The Influence of Raw Materials on the Stability of Grisaille Paint Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Analytical Techniques
3. Results and Discussion
3.1. Raw Materials Characterization
3.2. Grisaille Properties
3.2.1. Crystallographic Characterization
3.2.2. Colorimetry
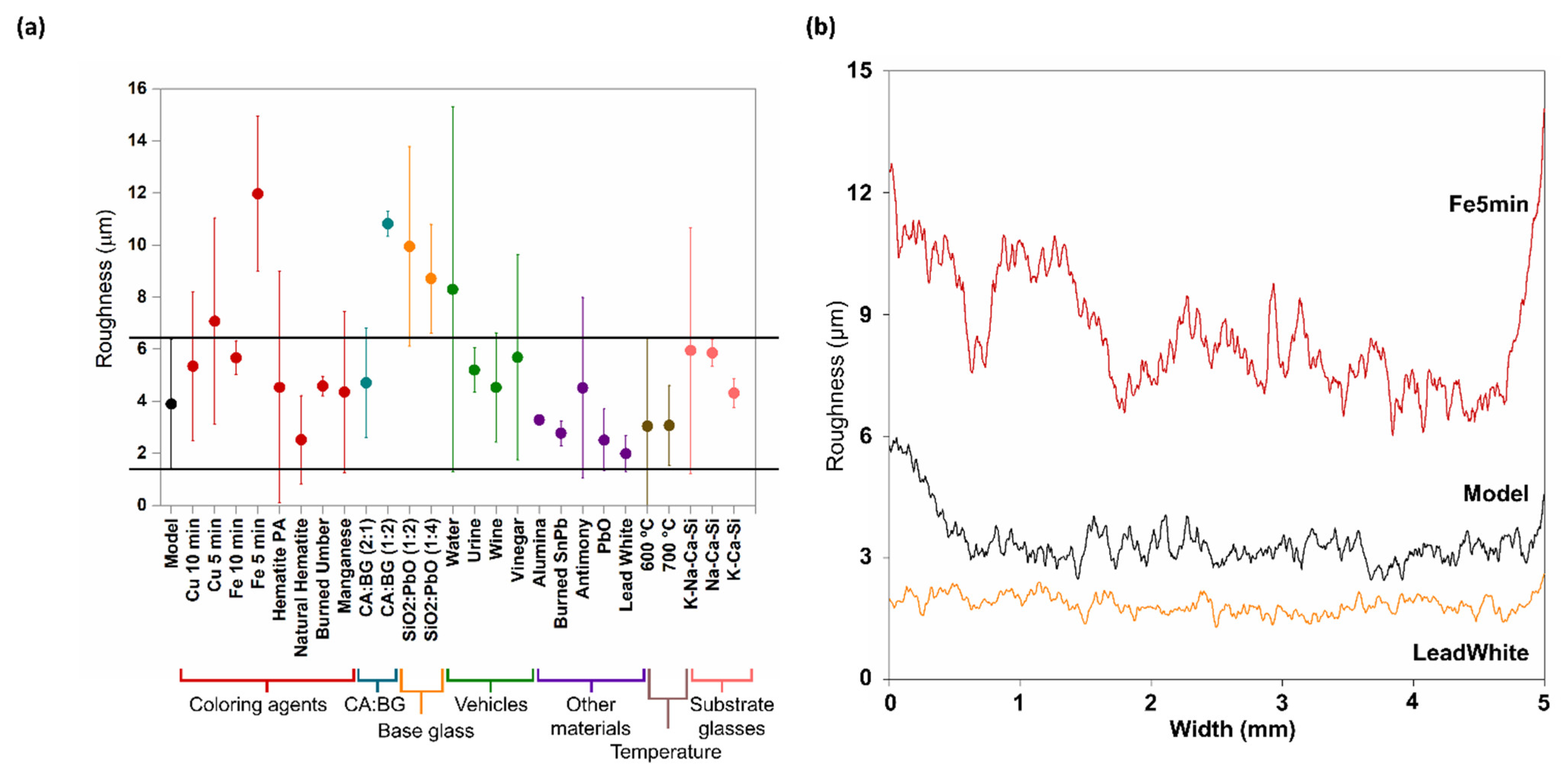
3.2.3. Roughness
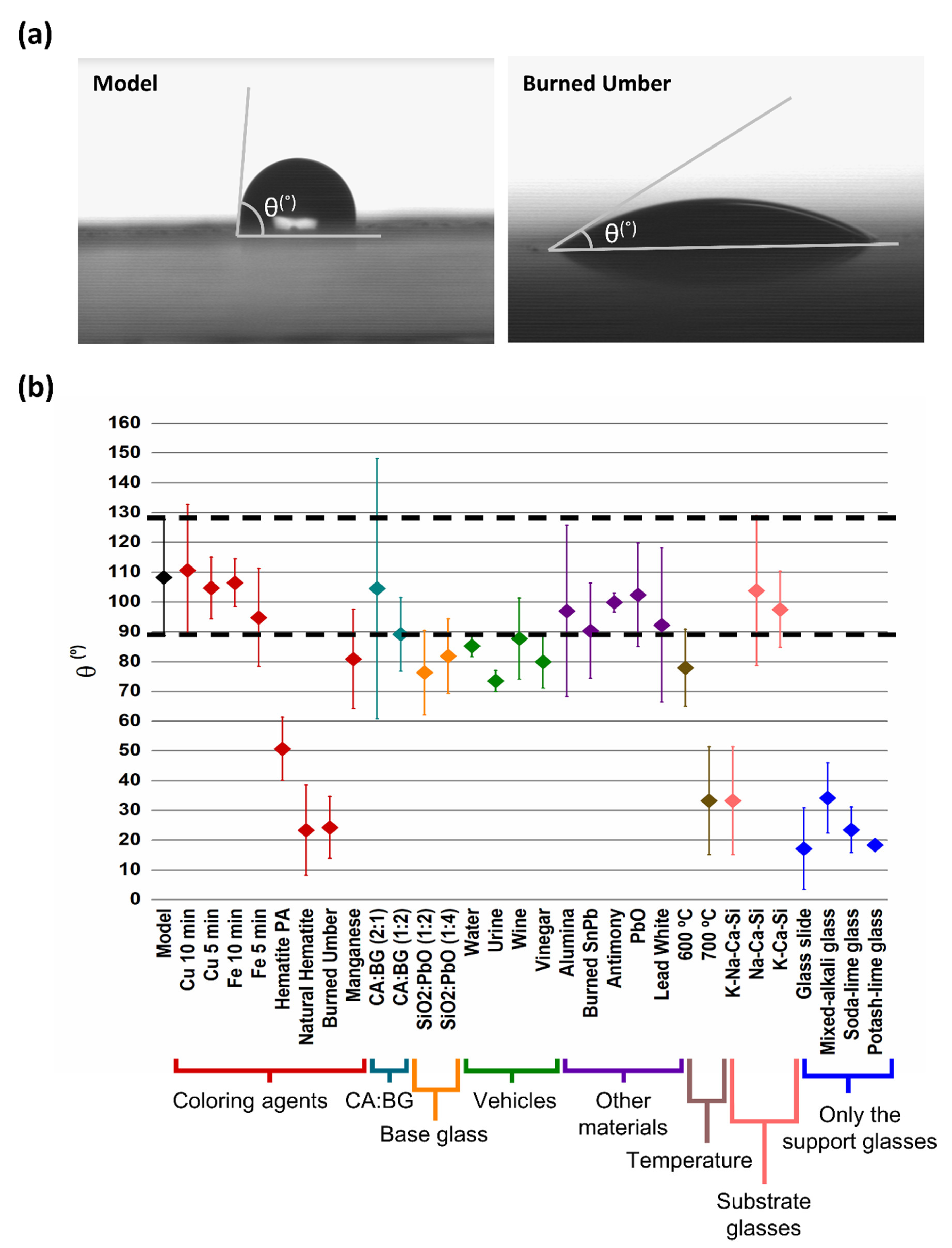
3.2.4. Contact Angle
3.3. Adherence Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Brand/Manufacturer |
|---|---|
| SiO2 | Sigma-Aldrich Chemistry |
| PbO | Sigma-Aldrich Chemistry0 |
| SnO2 | Alfa Aesar Chemicals |
| Fe2O3 | Riedel-de Haën–Honeywell Research Chemicals |
| MnO2 | Panreac Química SLU–ITW Reagents |
| Al2O3 | Fluka–Honeywell Research Chemicals |
| Sb2O3 | Alfa Aesar Chemicals |
| Burned Umber | Winsor and Newton |
| Gum Arabic | Debitus–Peintures pour verre |
Appendix B

















References
- Schalm, O. Characterization of Paint Layers in Stained-Glass Windows: Main Causes of the Degradation of Nineteenth Century Grisaille Paint Layers; Universiteit Antwerpen: Antwerpen, Belgium, 2000. [Google Scholar]
- Machado, C.; Machado, A.; Palomar, T.; Vilarigues, M. Grisaille in Historical Written Sources. J. Glass Stud. 2019, 61, 71–86. [Google Scholar]
- Vilarigues, M.; Machado, C.; Machado, A.; Costa, M.; Alves, L.C.; Cardoso, I.P.; Ruivo, A. Grisailles: Reconstruction and Characterization of Historical Recipes. Int. J. Appl. Glas. Sci. 2020, 11, 756–773. [Google Scholar] [CrossRef]
- Machado, C.; Vilarigues, M.; Palomar, T. Historical Grisailles Characterisation: A Literature Review. J. Cult. Herit. 2021, 49, 239–249. [Google Scholar] [CrossRef]
- Merrifield, M. Original Treatises on the Arts of Paintings; Dover Publications: Mineola, NY, USA, 1849; Volume 1. [Google Scholar]
- Bontemps, G. Guide du Verrier: Traité Historique et Pratique de la Fabrication des Verres, Cristaux, Vitraux; Librairie du Dictionnaire des Arts et Manufactures: Paris, France, 1868. [Google Scholar]
- Volf, M.B. Chemical Approach to Glass, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Foster, J. Lives of the Most Eminent Painters, Sculptors, and Architects: Translated from the Italian of Giorgio Vasari; Bohn, H.G., Ed.; Modern Library: New York, NY, USA, 1855. [Google Scholar]
- Verità, M. Paintwork in Medieval Stained-Glass Windows: Composition, Weathering, and Conservation. In Proceedings of the Forum for the Conservation and Restoration of Stained Glass, the Art of Collaboration: Stained-Glass Conservation in the Twenty-First Century, New York City, NY, USA, 1–3 June 2009; Shepard, M.B., Pilosi, L., Stroble, S., Eds.; Harvey Miller: London, UK, 2010; pp. 210–216. [Google Scholar]
- Rodrigues, A.; Coutinho, M.L.; Machado, C.; Alves, L.C.; Machado, A.; Vilarigues, M. An Overview of Germanic Grisailles through the Stained-Glass Collection at Pena Palace. Heritage 2022, 5, 1003–1023. [Google Scholar] [CrossRef]
- Pradell, T.; Molina, G.; Murcia, S.; Ibáñez, R.; Liu, C.; Molera, J.; Shortland, A. Materials, Techniques and Conservation of Historic Stained Glass “Grisailles”. Int. J. Appl. Glass Sci. 2016, 7, 41–58. [Google Scholar] [CrossRef]
- Verità, M.; Nicola, C.; Sommariva, G. The Stained Glass Windows of the Sainte Chapelle in Paris: Investigations on the Origin of the Loss of the Painted Work. AIHV Ann. 16 Congrès 2003, 347–351. [Google Scholar]
- Vilarigues, M.; Da Silva, R.C. Ion Beam and Infrared Analysis of Medieval Stained Glass. Appl. Phys. A Mater. Sci. Process. 2004, 79, 373–378. [Google Scholar] [CrossRef]
- Schalm, O.; Janssens, K.; Caen, J. Characterization of the Main Causes of Deterioration of Grisaille Paint Layers in 19th Century Stained-Glass Windows by J.-B. Capronnier. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 589–607. [Google Scholar] [CrossRef]
- Bettembourg, J.-M. Altération et Problèmes de Conservation Des Grisailles. Corpus Vitr. News Lett. 1984, 37/38, 5–7. [Google Scholar]
- Verità, M. Composition, Structure et Mécanisme de Détérioration Des Grisailles. Doss. Comm. R. Monum. Sites Fouill. 1996, 3, 61–68. [Google Scholar]
- Palomar, T.; Redol, P.; Almeida, I.C.; Pereira, E.; Vilarigues, M. The Influence of Environment in the Alteration of the Stained-Glass Windows in Portuguese Monuments. Heritage 2018, 1, 365–376. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary of Historical Pigments; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- DIN EN ISO 4287; Surface Roughness—Terminology: Part 1, Surface and Its Parameters. ISO: Geneva, Switzerland, 1998.
- Ariyathilaka, P.; Haddock, D.; Spindloe, C.; Tolley, M.K. Surface Roughness of NaCl Coating Used as Release Layers in Thin Film Production. J. Phys. Conf. Ser. 2018, 1079, 012017. [Google Scholar] [CrossRef]
- ISO 2409; European ISO Standard, Paints, and Varnishes—Cross Cut Test. ISO: Geneva, Switzerland, 1992.
- Wolbers, R. Cleaning Painted Surfaces; Archetype Publications Ltd.: London, UK, 2000. [Google Scholar]
- Redol, P. O Mosteiro Da Batalha e o Vitral Em Portugal Nos Séculos XV e XVI; da Batalha, C.M., Ed.; Câmara Municipal da Batalha: Batalha, Portugal, 2003. [Google Scholar]
- Eberhard, Z. Chemische Technologie des Glases; Cable, M., Translator; Society of Glass Technology: Sheffield, UK, 2013. [Google Scholar]
- Schmidt-Whitley, R.D.; Martinez-Clemente, M.; Revcolevschi, A. Growth and Microstructural Control of Single Crystal Cuprous Oxide Cu2O. J. Cryst. Growth 1974, 23, 113–120. [Google Scholar] [CrossRef]
- García-Heras, M.; Carmona, N.; Gil, C.; Villegas, M.A. Neorenaissance/Neobaroque Stained Glass Windows from Madrid: A Characterisation Study on Some Panels Signed by the Maumejean Fréres Company. J. Cult. Herit. 2005, 6, 91–98. [Google Scholar] [CrossRef]
- Beltrán, M.; Schibille, N.; Brock, F.; Gratuze, B.; Vallcorba, O.; Pradell, T. Modernist Enamels: Composition, Microstructure and Stability. J. Eur. Ceram. Soc. 2020, 40, 1753–1766. [Google Scholar] [CrossRef]
- Beltran, M.; Schibille, N.; Gratuze, B.; Vallcorba, O.; Bonet, J.; Pradell, T. Composition, Microstructure and Corrosion Mechanisms of Catalan Modernist Enamelled Glass. J. Eur. Ceram. Soc. 2021, 41, 1707–1719. [Google Scholar] [CrossRef]
- Weber, F.W. Artists’ Pigments: Their Chemical and Physical Properties; D. Van Nostrand Company: New York, NY, USA, 1923. [Google Scholar]
- Hudson Institute of Mineralogy. Melanotekite (mindat.org). Available online: https://www.mindat.org/min-2632.html (accessed on 30 June 2022).
- Ito, J.; Frondel, C. Syntheses of Lead Silicates: Larsenite, Barysilite and Related Phases. Am. Mineral. 1967, 52, 1077–1084. [Google Scholar]
- Bettembourg, J.-M. Composition Er Durabilité Des Grisailles. Sci. Technol. Conserv. Restaur. Oeuvres d’Art Patrim. 1991, 2, 47–55. [Google Scholar]
- Silvestri, A.; Molin, G.; Pomero, V. The Stained Glass Window of the Southern Transept of St. Anthony’s Basilica (Padova, Italy): Study of Glasses and Grisaille Paint Layers. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 81–87. [Google Scholar]
- Machado, C.; Vilarigues, M.; Palomar, T. Characterization of the Alteration of Debitus Grisailles. Stud. Conserv. 2021, 67, 413–422. [Google Scholar] [CrossRef]
- Cílová, Z.Z.; Kučerová, I.; Knížová, M.; Trojek, T. Corrosion Damage and Chemical Composition o Czech Stained Glass from 13th to 15th Century. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2015, 56, 153–162. [Google Scholar]
- Palomar, T. Chemical Composition and Alteration Processes of Glasses from the Cathedral of León (Spain). Bol. la Soc. Esp. Ceram. Vidr. 2018, 57, 101–111. [Google Scholar] [CrossRef]
- Shrimali, K.; Jin, J.; Hassas, B.V.; Wang, X.; Miller, J.D. The Surface State of Hematite and Its Wetting Characteristics. J. Colloid Interface Sci. 2016, 477, 16–24. [Google Scholar] [CrossRef]
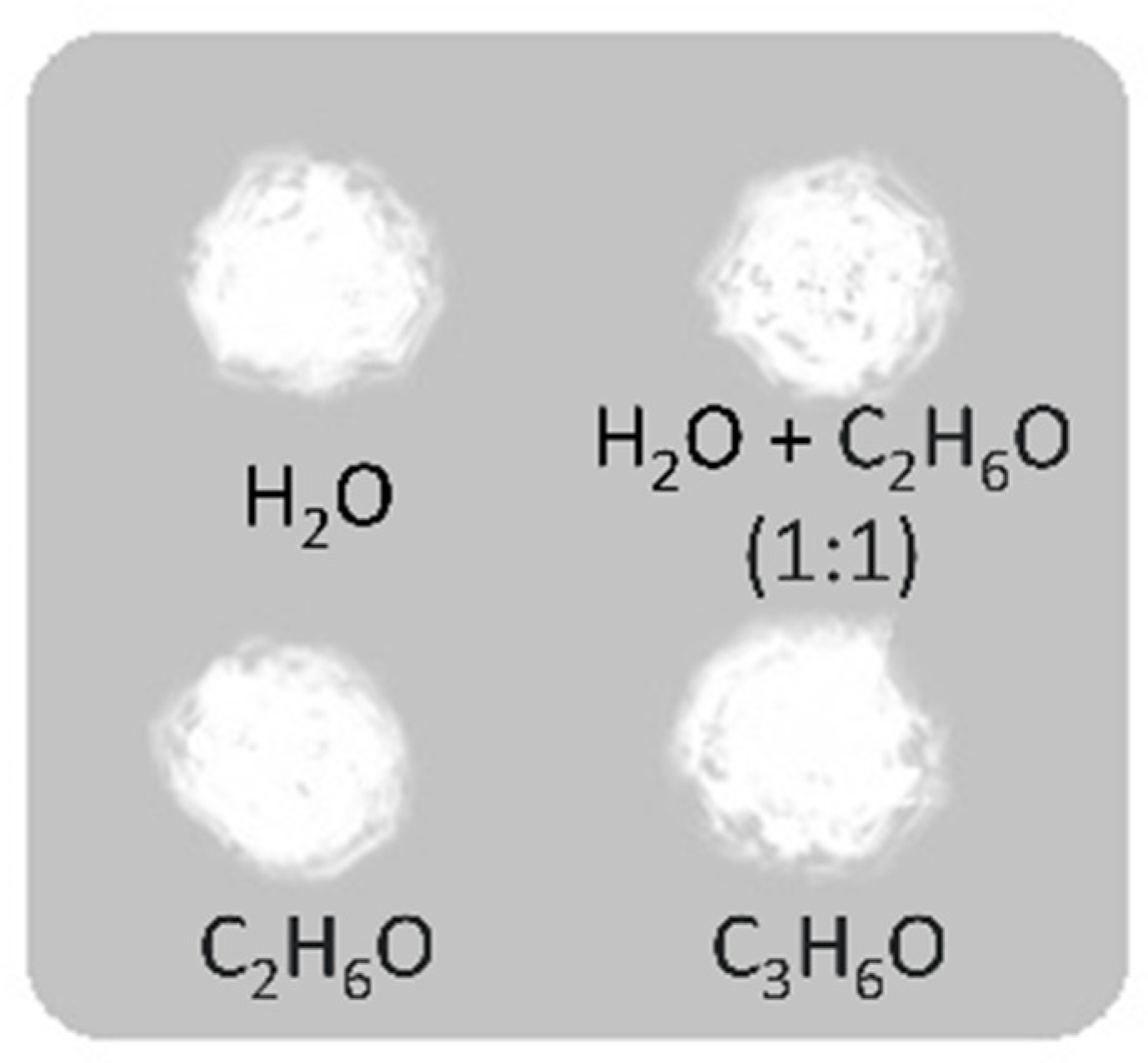

| Variables | Coloring Agents (CA) | Base Glass (BG) | (CA:BG) wt% | Vehicle | Other Materials | Substrate Glass | Temp. | Image of Final Grisaille |
|---|---|---|---|---|---|---|---|---|
| Model grisaille | Iron and copper (1:1 wt%) | Rocaille (SiO2 + PbO (1:3 wt%)) | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  |
| Coloring agents | Burned iron (10 min *) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  |
| Burned iron (5 min *) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Burned copper (10 min *) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Burned copper (5 min *) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Manganese (PA MnO2) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Hematite (PA Fe2O3) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Hematite (mineral) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Burned umber (pigment) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Base glass | Iron and copper (1:1 wt%) | SiO2 + PbO (1:2 wt%) | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  |
| Iron and copper (1:1 wt%) | SiO2 + PbO (1:4 wt%) | (1:1) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Vehicles | Iron and copper (1:1 wt%) | Rocaille | (1:1) | Water | - | Glass slide | 650 °C |  |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Urine | - | Glass slide | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Wine | - | Glass slide | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Vinegar | - | Glass slide | 650 °C |  | |
| Other materials | Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | Alumina (PA Al2O3) | Glass slide | 650 °C |  |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | Antimony (PA Sb2O3) | Glass slide | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | Burned lead and tin (Pb2SnO4) | Glass slide | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | Burned lead (PA PbO) | Glass slide | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | Lead white (2PbCO3·Pb(OH)2) | Glass slide | 650 °C |  | |
| Temperature | Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 600 °C |  |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | - | Glass slide | 700 °C |  | |
| CA:BG | Iron and copper (1:1 wt %) | Rocaille | (2:1) | Gum arabic + water | - | Glass slide | 650 °C |  |
| Iron and copper (1:1 wt%) | Rocaille | (1:2) | Gum arabic + water | - | Glass slide | 650 °C |  | |
| Substrate glasses | Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | - | Mixed-alkali glass (K-Na-Ca-Si) | 650 °C |  |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | - | Soda-lime glass (Na-Ca-Si) | 650 °C |  | |
| Iron and copper (1:1 wt%) | Rocaille | (1:1) | Gum arabic + water | - | Potash-lime glass (K-Ca-Si) | 650 °C |  |
| Na2O | Al2O3 | SiO2 | K2O | Fe2O3 | PbO | |
|---|---|---|---|---|---|---|
| SiO2:PbO (1:2) | - | 0.57 | 33.9 | - | - | 65.5 |
| SiO2:PbO (1:3) | - | 1.97 | 26.7 | - | - | 71.3 |
| SiO2:PbO (1:4) | 0.56 | 2.26 | 28.6 | 0.15 | 0.22 | 68.0 |
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | MnO | Fe2O3 | |
|---|---|---|---|---|---|---|---|---|---|
| Glass slide | 11.8 | 4.43 | 1.58 | 72.9 | ˂0.05 | 0.72 | 8.12 | ˂0.05 | ˂0.10 |
| Soda-lime glass (Na-Ca-Si) | 12.3 | 1.78 | 3.78 | 67.8 | ˂0.05 | 3.80 | 9.20 | ˂0.05 | ˂0.10 |
| Potash-lime glass (K-Ca-Si) | ˂0.10 | 1.79 | 2.97 | 52.5 | 1.75 | 20.7 | 19.9 | ˂0.05 | ˂0.10 |
| Mixed-alkali glass (K-Na-Ca-Si) | 9.49 | 3.89 | 3.29 | 62.1 | 0.14 | 6.88 | 13.4 | 0.37 | 0.40 |
| Crystallographic Phases | |
|---|---|
| Burned iron | Hematite (Fe2O3) |
| Burned copper | Cuprite (Cu2O), Tenorite (CuO) |
| Manganese PA | Pyrolusite (MnO2) |
| Hematite PA | Hematite (Fe2O3) |
| Natural hematite | Hematite (Fe2O3), Quartz (SiO2) |
| Burned umber | Hematite (Fe2O3) |
| Alumina PA | Corundum (Al2O3) |
| Antimony PA | Senarmontite (Sb2O3) |
| Burned SnPb | Cassiterite (SnO2), Massicot (PbO), Minium (Pb3O4) |
| Burned lead (PbO PA) | Massicot (PbO) |
| Lead white | Hydrocerussite (Pb3(CO3)2(OH)2) |
| Grisailles | Crystallographic Phases |
|---|---|
| Model | Hematite (Fe2O3); Cuprite (Cu2O); Tenorite (CuO) |
| Hematite PA | Hematite (Fe2O3) |
| Natural hematite | Hematite (Fe2O3); Quartz (SiO2) |
| Burned umber | Hematite (Fe2O3) |
| Burned lead | Hematite (Fe2O3); Cuprite (Cu2O); Tenorite (CuO); Iron barysilite (Pb8Fe(Si2O7)3) |
| Lead white | Hematite (Fe2O3); Cuprite (Cu2O); Tenorite (CuO); Iron barysilite (Pb8Fe(Si2O7)3) |
| Variable | Painted Samples | Adhesion Test | ||
|---|---|---|---|---|
| (Before Adhesion Tests) | (Reflected Light) | Adhesive Tape | Classification | |
| Model grisaille |  |  |  | 1 |
| Fe 5 min |  |  |  | 1 |
| Cu 5 min |  |  |  | 1 |
| Manganese |  |  |  | 3 |
| Hematite PA |  |  |  | 4 |
| Natural hematite |  |  |  | 3 |
| Burned umber |  |  |  | 4 |
| Burned SnPb |  |  |  | 1 |
| PbO |  |  |  | 2 |
| Lead white |  |  |  | 2 |
| 600 °C |  |  |  | 2 |
| 700 °C |  |  |  | 1 |
| CA:BG (2:1) |  |  |  | 2 |
| CA:BG (1:2) |  |  |  | 1 |
| Variable | Before | After | Swabs |
|---|---|---|---|
| Model |  |  |  |
| Fe 5 min |  |  |  |
| Cu 5 min |  |  |  |
| Manganese |  |  |  |
| Hematite PA |  |  |  |
| Natural hematite |  |  |  |
| Burned Umber |  |  |  |
| Burned SnPb |  |  |  |
| PbO |  |  |  |
| Lead White |  |  |  |
| 600 °C |  |  |  |
| 700 °C |  |  |  |
| CA:BG (2:1) |  |  |  |
| CA:BG (1:2) |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, C.; Vilarigues, M.; Pinto, J.V.; Palomar, T. The Influence of Raw Materials on the Stability of Grisaille Paint Layers. Appl. Sci. 2022, 12, 10515. https://doi.org/10.3390/app122010515
Machado C, Vilarigues M, Pinto JV, Palomar T. The Influence of Raw Materials on the Stability of Grisaille Paint Layers. Applied Sciences. 2022; 12(20):10515. https://doi.org/10.3390/app122010515
Chicago/Turabian StyleMachado, Carla, Márcia Vilarigues, Joana Vaz Pinto, and Teresa Palomar. 2022. "The Influence of Raw Materials on the Stability of Grisaille Paint Layers" Applied Sciences 12, no. 20: 10515. https://doi.org/10.3390/app122010515
APA StyleMachado, C., Vilarigues, M., Pinto, J. V., & Palomar, T. (2022). The Influence of Raw Materials on the Stability of Grisaille Paint Layers. Applied Sciences, 12(20), 10515. https://doi.org/10.3390/app122010515





