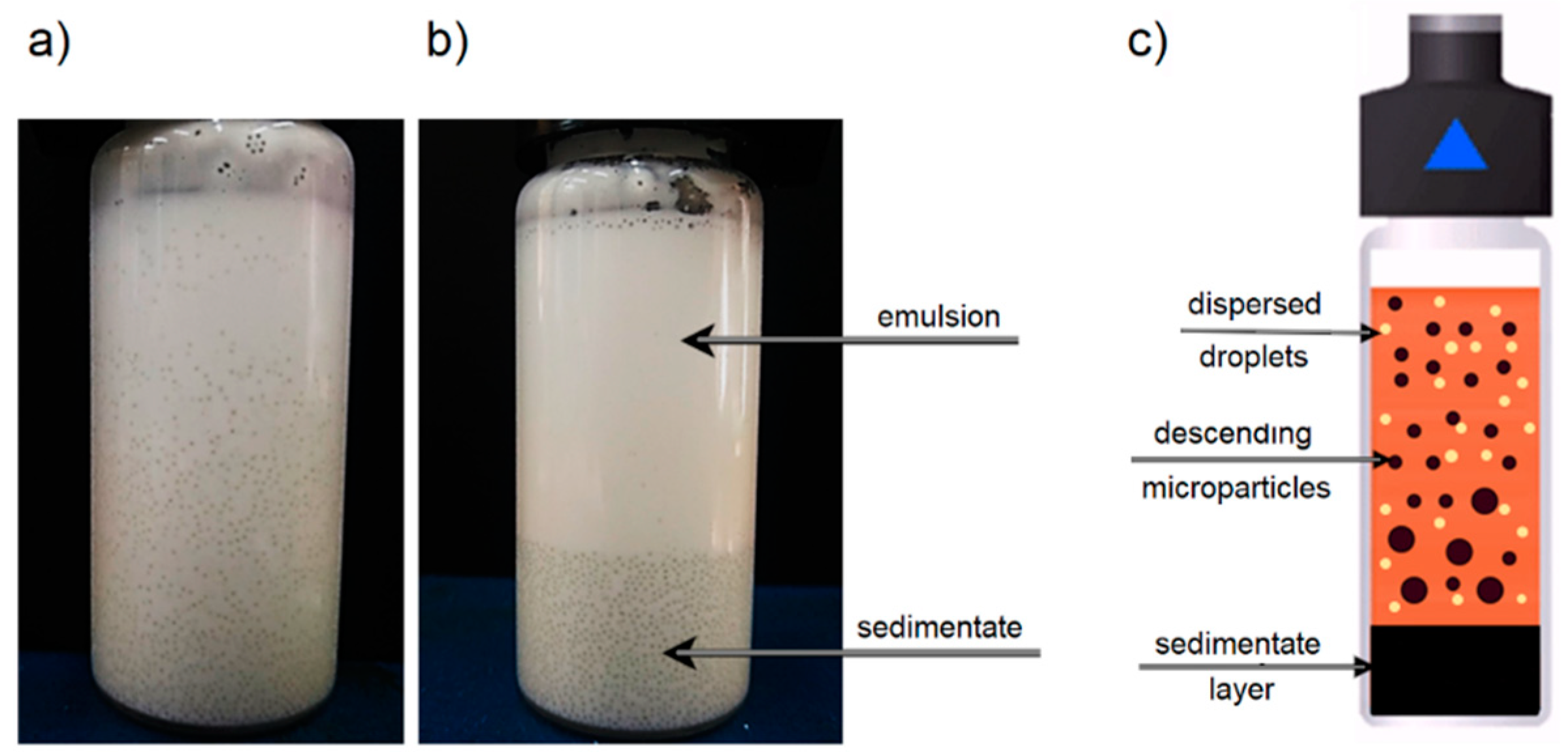
The analysis of sedimentation of solids in emulsion systems included the influence on the process of solid parameters, such as particle diameter size and microspheres concentration φz, and liquid parameters, such as emulsion concentration φe and average oil droplet size de.
3.1. Influence of Solid Parameters on the Sedimentation Process in Emulsion
To determine the effect of solid particle size on the sedimentation process, glass microspheres with five different grain size fractions were selected (see
Table 1). The sedimentation process was carried out according to the test procedure described earlier, which made it possible to obtain graphs of the dependence of the sediment layer growth h
s at time t. Applying Equation (1), it was also possible to calculate changes in velocity u during the process. The graphs of time dependence of h
s and u for sedimentation of 0.3 g/mL microspheres in 70% emulsion with average droplet diameter d
e1 are presented in
Figure 4a and
Figure 4b, respectively.
By analyzing the change in sediment layer height over time (
Figure 4a) for different grain size fractions, large differences in the alignment of experimental points can be observed. For the sedimentation of the smallest microspheres with a diameter range of 100–200 μm, the sediment layer growth is mild, and finally, the sediment layer height after 30 min h
sk was small and was about 2.5 mm. In addition, the sedimentation velocity for this fraction was small and relatively constant, as can be observed in
Figure 4b. For larger microspheres, with a diameter range of 200–300 μm, it can be observed that the value of h
s varies more steeply over time, and eventually, the height of h
sk was higher (6.42 mm). This also corresponds to higher sedimentation velocities. For the sedimentation of microspheres with a fraction of 300–400 μm, the final height of the sediment h
sk was the highest with a value of 10.34 mm. However, the shape of the h
s versus t curve is not as steep as for smaller beads. For larger microspheres, with a diameter range of 400–600 μm, it can be seen that the final sediment layer height h
sk was slightly smaller than that recorded for the preceding fraction. Differences can also be noticed in the shape of the h
s(t) curve. It can be observed that at the beginning of the process, there was a rapid increase in the value of h
s, while with the duration of the process, the increases were smaller and smaller. These results are also illustrated in the velocity vs. time plot (
Figure 4b). It can be seen here that initially, the sedimentation velocities take on high values, but as the process continues, these values decrease and eventually take on a fixed value. The trends observed here are further enhanced for the sedimentation process of microspheres with a diameter range of 600–800 μm. Here, a rapid increase in the height of the sediment layer is more clearly observed in the first phase of the process, but smaller and smaller changes are observed over time. Finally, the height of the sediment layer was lower than in the previous case.
To explain the observed trends, it is necessary to consider what happens to the phases in each case. It should be emphasized that we are dealing with sedimentation in a highly concentrated emulsion, where the packing of oil droplets is very high. Three cases can be distinguished here. The first refers to small particles, the second to medium particles, and the third to large particles. For each of these cases, the mechanism of movement of the microspheres will be different. To explain this, the diagram shown in
Figure 5 is used. In this diagram, oil droplets are shown in yellow and solid microspheres in brown.
When the size of microspheres is small (
Figure 5a), their weight is small. The gravity force in this case is not large enough to overcome the flow-resistance forces. The beads are not able to tear through the emulsion structure, so they remain trapped in it. Therefore, the final sediment height h
sk for small-sized microspheres is small. The situation is different when the microspheres are of larger size, as shown in
Figure 5b. Then their gravity is high enough to be able to break through the emulsion structure. The emulsion droplets move closer to each other, creating space for the solid to flow. In such a situation, the most intensive sedimentation process occurs, which explains the highest heights of the final sediment layer for medium-sized microspheres (300–400 μm fraction). It might seem that a further increase in particle size, i.e., a corresponding increase in particle weight, would result in an even more intense increase in the sedimentation process, but this is not the case. This is because, in the case of large particle sedimentation, although the weight is large enough to overcome flow resistance, the size of the particles itself becomes important. When they are large, in order to break through the emulsion structure, they cause oil droplets to come as close as possible to each other. These droplets start to form a compact structure. In a limited space, they form a barrier to the flow of solids, as shown schematically in
Figure 5c. This explains the fact that for beads of the largest size, a lower height of the sediment layer is ultimately observed compared to the medium sizes. Due to the fact that the sedimentation process took place from the whole volume, the particles closer to the bottom had a greater ease in overcoming this compact structure and therefore fell faster. This is explained by the fact that the sedimentation intensity is higher at the beginning of the process and is lower and lower in further stages.
When analyzing the structure of solid particles and oil droplets of emulsions, the number of individual phases in the system is not without significance. Therefore, analogous tests were carried out for the case when the concentration of micro particles in the system was lower and when it was higher than previously presented.
Figure 6 presents plots of (a) sediment layer height and (b) velocity versus time for sedimentation of 0.2 g/mL microspheres in 70% emulsion with d
e1 droplet diameter.
From the analysis of the curves of the dependence of h
s and u on t, shown in
Figure 6, it can be seen that the trend of these curves was similar to those obtained for sedimentation at a concentration of 0.3 g/mL. Obviously, the sediment layer heights obtained here will be smaller because fewer particles are in the system and may eventually form smaller layers. However, the highest sediment height h
sk was obtained for microspheres of smaller size than in the previous case, i.e., in the range of 200–300 μm. For the largest size of microspheres, the changes in h
s height are rapid in the first minutes of the process, but quickly, the process establishes itself, and the sediment height in the later stages did not change much anymore. It can be seen that the trend is more pronounced here than for sedimentation at 0.3 g/mL concentration.
Figure 7 shows analogous plots of the sediment layer growth (a) and velocity (b) relationship during the sedimentation process at the higher microspheres concentration of 0.4 g/mL.
For sedimentation at higher particle concentrations, some temporal turbulence can be observed in the sediment growth and velocity curves. The curves are not as smooth as in the previous cases. This may be indicative of clustered precipitation, where a larger number of particles fall together at one time. Obviously, the final sediment heights were relatively higher due to the larger number of particles in the system. The highest value of hsk was obtained for beads with sizes of 300–400 μm.
To explain the effect of the amount of sediment on the sedimentation process in the emulsion, the scheme presented in
Figure 8 was used.
Assume that the solid particle is large enough that its weight is able to overcome the emulsion structure, that is, to force the oil droplets to move aside to create a flow channel for the particle (
Figure 5b). Considering the number of particles in the system, three cases can be distinguished. In the first, shown in
Figure 8a, there is a small number of particles in the system. In this situation, the particles affect only the neighboring droplets, moving them from their track. The remaining emulsion droplets (which are not in direct contact with the particles) remain unaffected. In this case, the flow resistance is low; therefore, sedimentation can occur intensively, and in this case, the sediment layer grows rapidly. Another case occurs when the number of beads in the system is so large that in order for a particle to get through the emulsion structure, it must affect not only the neighboring droplets but also the remaining ones (
Figure 8b). In this case, the flow resistance is higher. However, there is enough space in the system to create a track for the particles to fall, so sedimentation also occurs in this case. However, when there are very many particles in the system, the oil droplets of the emulsion are so compressed by the particles that they form a barrier for the particles through which they cannot pass. This is schematically illustrated in
Figure 8c. In this case, the flow resistance is greatest. For sedimentation to occur, the particles must deform the spherical shape of the droplets.
An important conclusion from these studies is that there is a certain optimum particle size ratio relative to the emulsion droplets at given concentrations at which the sedimentation process occurs. When the particle size is too small, its weight is too small to overcome the resistance of the oil droplets. In this situation, the particles are trapped in the emulsion structure. When the particles are too large, the structure of the system becomes so compact that the oil droplets act as a barrier to the particles. Additionally, the amount of particles in the system is an important factor. When the number (concentration) of particles in the system is low enough, there is enough space for the particles to break through the emulsion structure. After exceeding a certain quantity, the structure of the system becomes compact, and the emulsion droplets block the particles’ descent.
3.2. Effect of Emulsion Parameters on the Sedimentation Process of Solids
When analyzing the sedimentation process of microspheres in highly concentrated emulsions, one should keep in mind that they exhibit non-Newtonian behavior. This property additionally influences the speed of sedimentation process. Rheological properties of emulsions depend on their concentration. It is estimated that oil-in-water emulsions with concentrations above 50% of internal phase start to exhibit non-Newtonian shear-thinning behavior. Increasing the concentration of the oil phase enhances these properties. Such liquids exhibit high viscosity at low shear rates. This increased viscosity can affect the sedimentation process, especially in the initial and final phases. To evaluate the influence of viscosity on the sedimentation process of microspheres in emulsions, an experimental study was carried out using emulsions with three different concentrations—60, 65, and 70%. The dependence of viscosity on shear rate is shown in
Figure A1. The differences between the curves are clear, and the emulsions with a concentration of 70% at a shear rate of 50 1/s show more than three times the viscosity of the emulsions with a concentration of 60%. Sedimentation of microspheres with a concentration of 0.2 g/mL was carried out in these liquids.
Figure 9a,b shows the plots of sediment layer height versus time and velocity versus time, respectively, for the precipitation of microspheres with a size range of 300–400 μm.
Comparing the curves of sediment layer height changes in time obtained at different emulsion concentrations (
Figure 9a), one can see that for the process at 70% concentration, the curve has a smoother shape than the others. The final obtained value of sediment height h
sk is also the smallest in this case. In addition, the sediment velocity reaches the smallest value for the emulsion with the highest concentration (
Figure 9b); however, it fluctuated somewhat in the first stage of the process. The shape of the obtained curves is a result of the fact that at higher concentrations, the number of oil droplets is higher, and consequently, the density of the system is also higher. In such systems, the solid particles have less space to move freely and therefore can become trapped in the structure of the medium. At the beginning of the process there is a cluster settling, and the sedimentation velocity is the highest, but with time, it decreases. Considering non-Newtonian shear-thinning properties, at low flow velocities, the increased viscosity of the system slows down the process. Therefore, for the emulsion with the highest concentration, after about 17 min, the sedimentation process is established, and the observed increase in sediment layer changes little, while for emulsions with lower concentrations, larger changes are observed after this time. However, the overall sedimentation rate depends on the available space in the system. At low concentrations of emulsions, there is enough space for particles to find enough space to sink (
Figure 10a), while at high concentrations, there is not enough space for particles to move (
Figure 10c).
Oil droplet size is another emulsion parameter that can affect the settling rate of particles suspended in the emulsion. To test the effect of this parameter on the process, emulsions with different oil droplet sizes were produced. The average droplet diameter corresponded to d
e1 = 8.24 μm, d
e2 = 11.52 μm, and d
e3 = 15.72 μm, respectively.
Figure 11 shows plots of the change in sediment height (a) and change in sedimentation velocity (b) over time with the descent of 0.3 g/mL microspheres with a diameter range of 300–400 μm for 70% emulsions with different oil droplet sizes.
As can be observed from the graphs presented in
Figure 11, the speed of the sedimentation process in emulsions is directly related to the size of the oil droplets. For the smallest droplets d
e1 (8.27 μm), the process rate was high, and the final sediment layer height was the largest. In contrast, for the largest droplets d
e3 (15.72 μm), the process was slowest, while the h
sk layer thickness was about four times smaller. That is, nearly doubling the average diameter of the emulsion droplets results in a fourfold increase in the sediment layer. The effect of emulsion oil droplet size on the rate of solid-particle fall is shown schematically in
Figure 12.
Assuming the same oil phase concentration, for smaller droplets, their number in the system is globally larger than for large droplets. However, smaller droplets have a greater ability to move relative to each other. For even not very high stresses induced by squeezing solid particles, the small droplets will be able to move and create free space for the particle to enter (
Figure 12a). For larger droplets (
Figure 12b), the stresses induced by the particles are not sufficient to move the droplets sideways and create a track for the particle to move through. In the case of very large droplets (
Figure 12c), the system forms a compact structure, and the solid particles become trapped between the droplets; hence, their ability to fall is impeded. Therefore, the largest sediment layers were observed for small droplets, while small layers were observed for emulsions characterized by large droplets.