5.1. The Improvement of Sample-Preparation Method
The method before improvement is the tablet-pressing method: to grind the irregular potassium bromide crystal block into powder in advance, press the potassium bromide powder into a wafer by using the tableting machine, and then heat the asphalt to be tested, which is coated on the potassium bromide wafer to complete the preparation of the test sample.
The method after improvement is the dissolution method: in the experiment, 1 g~2 g asphalt is taken from an SBS–asphalt sample and put into a glass container; trichloroethylene of approximately eight times the weight of asphalt is taken into the container to fully dissolve the asphalt. The asphalt standard samples need to be completely dissolved, and there is no insoluble matter in the solution. Then, the glass dropper is used to drop the dissolved SBS-modified asphalt onto the potassium bromide wafer. After trichloroethylene volatilizes, a thin and uniform transparent SBS-modified asphalt film will be formed on the potassium bromide wafer, as shown in
Figure 9.
Through comparative research, the dissolution method has the following advantages over the tablet-pressing method:
(1) It is necessary to heat the asphalt to the melting state in the tablet-pressing method. The repeated heating of asphalt will cause asphalt aging and then affect the test results. The dissolution method does not require heating and avoids such errors.
(2) Under manual operation, it is very difficult to coat the thick, uniform, transparent asphalt film on the potassium bromide wafer. The dissolution method makes use of the volatility of trichloroethylene and the tension of the solution itself, so the asphalt film obtained will be thinner and more uniform, which is more convenient for manual operation.
The experimental results by using the dissolution method are shown in
Figure 9 and
Table 8. The SBS modifier is YH-791. The SBS content of test sample is 4%.
According to
Figure 10, the linear correlation coefficient R
2 is 0.9982, which has a good linear correlation. According to
Table 6, it can be seen that the test error is 0.05%, which meets the allowable error range of ±0.1%. The accuracy is high enough, so it can be used as the basis for the quantitative determination of SBS content. From the above, it can be concluded that the dissolution method has more excellent accuracy, safety, convenience, and applicability, which can be used as an upgrade and improvement of the sample-preparation method in the infrared spectrum SBS content test technology.
5.2. The Improvement of Model Algorithm
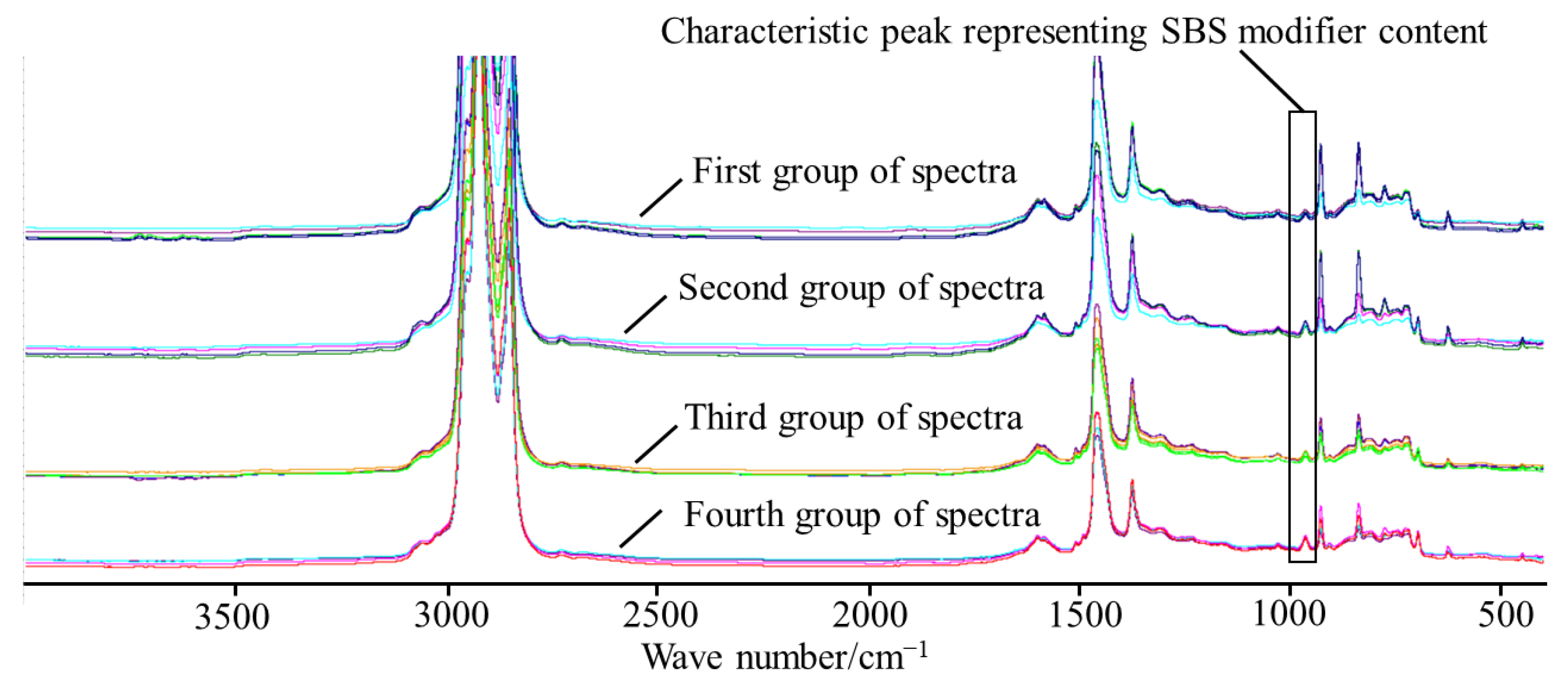
Reactive additives will reduce the number of =C-H bonds in polybutadiene, and the peak area ratio in the infrared spectrum will be significantly reduced. This paper analyzes the infrared spectra of SBS asphalt with different types of additives and proposes a new 699 cm
−1 characteristic peak representing SBS. The infrared spectrum of the first group of SBS-modified asphalt, whether it contains additives at 699 cm
−1, is shown in
Figure 11.
As can be seen from
Figure 11, the characteristic peak area of different types of SBS-modified asphalt basically does not change at 699 cm
−1. By analyzing the infrared spectrum and the structure of the SBS modifier, the characteristic peak at 699 cm
−1 was determined as a new characteristic peak to detect the SBS content. The 699 cm
−1 is the characteristic peak of styrene, which belongs to the out-of-plane vibration of the C-H bond in the benzene ring in styrene. The benzene ring is not a C=C unsaturated double-bond structure, but an overall large π-bond structure, which leads to a relative stability overall, and chemical reactions occur with difficulty. Therefore, when 699 cm
−1 is used as the characteristic peak of SBS-modified asphalt, the test is not easily covered by the additives.
Four groups of SBS-modified asphalt standard samples with different content of 2%, 3%, 4%, 5%, and 6% were detected by the infrared spectrum. The standard curve is established by linear fitting with the characteristic peak area ratio A699/(A699 + A1377) and SBS content as shown in
Figure 12.
The linear correlation coefficients R2 of the four groups are 0.9951, 0.9998, 0.9979, and 0.9989. The R2 are all above 0.99, which proves that the curves fit well and meet the test requirements.
The standard curve in
Figure 12 is used to test the 4% SBS-modified asphalt samples without additives, and the test results are shown in
Table 9.
It can be seen from
Table 9 that the errors of four groups are −0.06%, −0.01%, +0.09%, and +0.04%, all within the required error range of ±0.1%. It proves that the standard curve model is accurate and can be used for the SBS content test.
Four groups of 4% SBS-modified asphalt with additives ①, ②, ③, and ④ were tested by the infrared spectrum.
Table 8 shows the detection results after calculating the peak area ratio A699(A699 + A1377) and substituting them into the standard curve in
Figure 11.
After considering the influence caused by the quality of the additives, the actual SBS modifier content is shown in
Table 10: the content of No. ① compatibilizer was 2.7%, resulting in the actual SBS modifier content of 3.89%, and the actual errors of the four groups were −0.07%, −0.01%, +0.05%, and +0.10%, and all of them are within ±0.1%. The content of No. ② stabilizer was 0.2%, resulting in the actual SBS modifier content of 3.99%, and the actual errors of the four groups were −0.04%, +0.01%, +0.08%, and +0.10%, all of them are within ±0.1%. The content of No. ③ compatibilizer was 3.0%, resulting in the actual SBS modifier content of 3.88%, and the actual errors of the four groups respectively were −0.03%, −0.07%, +0.10%, and −0.07%, all of them are within ±0.1%. The content of No. ④ stabilizer was 0.25%, resulting in the actual SBS modifier content of 3.99%, and the actual errors of the four groups were −0.08%, −0.10%, +0.10%, and −0.07%, all of them are within ±0.1%. It proves that the addition of additives has no effect on the content determination of the SBS modifier when using the peak area ratio of A699/(A699 + A1377). This improved model algorithm can simplify the boundary conditions of sample preparation. It is no longer necessary to consider the influence of additives, and no need to add additives when preparing standard samples, which greatly improves the application convenience of the test technology.