Al-Fe-Ni Metallic Glasses via Mechanical Alloying and Its Consolidation
Abstract
:1. Introduction
2. Materials and Methods
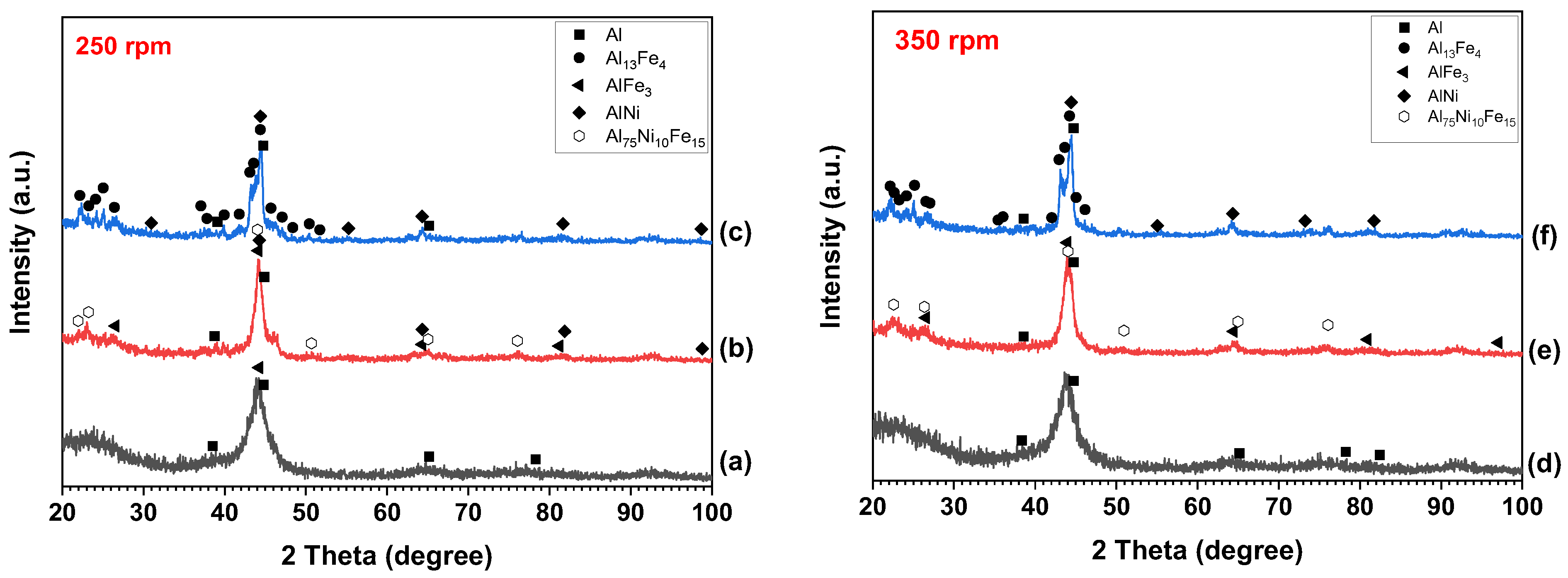
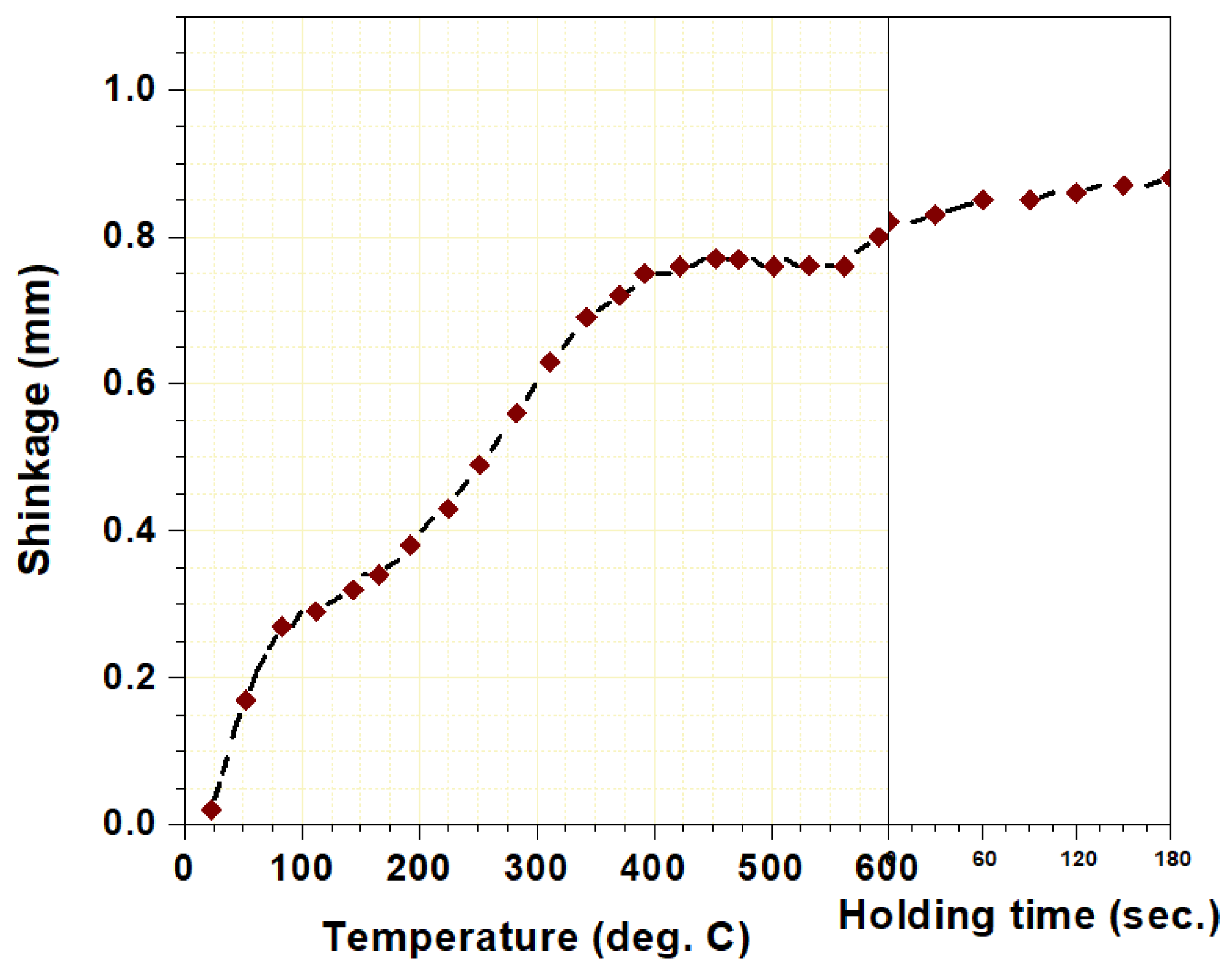
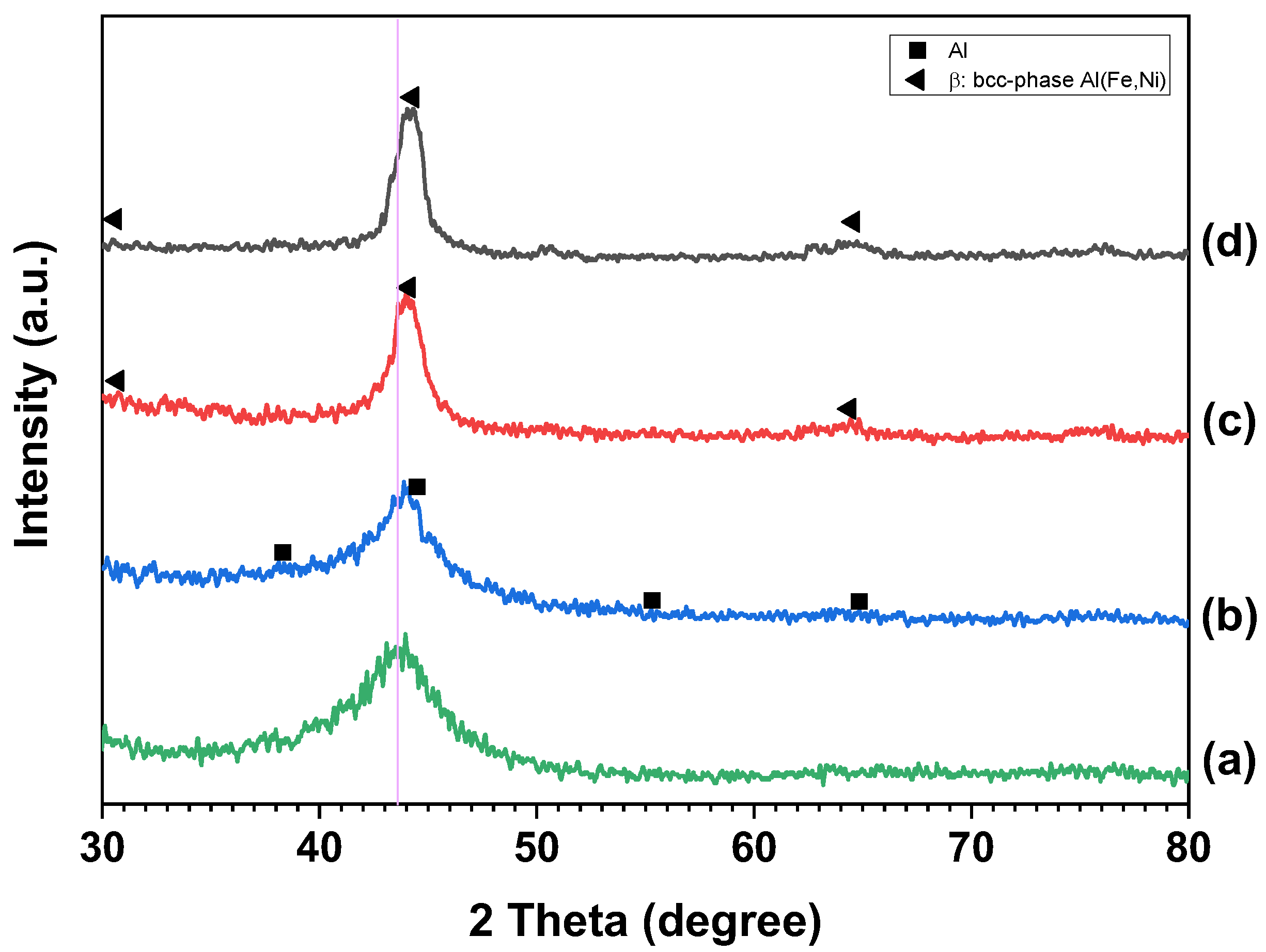
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, A. Amorphous, nanoquasicrystalline and nanocrystalline alloys in Al-based systems. Prog. Mater. Sci. 1998, 43, 365–520. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Metallic Glasses. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.; Zhu, S.; Liu, C.T.; Al-Marzouki, F. Development and Applications of Highly Functional Al-based Materials by Use of Metastable Phases. Mater. Res. 2015, 18, 1414–1425. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.J.; Yao, J.H.; Chao, Y.S.; Wang, J.Q.; Ma, E. Developing aluminum-based bulk metallic glasses. Philos. Mag. 2010, 90, 3215–3231. [Google Scholar] [CrossRef]
- Basu, J.; Ranganathan, S. Bulk metallic glasses: A new class of engineering materials. Sadhana 2003, 28, 783–798. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, D.H. Design of Bulk metallic glasses with high glass forming ability and enhancement of plasticity in metallic glass matrix composites: A review. Met. Mater. Int. 2005, 11, 19–27. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Hebert, R.J.; Tong, W.S. Amorphization and nanostructure synthesis in Al alloys. Intermetallics 2002, 10, 1079–1088. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Hebert, R.J. Amorphous aluminum alloys—synthesis and stability. JOM 2002, 54, 34–39. [Google Scholar] [CrossRef]
- He, Y.; Shiflet, G.J.; Poon, S.J. Synthesis and properties of aluminum-based metallic glasses containing rare earths. J. Alloys Compd. 1994, 207–208, 349–354. [Google Scholar] [CrossRef]
- Masumoto, T. Recent progress in amorphous metallic materials in Japan. Mater. Sci. Eng. A 1994, 179–180, 8–16. [Google Scholar] [CrossRef]
- Scudino, S.; Surreddi, K.B.; Nguyen, H.V.; Liu, G.; Gemming, T.; Sakaliyska, M.; Kim, J.S.; Vierke, J.; Wollgarten, M.; Eckert, J. High-strength Al87Ni8La5 bulk alloy produced by spark plasma sintering of gas atomized powders. J. Mater. Res. 2011, 24, 2909–2916. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Kusabiraki, K.; Saji, S. Effect of Ni addition on formation of amorphous and nanocrystalline phase during mechanical alloying of Al–25 at.%Fe–(5,10) at.%Ni powders. Mater. Res. Bull. 2002, 37, 1307–1313. [Google Scholar] [CrossRef]
- Krasnowski, M.; Kulik, T. Nanocrystalline and amorphous Al–Fe alloys containing 60–85% of Al synthesised by mechanical alloying and phase transformations induced by heating of milling products. Mater. Chem. Phys. 2009, 116, 631–637. [Google Scholar] [CrossRef]
- Inoue, A.; Amiya, K.; Yoshii, I.; Kimura, H.M.; Masumoto, T. Production of Al-Based Amorphous Alloy Wires with High Tensile Strength by a Melt Extraction Method. Mater. Trans. JIM 1994, 35, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Kimura, H. Fabrications and mechanical properties of bulk amorphous, nanocrystalline, nanoquasicrystalline alloys in aluminum-based system. J. Light Met. 2001, 1, 31–41. [Google Scholar] [CrossRef]
- Babilas, R.; Spilka, M.; Młynarek, K.; Łoński, W.; Łukowiec, D.; Radoń, A.; Kądziołka-Gaweł, M.; Gębara, P. Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys. Materials 2021, 14, 1581. [Google Scholar] [CrossRef] [PubMed]
- Batraev, I.S.; Wolf, W.; Bokhonov, B.B.; Ukhina, A.V.; Kuchumova, I.D.; Pal, A.K.; Bataev, I.A.; Ulianitsky, V.Y.; Dudina, D.V.; Botta, W.J.; et al. Structural transformations of a gas-atomized Al62.5Cu25Fe12.5 alloy during detonation spraying, spark plasma sintering and hot pressing. Sci. Sinter. 2021, 53, 379–386. [Google Scholar] [CrossRef]
- Nowosielski, R.; Babilas, R. Fabrication of bulk metallic glasses by centrifugal casting method. J. Achiev. Mater. Manuf. Eng. 2007, 20, 487–490. [Google Scholar]
- El-Eskandarany, M.S. (Ed.) 10-Mechanically induced solid-state amorphization. In Mechanical Alloying, 2nd ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 228–305. [Google Scholar]
- Urban, P.; Ternero, F.; Caballero, E.S.; Nandyala, S.; Montes, J.M.; Cuevas, F.G. Amorphous Al-Ti Powders Prepared by Mechanical Alloying and Consolidated by Electrical Resistance Sintering. Metals 2019, 9, 1140. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [Green Version]
- Yaykasli, H.; Avar, B.; Panigrahi, M.; Gogebakan, M.; Eskalen, H. Investigation of the Microstructural, Morphological, and Magnetic Properties of Mechanically Alloyed Co60Fe18Ti18Si4 Powders. Arab. J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Avar, B.; Chattopadhyay, A.K.; Simsek, T.; Simsek, T.; Ozcan, S.; Kalkan, B. Synthesis and characterization of amorphous-nanocrystalline Fe70Cr10Nb10B10 powders by mechanical alloying. Appl. Phys. A 2022, 128, 537. [Google Scholar] [CrossRef]
- Avar, B.; Ozcan, S. Characterization and amorphous phase formation of mechanically alloyed Co60Fe5Ni5Ti25B5 powders. J. Alloys Compd. 2015, 650, 53–58. [Google Scholar] [CrossRef]
- GÖĞEbakan, M.; Avar, B. Structural evolutions of the mechanically alloyed Al70Cu20Fe10 powders. Pramana 2011, 77, 735–747. [Google Scholar] [CrossRef]
- Gogebakan, M.; Avar, B. Quasicrystalline phase formation during heat treatment in mechanically alloyed Al65Cu20Fe15 alloy. Mater. Sci. Technol. 2010, 26, 920–924. [Google Scholar] [CrossRef]
- Oanh, N.T.H.; Viet, N.H.; Dudina, D.V.; Jorge, A.M.; Kim, J.-S. Structural characterization and magnetic properties of Al82Fe16TM2 (TM: Ti, Ni, Cu) alloys prepared by mechanical alloying. J. Non-Cryst. Solids 2017, 468, 67–73. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Choi, P.P.; Kim, J.S.; Nguyen, O.T.H.; Kwon, D.H.; Kwon, Y.S.; Kim, J.C. Al-La-Ni-Fe bulk metallic glasses produced by mechanical alloying and spark-plasma sintering. Mater. Sci. Eng. A 2007, 449–451, 1119–1122. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, X.; Wang, D.; Qin, Y.; Han, F. Bulk amorphous Al75V12.5Fe12.5−xCux alloys fabricated by consolidation of mechanically alloyed amorphous powders. J. Alloy. Compd. 2014, 586, 645–649. [Google Scholar] [CrossRef]
- Krapivka, N.A.; Firstov, S.A.; Karpets, M.V.; Myslivchenko, A.N.; Gorban’, V.F. Features of phase and structure formation in high-entropy alloys of the AlCrFeCoNiCuxsystem (x = 0, 0.5, 1.0, 2.0, 3.0). Phys. Met. Metallogr. 2015, 116, 467–474. [Google Scholar] [CrossRef]
- Mu, J.; Fu, H.; Zhu, Z.; Wang, A.; Li, H.; Hu, Z.; Zhang, H. Synthesis and Properties of Al-Ni-La Bulk Metallic Glass. Adv. Eng. Mater. 2009, 11, 530–532. [Google Scholar] [CrossRef]
- Abedi, M.; Sovizi, S.; Azarniya, A.; Giuntini, D.; Seraji, M.E.; Hosseini, H.R.M.; Amutha, C.; Ramakrishna, S.; Mukasyan, A. An analytical review on Spark Plasma Sintering of metals and alloys: From processing window, phase transformation, and property perspective. Crit. Rev. Solid State Mater. Sci. 2022, 1–46. [Google Scholar] [CrossRef]
- Bubesh Kumar, D.; Selva babu, B.; Aravind Jerrin, K.M.; Joseph, N.; Jiss, A. Review of Spark Plasma Sintering Process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 993, 012004. [Google Scholar] [CrossRef]
- Cavaliere, P.; Sadeghi, B.; Shabani, A. Spark Plasma Sintering: Process Fundamentals. In Spark Plasma Sintering of Materials: Advances in Processing and Applications; Cavaliere, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–20. [Google Scholar]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark Plasma Sintering of Metals and Metal Matrix Nanocomposites: A Review. J. Nanomater. 2012, 2012, 983470. [Google Scholar] [CrossRef] [Green Version]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process. J. Am. Ceram. Soc. 2011, 94, 1–19. [Google Scholar] [CrossRef]
- Yan, L.; Yan, B.; Jian, Y. Fabrication of Fe-Si-B Based Amorphous Powder Cores by Spark Plasma Sintered and Their Magnetic Properties. Materials 2022, 15, 1603. [Google Scholar] [CrossRef] [PubMed]
- Duc Dung, D.; Cho, S. Anomalous Hall effect in epitaxially grown ferromagnetic FeGa/Fe3Ga hybrid structure: Evidence of spin carrier polarized by clusters. J. Appl. Phys. 2013, 113, 17C734. [Google Scholar] [CrossRef]
- Binh, D.N.; Oanh, N.T.H.; Viet, N.H. The effect of Ni and Ti additions on the glass forming ability and magnetic properties of Al-Fe-Y alloy prepared by mechanical alloying. J. Non-Cryst. Solids 2022, 583, 121478. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, S.; Liao, B.; Zhao, S.; Dai, X.; Chen, D. Effect of milling time on the microstructure and magnetic properties of amorphous Ti50Fe50 alloys prepared by mechanical alloying. J. Mater. Res. Technol. 2019, 8, 3929–3935. [Google Scholar] [CrossRef]
- Phong, P.T.; Phuc, N.X.; Nam, P.H.; Chien, N.V.; Dung, D.D.; Linh, P.H. Size-controlled heating ability of CoFe2O4 nanoparticles for hyperthermia applications. Phys. B Condens. Matter 2018, 531, 30–34. [Google Scholar] [CrossRef]
- Taghvaei, A.H.; Stoica, M.; Khoshkhoo, M.S.; Thomas, J.; Vaughan, G.; Janghorban, K.; Eckert, J. Microstructure and magnetic properties of amorphous/nanocrystalline Co40Fe22Ta8B30 alloy produced by mechanical alloying. Mater. Chem. Phys. 2012, 134, 1214–1224. [Google Scholar] [CrossRef]
- Abrosimova, G.; Chirkova, V.; Pershina, E.; Volkov, N.; Sholin, I.; Aronin, A. The Effect of Free Volume on the Crystallization of Al87Ni8Gd5 Amorphous Alloy. Metals 2022, 12, 332. [Google Scholar] [CrossRef]
- Paustovskii, A.V.; Gubin, Y.V.; Kunitskii, Y.A. On the Relationship between Residual Stresses and Structural Characteristics of Amorphous Alloys. Mater. Sci. 2001, 37, 73–79. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Shokrollahi, H. Magnetic and structural properties of amorphous/nanocrystalline Fe42Ni28Zr8Ta2B10C10 soft magnetic alloy produced by mechanical alloying. Adv. Powder Technol. 2013, 24, 1100–1108. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Bian, X.; Wang, L.; Zhang, J.; Li, R.; Huang, T. Influences of Similar Elements on Glass Forming Ability and Magnetic Properties in Al-Ni-La Amorphous Alloy. J. Mater. Sci. Technol. 2010, 26, 146–150. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Oanh, N.T.H.; Quynh, P.N.D.; Lap, T.Q.; Kim, J.S. Thermal Stability of Amorphous Al-Fe-Y Prepared by Mechanical Alloying. Mater. Sci. Forum 2015, 804, 271–274. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J.H. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor alloying additions. J. Alloy. Compd. 2017, 707, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Wang, Q.; Qiang, J.B.; Wang, Y.M.; Jiang, N.; Han, G.; Li, Y.H.; Wu, J.; Xia, J.H. From clusters to phase diagrams: Composition rules of quasicrystals and bulk metallic glasses. J. Phys. D Appl. Phys. 2007, 40, R273–R291. [Google Scholar] [CrossRef]
- Viet, N.H.; Oanh, N.T.; Kim, J.-S.; Jorge, A.M. Crystallization Kinetics and Consolidation of Al82La10Fe4Ni4 Glassy Alloy Powder by Spark Plasma Sintering. Metals 2018, 8, 812. [Google Scholar] [CrossRef] [Green Version]
- Rudinsky, S.; Aguirre, J.M.; Sweet, G.; Milligan, J.; Bishop, D.P.; Brochu, M. Spark plasma sintering of an Al-based powder blend. Mater. Sci. Eng. A 2015, 621, 18–27. [Google Scholar] [CrossRef]

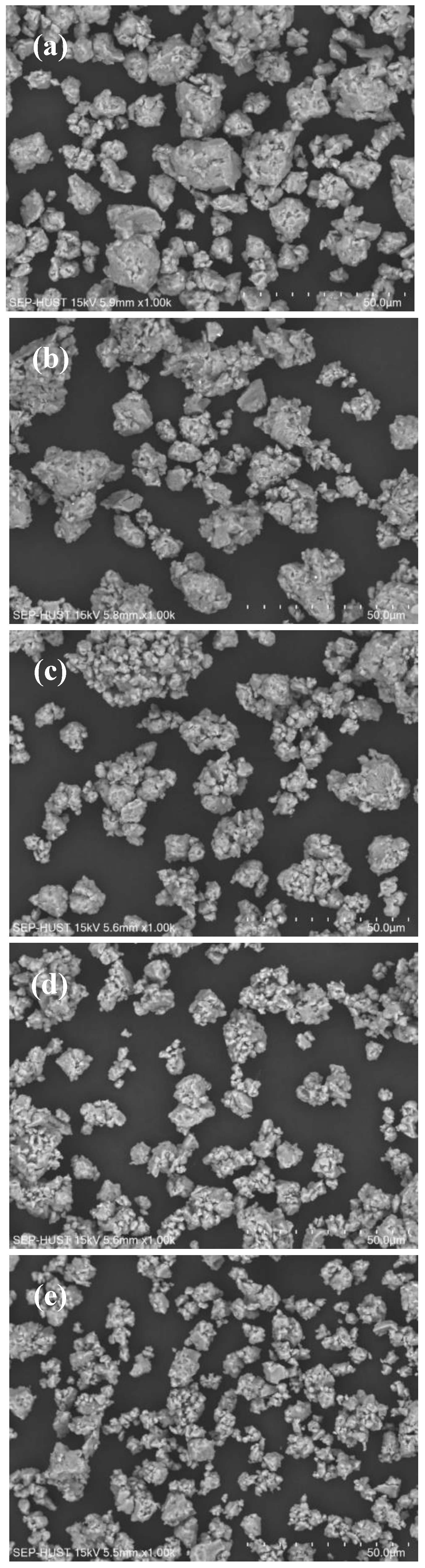

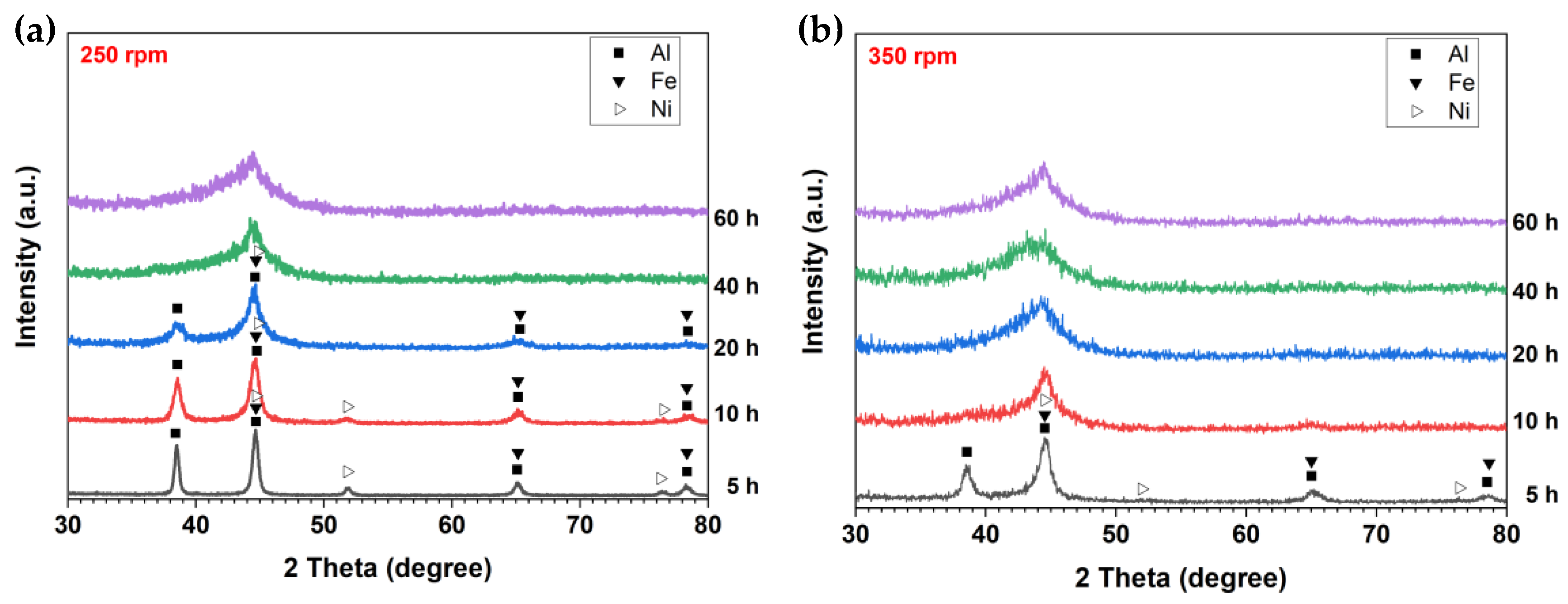
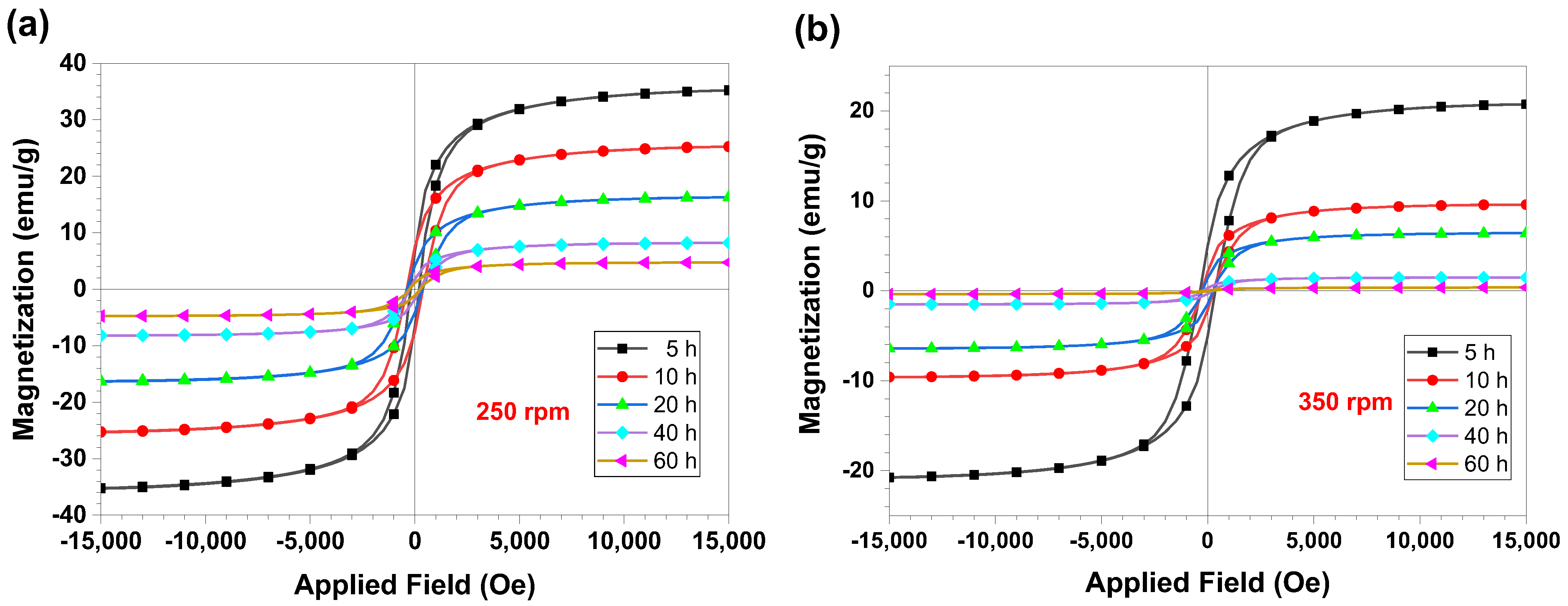
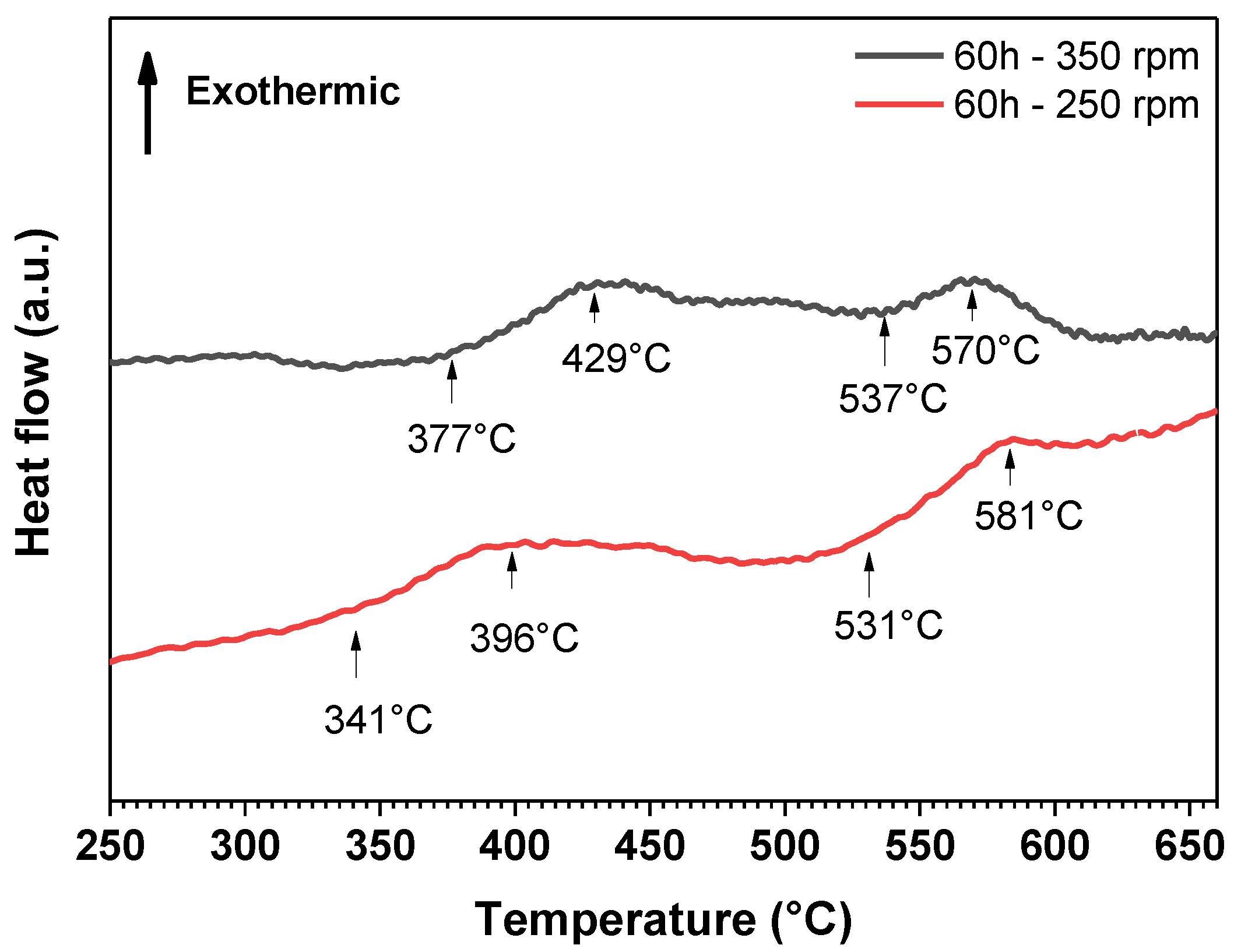

| Atom | Atomic Weight, g/mol | at.% | wt.% |
|---|---|---|---|
| Al | 26.98 | 82 | 68.52 |
| Fe | 55.84 | 14 | 24.21 |
| Ni | 58.69 | 4 | 7.27 |
| Milling Time (h) | The Portion of Particles with Diameters Smaller than Cumulative 10, 50 and 90% | |||||
|---|---|---|---|---|---|---|
| MA at Speed of 250 rpm | MA at Speed of 350 rpm | |||||
| D10 (μm) | D50 (μm) | D90 (μm) | D10 (μm) | D50 (μm) | D90 (μm) | |
| 5 | 15.4 | 26.6 | 37.8 | 11.4 | 23.3 | 47.8 |
| 10 | 11.1 | 21.8 | 37.0 | 11.0 | 21.2 | 39.3 |
| 20 | 6.4 | 11.8 | 21.4 | 11.4 | 22.8 | 37.0 |
| 40 | 8.7 | 18.0 | 41.8 | 7.9 | 13.6 | 22.2 |
| 60 | 6.2 | 11.1 | 19.4 | 5.8 | 9.5 | 14.6 |
| MA Speed (rpm) | Milling Time, h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 05 | 10 | 20 | 40 | 60 | ||||||
| Hc, (Oe) | Ms, (emu/g) | Hc, (Oe) | Ms, (emu/g) | Hc, (Oe) | Ms, (emu/g) | Hc, (Oe) | Ms, (emu/g) | Hc, (Oe) | Ms, (emu/g) | |
| 250 | 204.00 | 35.27 | 384.64 | 25.28 | 372.97 | 16.30 | 246.05 | 8.20 | 258.05 | 4.75 |
| 350 | 366.38 | 20.76 | 270.00 | 9.59 | 259.80 | 6.42 | 221.44 | 1.49 | 173.10 | 0.37 |
| Alloys | Compression Strength (MPa) | Relative Density (%) |
|---|---|---|
| Al82Fe14Ni4 (450 °C) | 600 | 88.4% |
| Al82Fe14Ni4 (500 °C) | 650 | 92.6% |
| Al82Fe14Ni4 (600 °C) | 710 | 97.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binh, D.N.; Oanh, N.T.H.; Viet, N.H. Al-Fe-Ni Metallic Glasses via Mechanical Alloying and Its Consolidation. Appl. Sci. 2022, 12, 10561. https://doi.org/10.3390/app122010561
Binh DN, Oanh NTH, Viet NH. Al-Fe-Ni Metallic Glasses via Mechanical Alloying and Its Consolidation. Applied Sciences. 2022; 12(20):10561. https://doi.org/10.3390/app122010561
Chicago/Turabian StyleBinh, Do Nam, Nguyen Thi Hoang Oanh, and Nguyen Hoang Viet. 2022. "Al-Fe-Ni Metallic Glasses via Mechanical Alloying and Its Consolidation" Applied Sciences 12, no. 20: 10561. https://doi.org/10.3390/app122010561






