Nanotechnology-Based Strategies for Treatment of Obesity, Cancer and Anti-microbial Resistance: Highlights of the Department of Science and Innovation/Mintek Nanotechnology Innovation Centre Biolabels Research Node at the University of the Western Cape
Abstract
:1. Introduction
2. History of Nanotechnology in SA
3. BRN Perspective on Nanotechnology
Burden of Diseases in SA
4. Advances in Nanotechnology towards Development of Nano-Based Therapies
4.1. AuNPs in the Targeted Treatment of Obesity and Cancer
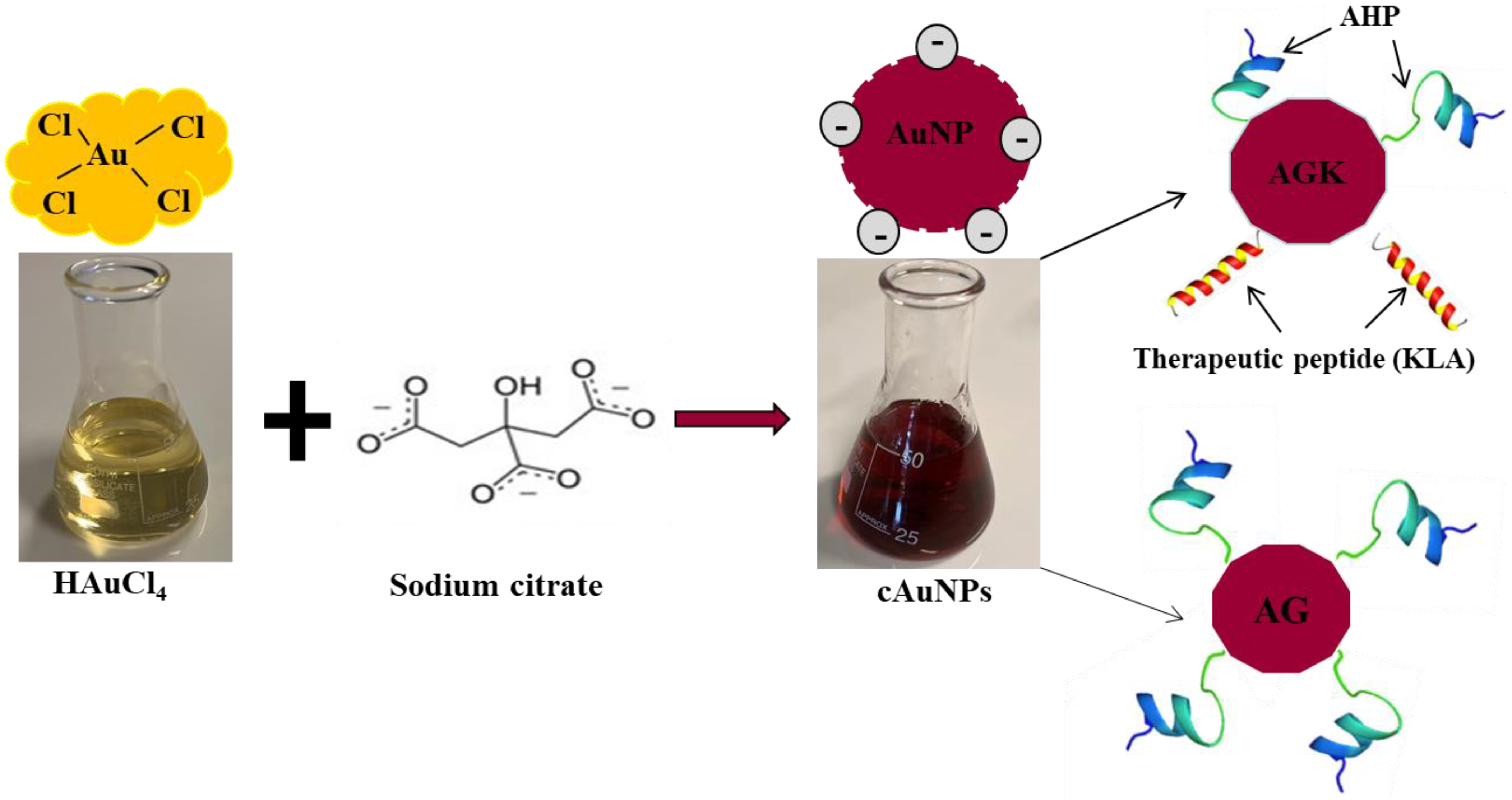
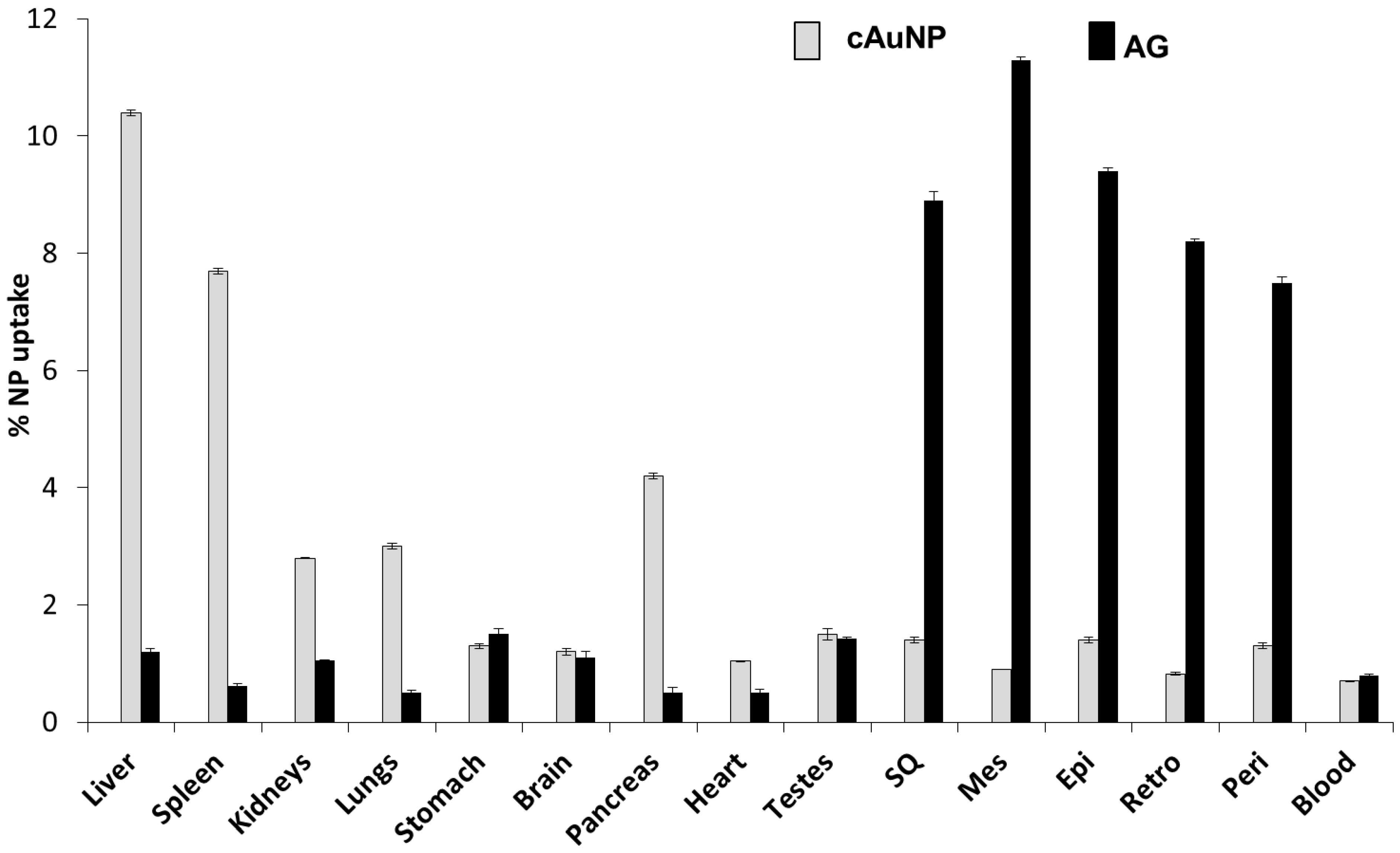
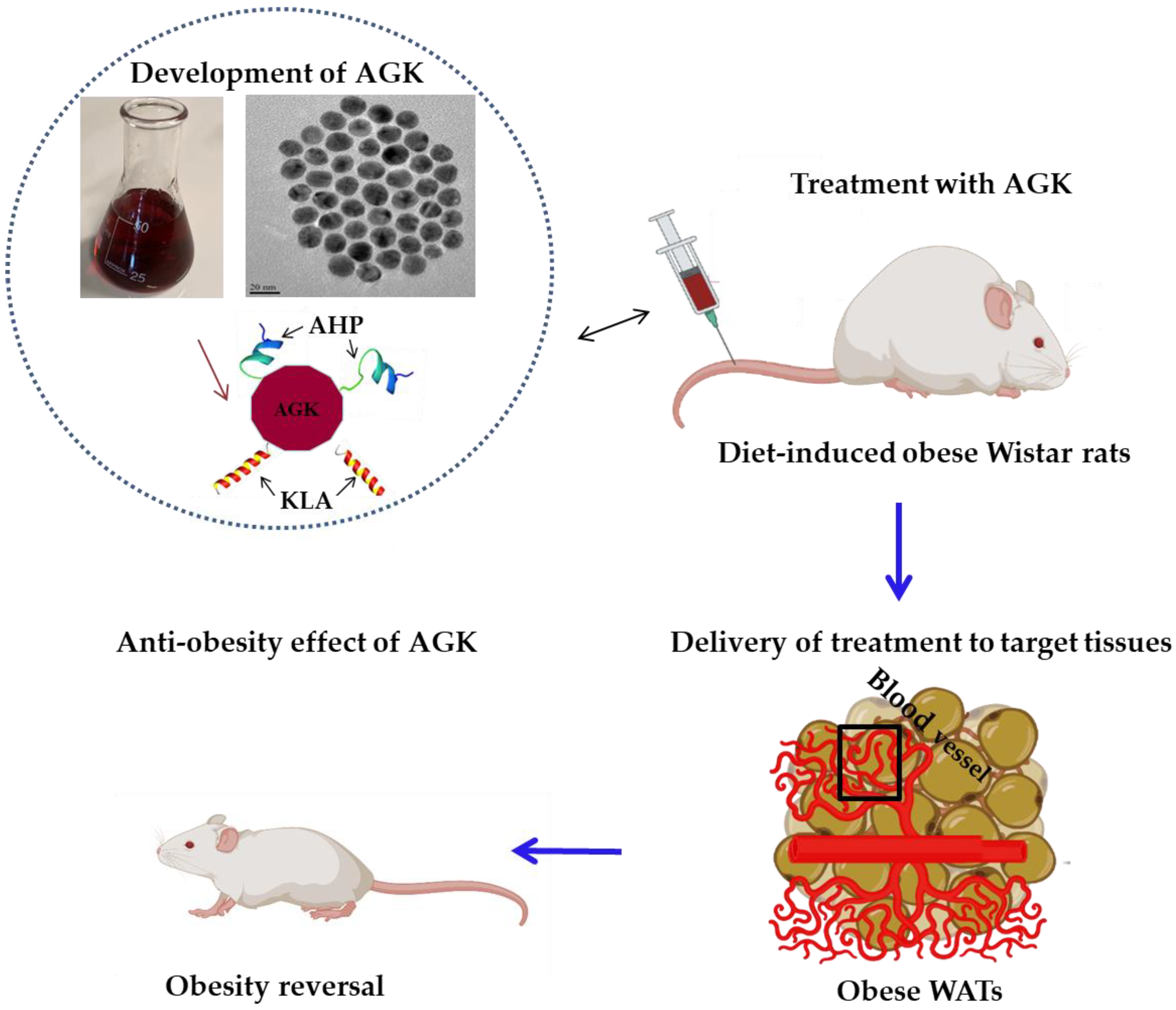
4.1.1. Targeted AuNPs as Drug-Delivery Agents for Anti-Obesity Drugs
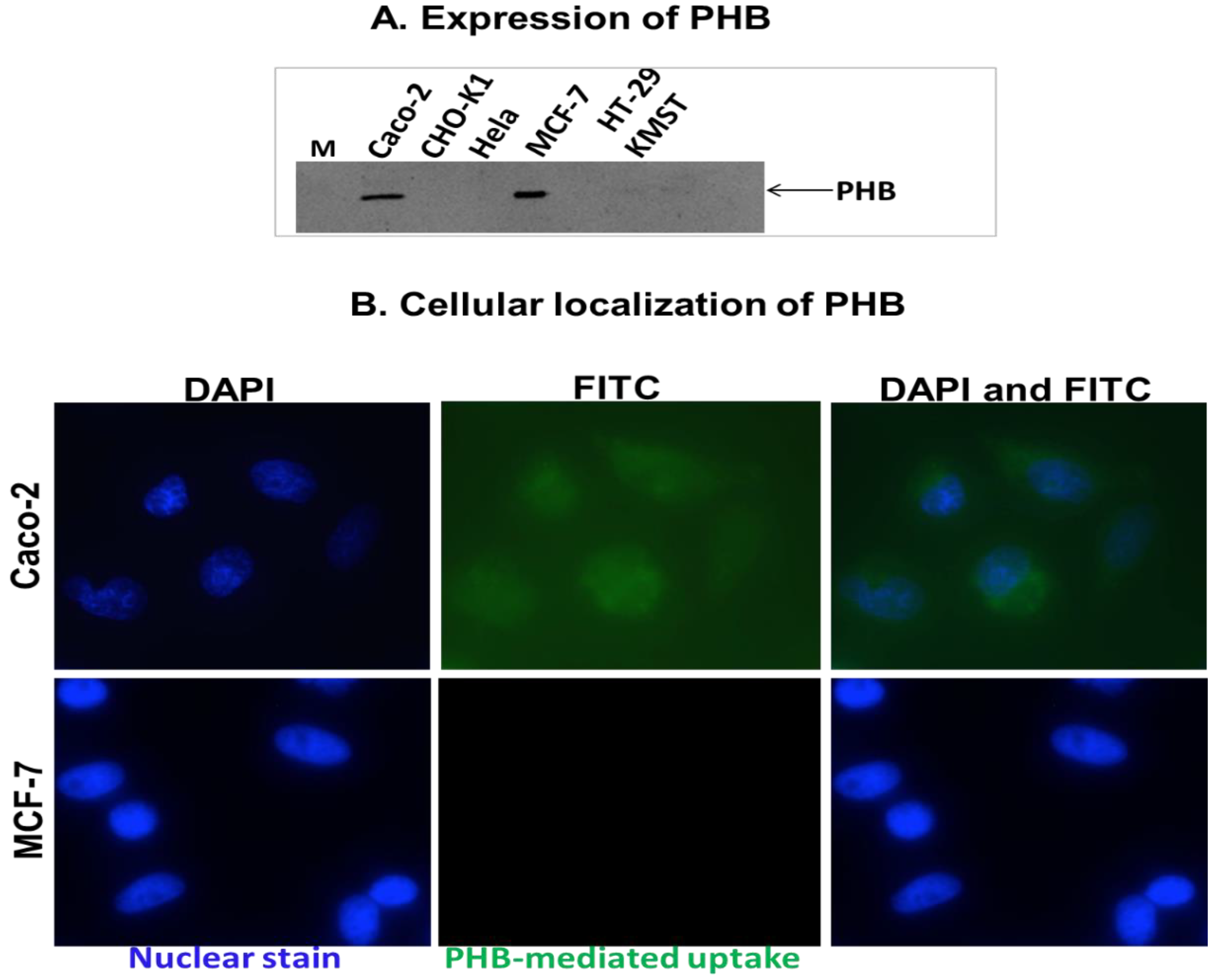
4.1.2. PHB-Targeted AuNPs for Colon Cancer Treatment
4.1.3. PHB-Targeted AuNRs as Photothermal Agents
5. Novel Treatment Strategies Using Phyto-Nanomedicines
5.1. Phyto-Nanomedicines for the Treatments of Cancer
5.2. Phyto-Nanomedicines for the Treatments of Anti-bacterial Resistance
5.3. Phyto-Nanomedicines for the Treatments of Autoimmune Disorders
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, J. Nanotechnology in Medicine and Healthcare: Possibilities, Progress and Problems. S. Afr. J. Bioeth. Law 2015, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Dube, A.; Ebrahim, N. The Nanomedicine Landscape of South Africa. Nanotechnol. Rev. 2017, 6, 339–344. [Google Scholar] [CrossRef]
- Makhoba, X.; Pouris, A. Bibliometric Analysis of the Development of Nanoscience Research in South Africa. S. Afr. J. Sci. 2017, 113, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Saidi, T.; Douglas, T.S. Nanotechnology in South Africa-Challenges in Evaluating the Impact on Development. S. Afr. J. Sci. 2017, 113, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Statistics SA. Mortality and Causes of Death in South Africa, 2015: Findings from Death Notification. Available online: http://www.statssa.gov.za/publications/P03093/P030932015.pdf (accessed on 7 January 2020).
- Sibuyi, N.R.S.; Meyer, M.; Onani, M.O.; Skepu, A.; Madiehe, A.M. Vascular Targeted Nanotherapeutic Approach for Obesity Treatment. Int. J. Nanomed. 2018, 13, 7915–7929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, M.; Onani, M.O.; Dube, A.; Madiehe, A.M. Nanotechnology Advances towards Development of Targeted-Treatment for Obesity. J. Nanobiotechnol. 2019, 17, 122. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 2 March 2022).
- Cancer Association of South Africa (CANSA) Fact Sheet on the Top Ten Cancers per Population Group. Available online: https://www.cansa.org.za/files/2017/11/Fact-Sheet-Top-Ten-Cancers-per-Population-Group-NCR-2013-web-November-2017.pdf (accessed on 7 January 2022).
- Sibuyi, N.R.S.; Thovhogi, N.; Gabuza, K.B.; Meyer, M.D.; Drah, M.; Onani, M.O.; Skepu, A.; Madiehe, A.M.; Meyer, M. Peptide-Functionalized Nanoparticles for the Selective Induction of Apoptosis in Target Cells. Nanomedicine 2017, 12, 1631–1645. [Google Scholar] [CrossRef]
- Jin, K.T.; Lu, Z.B.; Chen, J.Y.; Liu, Y.Y.; Lan, H.R.; Dong, H.Y.; Yang, F.; Zhao, Y.Y.; Chen, X.Y. Recent Trends in Nanocarrier-Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-RhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [Green Version]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Os, T.; Weber, W. Overcoming Physiological Barriers to Nanoparticle Delivery-Are We There Yet? Front. Bioeng. Biotechnol. 2019, 7, 415. [Google Scholar] [CrossRef]
- Jiang, C.; Cano-Vega, M.A.; Yue, F.; Kuang, L.; Narayanan, N.; Uzunalli, G.; Merkel, M.P.; Kuang, S.; Deng, M. Dibenzazepine-Loaded Nanoparticles Induce Local Browning of White Adipose Tissue to Counteract Obesity. Mol. Ther. 2017, 25, 1718–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Jeong, H.S.; Lee, D.H.; Beack, S.; Kim, T.; Lee, G.-H.; Park, W.C.; Kim, C.; Kim, K.S.; Hahn, S.K. Targeted Hyaluronate–Hollow Gold Nanosphere Conjugate for Anti-Obesity Photothermal Lipolysis. ACS Biomater. Sci. Eng. 2017, 3, 3646–3653. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Chen, G.; Cui, X.; Zhang, Y.; Li, M.; Liao, Y.; Zhang, X.; Qin, G.; Yan, F.; et al. Bio-Barcode Detection Technology and Its Research Applications: A Review. J. Adv. Res. 2019, 20, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mbugua, S.N.; Sibuyi, N.R.S.; Njenga, L.W.; Odhiambo, R.A.; Wandiga, S.O.; Meyer, M.; Lalancette, R.A.; Onani, M.O. New Palladium(II) and Platinum(II) Complexes Based on Pyrrole Schiff Bases: Synthesis, Characterization, X-Ray Structure, and Anticancer Activity. ACS Omega 2020, 5, 14942–14954. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Jeong, S.; Choi, H.J.; Eun, H.; Jo, M.G.; Woo, Y.; Seokjoon, K.; Yonghwan, K.; Miran, L.; Park, K.K.S. Target-Induced Aggregation of Gold Nanoparticles for Colorimetric Detection of Bisphenol A. J. Nanomater. 2019, 2019, 3676384. [Google Scholar] [CrossRef] [Green Version]
- Vechia, I.C.D.; Steiner, B.T.; Freitas, M.L.; dos Santos Pedroso Fidelis, G.; Galvani, N.C.; Ronchi, J.M.; Possato, J.C.; Fagundes, M.Í.; Rigo, F.K.; Feuser, P.E.; et al. Comparative Cytotoxic Effect of Citrate-Capped Gold Nanoparticles with Different Sizes on Noncancerous and Cancerous Cell Lines. J. Nanopart. Res. 2020, 22, 133. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors Controlling the Pharmacokinetics, Biodistribution and Intratumoral Penetration of Nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Thovhogi, N.; Sibuyi, N.; Meyer, M.; Onani, M.; Madiehe, A. Targeted Delivery Using Peptide-Functionalised Gold Nanoparticles to White Adipose Tissues of Obese Rats. J. Nanopart. Res. 2015, 17, 112. [Google Scholar] [CrossRef]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of Obesity by Targeted Ablation of Adipose Tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, K.F.; Christianson, D.R.; Hanley, P.W.; Driessen, W.H.P.; Bernacky, B.J.; Baze, W.B.; Wen, S.; Tian, M.; Ma, J.; Kolonin, M.G.; et al. A Peptidomimetic Targeting White Fat Causes Weight Loss and Improved Insulin Resistance in Obese Monkeys. Sci. Transl. Med. 2011, 3, 108ra112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, M.N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Harashima, H. Vascular-Targeted Nanotherapy for Obesity: Unexpected Passive Targeting Mechanism to Obese Fat for the Enhancement of Active Drug Delivery. J. Control. Release 2012, 163, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosibo, N.M.; Keter, F.K.; Skepu, A.; Tshikhudo, R.T.; Revaprasadu, N. Facile Attachment of TAT Peptide on Gold Monolayer Protected Clusters: Synthesis and Characterization. Nanomaterials 2015, 5, 1211–1222. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Chen, Y.; Zhang, L.; Li, Y.; Li, H.; Zhang, H.; Tian, Y. SERS Assay for Pyrophosphate Based on Its Competitive Binding to Cu(II) Ion on Silver Nanoparticles Modified with Cysteine and Rhodamine 6G. Microchim. Acta 2017, 184, 595–601. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, Long-Term Toxicity, and Mechanistic Studies of Gold Nanorods Photothermal Therapy of Cancer in Xenograft Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; Day, E.S. Gold Nanoparticle-Mediated Photothermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Zhao, R.; Zheng, G.; Fan, L.; Shen, Z.; Jiang, K.; Guo, Y.; Shao, J.-W. Carrier-Free Nanodrug by Co-Assembly of Chemotherapeutic Agent and Photosensitizer for Cancer Imaging and Chemo-Photo Combination Therapy. Acta Biomater. 2018, 70, 197–210. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Hong, X.; Cheng, Y.; Tian, Y.; Zhao, S.; Liu, W.; Tang, Y.; Zhao, R.; Song, L.; et al. Adjuvant Photothermal Therapy Inhibits Local Recurrences after Breast-Conserving Surgery with Little Skin Damage. ACS Nano 2018, 12, 662–670. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.D.; Meyer, M. Peptide Functionalised Gold Nanorods for the Selective Eradication of Target Cells Using Photothermal Therapy. Available online: http://etd.uwc.ac.za/xmlui/handle/11394/6771?show=full (accessed on 7 January 2020).
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Gold Nanoparticles Synthesized Using Extracts of Cyclopia Intermedia, Commonly Known as Honeybush, Amplify the Cytotoxic Effects of Doxorubicin. Nanomaterials 2021, 11, 132. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Mohandoss, D.K.D.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New Approaches in Breast Cancer Therapy through Green Nanotechnology and Nano-Ayurvedic Medicine–Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [Green Version]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their in Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial Activity of Biogenic Silver and Gold Nanoparticles Synthesized from Salvia africana-lutea and Sutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef]
- Pantidos, N.; Horsfall, L.E. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 5, 5. [Google Scholar] [CrossRef]
- Elbagory, A.; Meyer, M.; Cupido, C.; Hussein, A.A. Inhibition of Bacteria Associated with Wound Infection by Biocompatible Green Synthesized Gold Nanoparticles from South African Plant Extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [Green Version]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green Synthesis of Gold Nanoparticles Using Acai Berry and Elderberry Extracts and Investigation of Their Effect on Prostate and Pancreatic Cancer Cells. Nanobiomedicine 2021, 8, 184954352199531. [Google Scholar] [CrossRef] [PubMed]
- Madiehe, A.M.; Moabelo, K.L.; Modise, K.; Sibuyi, N.R.S.; Meyer, S.; Dube, A.; Onani, M.O.; Meyer, M. Catalytic Reduction of 4-Nitrophenol and Methylene Blue by Biogenic Gold Nanoparticles Synthesized Using Carpobrotus edulis Fruit (Sour Fig) Extract. Nanomater. Nanotechnol. 2022, 12. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects Of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Madiehe, A.M.; du Preez, M.G. The Antimicrobial Activity of Biogenic Silver Nanoparticles Synthesized from Extracts of Red and Green European Pear Cultivars. Artif. Cells Nanomed. Biotechnol. 2021, 49, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Mmola, M.; Le Roes-Hill, M.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.E.; Beukes, D.R.; Antunes, E. Enhanced Antimicrobial and Anticancer Activity of Silver and Gold Nanoparticles Synthesised Using Sargassum incisifolium Aqueous Extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef] [Green Version]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced Anti-Bacterial Activity of Biogenic Silver Nanoparticles Synthesized from Terminalia mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef] [Green Version]
- Anadozie, S.O.; Adewale, O.B.; Meyer, M. In Vitro Anti-Oxidant and Cytotoxic Activities of Gold Nanoparticles In Vitro Anti-Oxidant and Cytotoxic Activities of Gold Nanoparticles Synthesized from an Aqueous Extract of the Xylopia aethiopica Fruit. Nanotechnology 2021, 32, 315101. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-Based Delivery Systems for Antimicrobial Peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.O.; Meyer, S.; Ahmed, O.; Geerts, G.; Madiehe, M.A.; Meyer, M.; Sibuyi, N.R.S. Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 1799. [Google Scholar] [CrossRef] [PubMed]
- Thovhogi, N.; Sibuyi, N.R.S.; Onani, M.O.; Meyer, M.; Madiehe, A.M. Peptide-Functionalized Quantum Dots for Potential Applications in the Imaging and Treatment of Obesity. Int. J. Nanomed. 2018, 13, 2551–2559. [Google Scholar] [CrossRef] [Green Version]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Martin, D.R.; Sibuyi, N.R.; Dube, P.; Fadaka, A.O.; Cloete, R.; Onani, M.; Madiehe, A.M.; Meyer, M. Aptamer-Based Diagnostic Systems for the Rapid Screening of TB at the Point-of-Care. Diagnostics 2021, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
| Plant Name | Traditional or Medicinal Use | MNPs Type | Properties of the NPs | NP Activity | Ref |
|---|---|---|---|---|---|
| Acai berry (Euterpe oleraceae) | Boost digestion and cardiovascular health | AuNPs | Size: 168 ± 3.0 to 197 ± 21.5 nm Zeta: −26 ± 0.4 to −36 ± 0.8 mV Shape: Various | Anti-cancer activity | [46] |
| Elderberry (Sambucus nigra) | Fever, rheumatism, laxative, diuretic | AuNPs | Size: 94 ± 1.4 to 195 ± 3.1 nm Zeta: −28 ± 0.5 to −31 ± 0.4 mV Shape: Various | Anti-cancer activity | [46] |
| Carpobrotus edulis | Treatment of tuberculosis, other respiratory infections, toothache, earache, facial eczema, wounds, burns, hypertension, and diabetes mellitus | AuNPs | Size: 108.0 ± 0.2 nm Shape: Spherical | Catalytic activity | [47] |
| Cotyledon orbiculata | Treatment of skin rashes, abscesses, inflammation, boils, and acne | AgNPs | Size: 106 ± 2 to 137 ± 2 nm PDI: 0.07 ± 0.02 to 0.15 ± 0.33 Zeta: −18 ± 1.0 to −20 ± 1.0 mV Shape: Spherical | Anti-bacterial and Anti-inflammatory activity | [48] |
| Cyclopia intermedia (Honeybush, HB) | Treatment of infections, coughs, sore throat, colds, osteoporosis, prevention of cancer and asthma | AuNPs | Size: 66.74 ± 9.7 nm PDI: 0.57 ± 0.01 Zeta: −23.45 ± 1.4 mV Shape: Spherical and triangular | Anti-cancer activity | [36] |
| Galenia Africana | Treatment of venereal sores, asthma, coughs, eye infections, skin diseases | AuNPs | Size: 44 ± 29 Zeta: 11 ± 1 mV Shape: Spherical | Anti-microbial activity | [43] |
| H. hemerocallidea | Treatment of diabetes, urinary infections, cancer, skin wounds, rashes and management of HIV/AIDS | AuNPs | Size: 51 ± 34 nm Zeta: 26 ± 6 mV Shape: Spherical | Anti-microbial and immunomodulation activity | [43,49] |
| Pyrus communis L. (European pear) | Treat fever, suppress cough, detoxify abscesses, and alcohol poisoning | AgNPs | Size: 117.2 to 190 nm PDI: 0.244 to 0.545 Zeta: −1.1 to −9.5 mV Shape: Spherical | Anti-bacterial activity | [50] |
| Salvia Africana L. | Treatment of skin and gastric disorders | AgNPs | Size: 34.63 ± 4.53 nm PDI: 0.63 ± 0.03 Zeta: −41.1 ± 2.00 mV Shape: Mostly spherical and some polygonal | Anti-microbial activity | [41] |
| Sargassum incisifolium | Unknown | AgNPs | Size: 76.29 to 316.3 nm PDI: 0.264 to 0.568 Zeta: −26.4 ± 17.8 to –44.1 ± 11.0 mV Shape: Spherical | Anti-microbial and Anti-cancer activities | [51] |
| AuNPs | Size: 37.46 to 92.85 nm PDI: 0.158 to 0.551 Zeta: −39.3 ±13.5 to –56.3 ± 13.9 mV Shape: Spherical | ||||
| Sutherlandia frutescence | Treatment of cancer | AuNPs | Size: 261.2 ± 10.40 PDI: 0.612 ± 0.02 Zeta: −35.7 ± 1.53 Shape: Spherical | Anti-microbial activity | [41] |
| Terminalia mantaly | Treatment of cancer, dysentery, diabetes, mycosis, and bacterial infections | AgNPs | Size: 11 to 83 nm PDI: 0.40 to 0.88 Zeta: −12 to −37 mV Shape: Various | Anti-microbial activity | [52] |
| AuNPs | Size: 39 to 79 nm PDI: 0.30 to 0.80 Zeta: −10 to −37 mV Shape: Various | Anti-cancer activity | [40] | ||
| Xylopia aethiopica | Treatment of uterine fibroids, bronchitis, and kidney disease | AuNPs | Size: 17.77 ± 0.56 nm PDI: 0.21 Zeta: −29.9 mV Shape: Various | Anti-cancer activity | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, S.; Skepu, A.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Nanotechnology-Based Strategies for Treatment of Obesity, Cancer and Anti-microbial Resistance: Highlights of the Department of Science and Innovation/Mintek Nanotechnology Innovation Centre Biolabels Research Node at the University of the Western Cape. Appl. Sci. 2022, 12, 10512. https://doi.org/10.3390/app122010512
Sibuyi NRS, Moabelo KL, Meyer S, Skepu A, Onani MO, Madiehe AM, Meyer M. Nanotechnology-Based Strategies for Treatment of Obesity, Cancer and Anti-microbial Resistance: Highlights of the Department of Science and Innovation/Mintek Nanotechnology Innovation Centre Biolabels Research Node at the University of the Western Cape. Applied Sciences. 2022; 12(20):10512. https://doi.org/10.3390/app122010512
Chicago/Turabian StyleSibuyi, Nicole Remaliah Samantha, Koena Leah Moabelo, Samantha Meyer, Amanda Skepu, Martin Opiyo Onani, Abram Madimabe Madiehe, and Mervin Meyer. 2022. "Nanotechnology-Based Strategies for Treatment of Obesity, Cancer and Anti-microbial Resistance: Highlights of the Department of Science and Innovation/Mintek Nanotechnology Innovation Centre Biolabels Research Node at the University of the Western Cape" Applied Sciences 12, no. 20: 10512. https://doi.org/10.3390/app122010512





