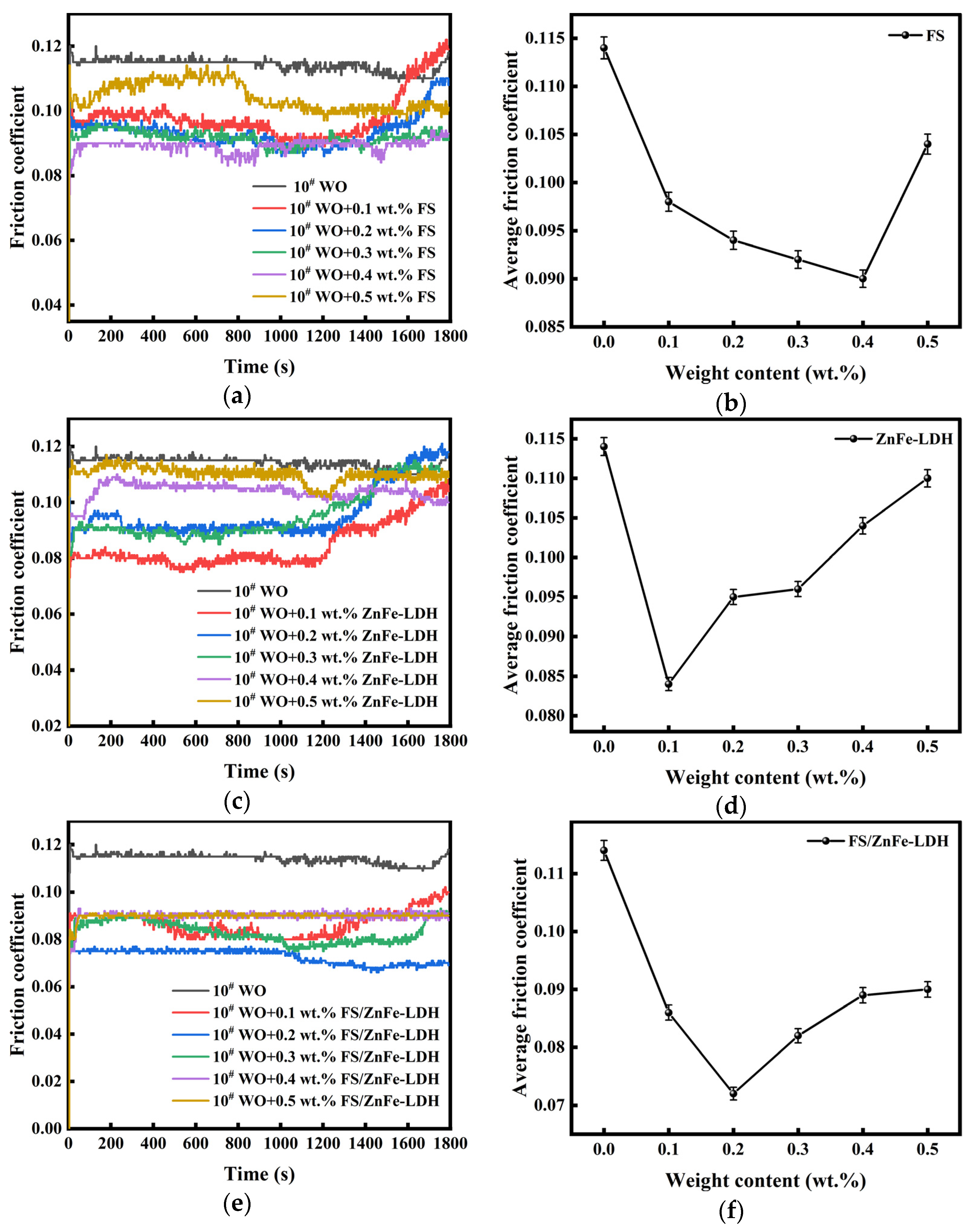
3.3. Influence of the Three Lubricating Materials on the Anti-Friction Performance of 10# WO
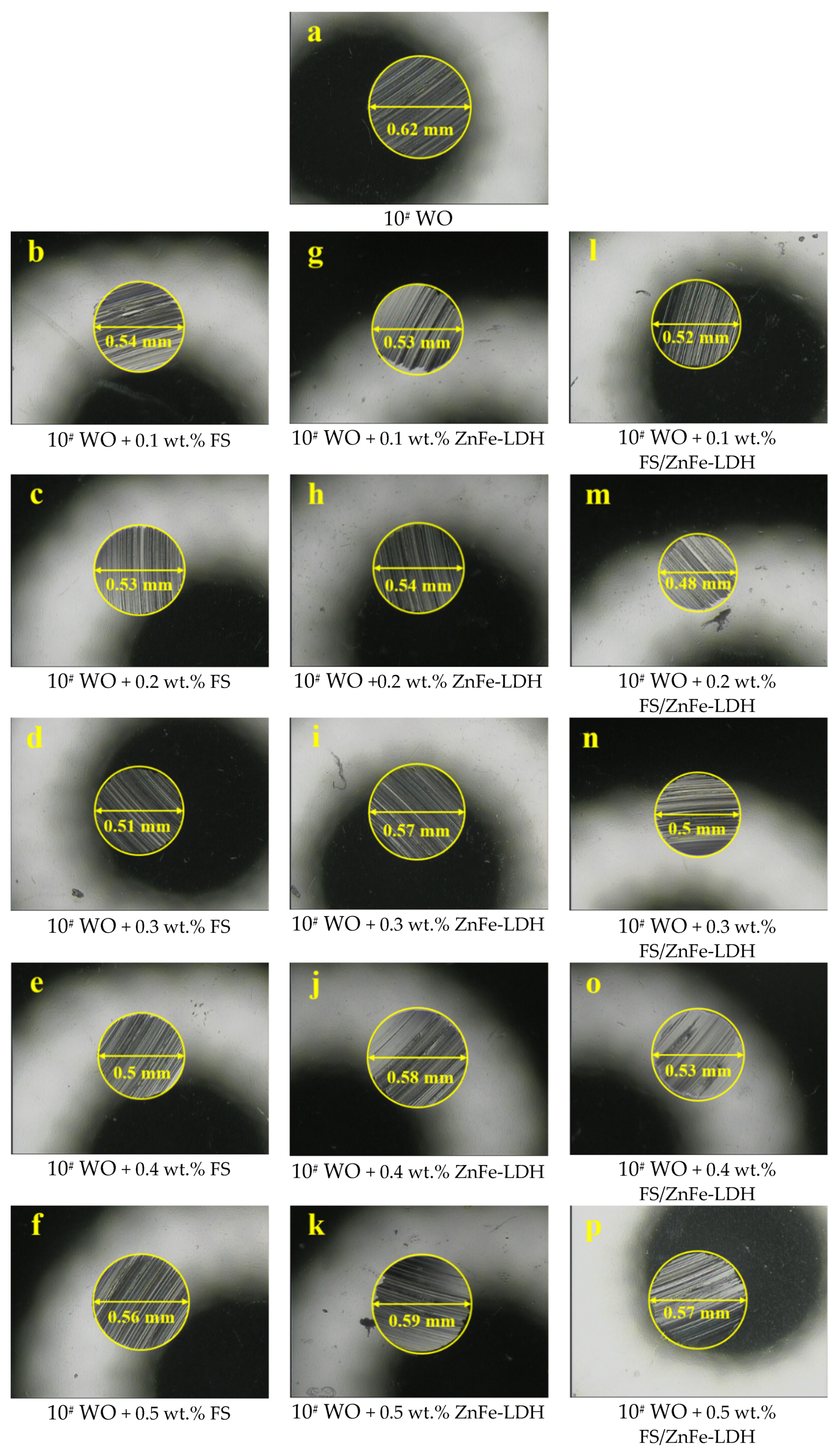
3.4. Influence of the Three Lubricating Materials on the Anti-Wear Properties of 10# WO
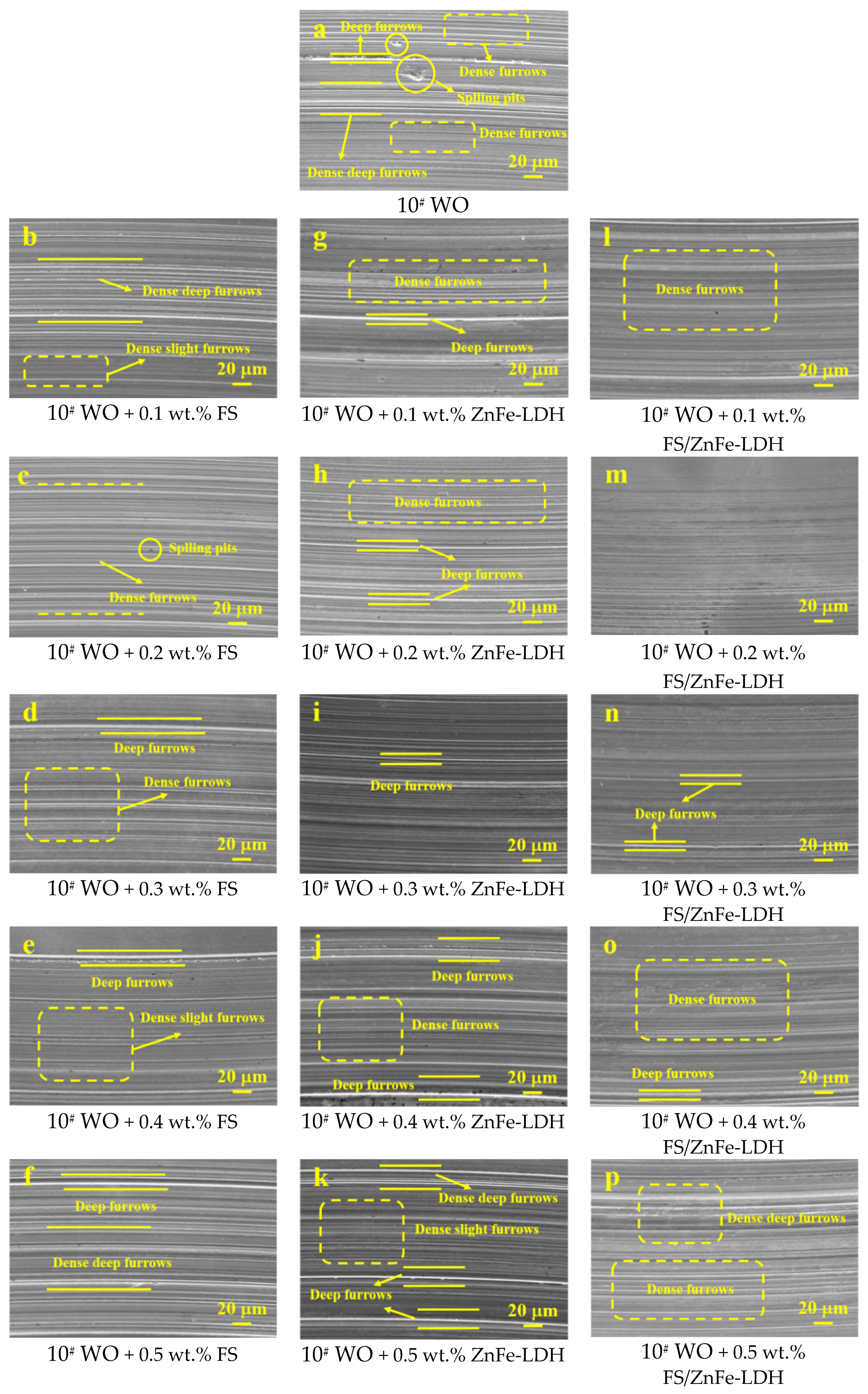
3.5. Analysis of Wear Scar Surface Morphology and Anti-Friction and Anti-Wear Mechanism
4. Conclusions
- FS is an agglomeration composed of amorphous carbon and graphite crystallite. ZnFe-LDH is mainly composed of Zn and Fe hydroxide nanosheets with a high degree of crystallinity. FS/ZnFe-LDH is a “sandwich layer” material composed of nanosheets and soot. FS/ZnFe-LDH may significantly improve the lubricating performance of 10# WO compared to FS and ZnFe-LDH.
- The anti-friction and anti-wear capabilities of the three lubricating materials prepared were different. FS/ZnFe-LDH had the best lubrication performance, followed by FS, and finally ZnFe-LDH. FS/ZnFe-LDH has the “sandwich layer” structure, and the surface is grafted with long carbon chains that can improve oil solubility, so FS/ZnFe-LDH had the best lubrication performance.
- In the friction process, FS/ZnFe-LDH participated in the formation of friction lubrication film, which contains the four elements C, O, Zn, and Fe. The friction surface was protected and repaired. Therefore, as a lubricant additive, FS/ZnFe-LDH effectively improves the lubricating performance of 10# WO.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, L.L.; Cao, Z.; Dai, M. Material metabolism and carbon emission reduction strategies of passenger cars in China’s mainland. Resour. Sci. 2021, 43, 501–512. [Google Scholar] [CrossRef]
- Sun, W.C.; Jiang, M.Q. Coal-based synthetic fuel and its application and technical progress in automobile engines. J Automot. Saf. Energy 2021, 12, 1–17. [Google Scholar]
- Kang, K.; Liu, T.X.; Wang, J.; Qin, J. On the characteristic of Coal Fischer-Tropsch synthesis diesel soot. J. Henan Univ. (Nat. Sci.) 2021, 51, 75–82. [Google Scholar]
- Su, P.; Xiong, Y.; Liu, X.; Yang, H.; Fan, L.J.; Liu, P. The Role of Diesel Soot in the Tribological Behavior of 150SN Base Oil. China Pet. Process. Petrochem. Technol. 2017, 19, 89–95. [Google Scholar]
- Huang, G.W.; Yu, Q.L.; Ma, Z.F.; Cai, M.R.; Zhou, F.; Liu, W. Fluorinated Candle Soot as the Lubricant Additive of Perfluoropolyether. Tribol. Lett. 2017, 65, 28. [Google Scholar] [CrossRef]
- Hu, E.Z.; Hu, K.H.; Xu, Z.Y.; Hu, X.G.; Dearn, K.D.; Xu, Y.; Xu, Y.F.; Xu, L. Investigation into the morphology, composition, structure and dry tribological behavior of rice husk ceramic particles. Appl. Surf. Sci. 2016, 366, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.Z.; Hu, K.H.; Dearn, K.D.; Hu, X.G.; Xu, Y.F.; Yu, D.R.; Gu, H.M.; Tang, Y.C. Tribological performance of rice husk ceramic particles as a solid additive in liquid paraffin. Tribol. Int. 2016, 103, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.Z.; Dearn, K.; Yang, B.X.; Song, R.H.; Xu, Y.F.; Hu, X.G. Tribofilm formation and characterization of lubricating oils with biofuel soot and inorganic fluorides. Tribol. Int. 2017, 107, 163–172. [Google Scholar] [CrossRef]
- Guo, M.F.; Cai, Z.B.; Zhang, Z.C.; Zhu, M.H. Characterization and lubrication performance of diesel soot nanoparticles as oil lubricant additives. Rsc Adv. 2015, 5, 101965–101974. [Google Scholar] [CrossRef]
- Zhang, B.B.; Duan, J.Z.; Huang, Y.L.; Hou, B.R. Double layered superhydrophobic PDMS-Candle soot coating with durable corrosion resistance and thermal-mechanical robustness. J. Mater. Sci. Technol. 2021, 71, 1–11. [Google Scholar] [CrossRef]
- Su, F.H.; Chen, G.F.; Huang, P. Lubricating performances of graphene oxide and onion-like carbon as water-based lubricant additives for smooth and sand-blasted steel discs. Friction 2020, 8, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.F.; Shen, M.X.; Cai, Z.B. Nano Diesel Soot Particles Reduce Wear and Friction Performance Using an Oil Additive on a Laser Textured Surface. Coatings 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Guo, J.H.; Cao, X.A.; Hu, E.Z.; Hu, K.H. Effects of carbon soot from the combustion of diesel fuels on the tribological properties of lubricating oil and diesel fuels. Ind. Lubr. Tribol. 2018, 70, 532–537. [Google Scholar] [CrossRef]
- Li, S.; Bai, Z.; Zhao, D. Characterization and friction performance of Zn/Mg/Al-CO3 layered double hydroxides. Appl. Surf. Sci. 2013, 284, 7–12. [Google Scholar] [CrossRef]
- Li, G.J.; Zhang, Z.L.; Bai, Z.M. Friction performance of hydrotalcite as lubrication oil (grease) additive and its influence factors. J. Mater. Eng. 2021, 49, 48–55. [Google Scholar]
- Li, S.; Qin, H.; Zuo, R.; Bai, Z. Tribological performance of Mg/Al/Ce layered double hydroxides nanoparticles and intercalated products as lubricant additives. Appl. Surf. Sci. 2015, 353, 643–650. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Wang, H.; Liu, Y. Superior extreme pressure properties of different layer LDH nanoplatelets used as boundary lubricants. Appl. Surf. Sci. 2020, 530, 147203. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Guo, F.; Sheng, H.; Xia, K.; Liu, W.; Wen, J.; Shi, Y.; Erdemir, A.; Luo, J. Catalytically Active Oil-Based Lubricant Additives Enabled by Calcining Ni-Al Layered Double Hydroxides. J. Phys. Chem. Lett. 2020, 11, 113–120. [Google Scholar] [CrossRef]
- Tsiganov, A.; Krivonogova, A.; Nikityuk, T.; Smirnova, O.; Gorokhovsky, A. Synthesis, structure and tribological properties of nanocomposite materials in the system of potassium polytitanate-layered double hydroxide–Serpentinite. In Proceedings of the 13th International Conference on Mechanical Engineering, Novosibirsk, Russia, 12–14 December 2019. [Google Scholar]
- Li, S.; Bhushan, B. Lubrication performance and mechanisms of Mg/Al-, Zn/Al-, and Zn/Mg/Al-layered double hydroxide nanoparticles as lubricant additives. Appl. Surf. Sci. 2016, 378, 308–319. [Google Scholar] [CrossRef]
- Ba, Z.W.; Han, Y.Y.; Qiao, D.; Feng, D.P.; Huang, G.W. Phoshate Interlayered LDH as Nanolubricant Additive. Tribology 2018, 38, 373–382. [Google Scholar]
- Aguilar, O.; Tzompantzi, F.; Pérez-Hernández, R.; Gómez, R.; Hernández-Gordillo, A. Novel preparation of ZnS from Zn5(CO3)2(OH)6 by the hydro- or solvothermal method for H2 production. Catal. Today 2017, 287, 91–98. [Google Scholar] [CrossRef]
- Parveen, M.F.; Umapathy, S.; Dhanalakshmi, V.; Anbarasan, R. Synthesis and Characterization of Nano-sized Zn(OH)(2) and Zn(OH)(2)/PVA Nano-composite. Compos. Interfaces 2010, 17, 757–774. [Google Scholar] [CrossRef]
- Tzompantzi-Flores, C.; Cesar Castillo-Rodriguez, J.; Gomez, R.; Tzompantzi, F.; Perez-Hernandez, R.; De la Luz Tlapaya, V.; Eduardo Santolalla-Vargas, C. Synthesis and characterization of ZnZr composites for the photocatalytic degradation of phenolic molecules: Addition effect of ZrO2 over hydrozincite Zn5(OH)6(CO3)2. J. Chem. Technol. Biotechnol. 2019, 94, 3428–3439. [Google Scholar] [CrossRef]
- Maria Rodriguez-Chiang, L.; Llorca, J.; Dahl, O.P. Effect of Fe-Zn-Mg-Al hydrotalcites on the methane potential of synthetic sulfate-containing wastewater. J. Water Process Eng. 2016, 10, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Zhou, X.; Lund, P.; Chen, C.; Fan, L. Electrochemical impact of the carbonate in ceria-carbonate composite for low temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2021, 46, 9898–9904. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Yang, Y.; Li, Z.; Zhang, L.; Huang, Q. Mechanochemical synthesis of Fe2O3/Zn-Al layered double hydroxide based on red mud. J. Hazard. Mater. 2020, 394, 122566. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.A.N.H.; Yang, H. Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Yuan, X.; Li, W. Graphitic-C3N4 modified ZnAl-layered double hydroxides for enhanced photocatalytic removal of organic dye. Appl. Clay Sci. 2017, 138, 107–113. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/Fe(III) cycle under visible light irradiation. Appl. Catal. B-Environ. 2020, 263, 118282. [Google Scholar] [CrossRef]
- Salehi, F.M.; Morina, A.; Neville, A. The effect of soot and diesel contamination on wear and friction of engine oil pump. Tribol. Int. 2017, 115, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.X.; Yao, M.H.; Cai, M.R.; Zhou, F. Tribological Properties of Cetyltrimethyl Ammonium Bromide Modified Candle Soot as Effective Lubricant Additive in Oil. Tribology. 2014, 34, 428–436. [Google Scholar]




| Physicochemical Properties | F-T Diesel | 0# Diesel | Test Standard | |
|---|---|---|---|---|
| Density (kg/m3, 20 °C) | 773.2 | 737.8 | GB/T 1884 | |
| Kinematic viscosity (mm2, 20 °C) | 3.595 | 4.71 | GB/T 265 | |
| Closed flash point (mg/100 mL) | 78.5 | 55 | GB/T 261 | |
| Oxidation stability sediment | 2.0 | 2.5 | SH/T 0175 | |
| Distillation Range | 50% recycling temperature | 286.5 | 273 | GB/T 6536 |
| 90% recycling temperature | 335.0 | 339 | ||
| 95% recycling temperature | 347.0 | 355 | ||
| Sulfur content (mass fraction), mg/kg | <1.0 | 0.81 | SH/T 0689 | |
| Acidity (calculated by KOH), mg/100 mL | not detected | 5.64 | GB/T 258 | |
| 10% residual carbon after steaming (mass fraction), % | not detected | 0.3 | GB/T 17144 | |
| Ash content (volume fraction), % | not detected | 0.01 | GB/T 508 | |
| Copper sheet corrosion (50 °C, 3 h), grade | 1a | 1a | GB/T 5096 | |
| Moisture (volume fraction), % | none | trace | GB/T 260 | |
| Total pollutant content, mg/kg | / | / | GB/T 33400 | |
| Lubricity, wear scar diameter, µm | 346 | / | SH/T 0765 | |
| Solidifying point, °C | 5 | 0 | GB/T 510 | |
| Condensation point, °C | 4 | 4 | SH/T 0248 | |
| Cetane value | / | 57.6 | GB/T 386 | |
| Cetane Index | 79 | 46 | GB/T 11139 | |
| Polycyclic aromatic hydrocarbon content (mass fraction), % | 773.2 | / | SH/T 0606 | |
| Fatty acid methyl ester content (volume fraction), % | / | / | NB/SH/T 0916 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, T. Study on the Tribological Properties of F-T DS/ZnFe-LDH Composite Lubricating Material. Appl. Sci. 2022, 12, 599. https://doi.org/10.3390/app12020599
Wang J, Liu T. Study on the Tribological Properties of F-T DS/ZnFe-LDH Composite Lubricating Material. Applied Sciences. 2022; 12(2):599. https://doi.org/10.3390/app12020599
Chicago/Turabian StyleWang, Jian, and Tianxia Liu. 2022. "Study on the Tribological Properties of F-T DS/ZnFe-LDH Composite Lubricating Material" Applied Sciences 12, no. 2: 599. https://doi.org/10.3390/app12020599





