Alginate/Polypyrrole Hydrogels as Potential Extraction Phase for Determination of Atrazine, Caffeine, and Progesterone in Aqueous Samples
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
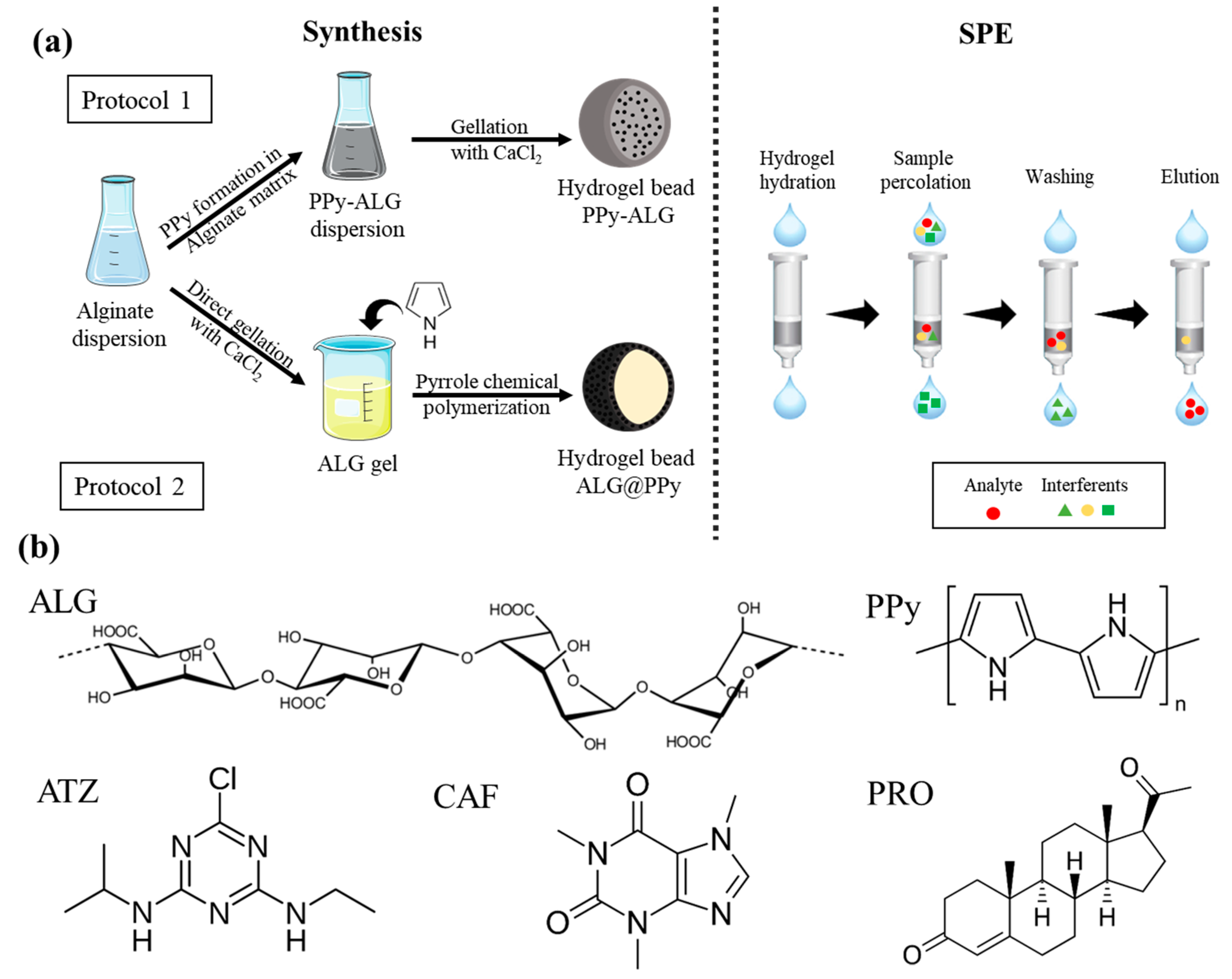
2.2. Preparation of the Alginate/Polypyrrole Hydrogels Disks and Beads
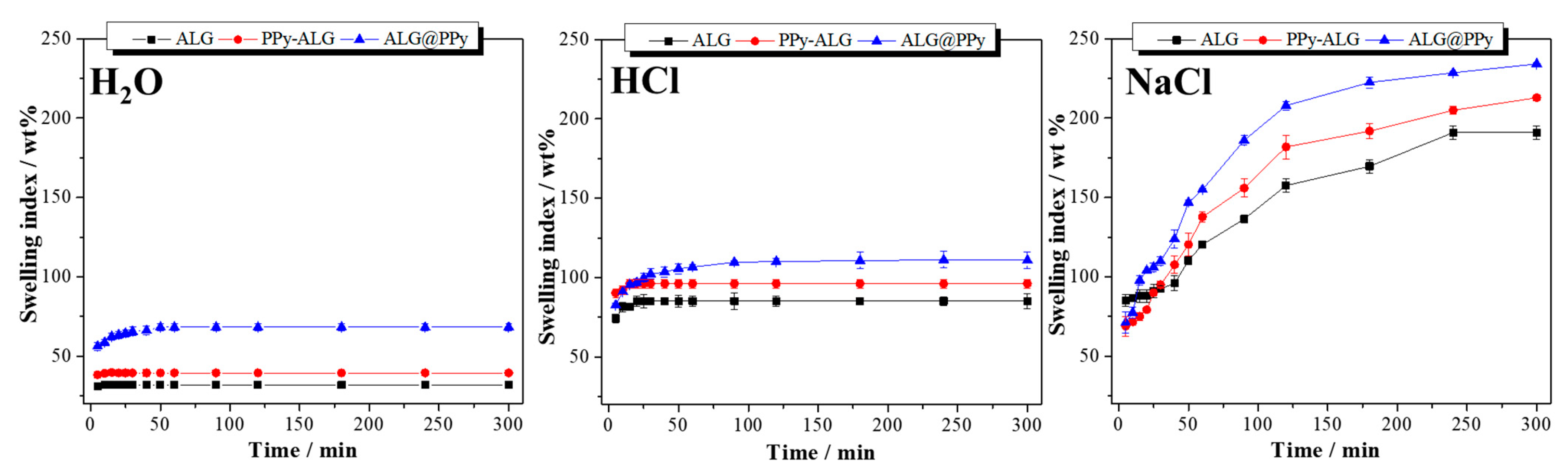
2.3. Chemical, Physical Chemical, and Morphological Properties of Hydrogels
2.4. Application of Hydrogel Beads as Extraction Phases for Determination of Atrazine, Caffeine, and Progesterone
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, M.A.; Sahiner, N. Cationic Hydrogels for Toxic Arsenate Removal from Aqueous Environment. J. Environ. Manag. 2008, 88, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mat. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Appl. Sci. 2019, 9, 1638. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, R.; Zheng, B.; Cai, C.; Chen, Z.; Li, H.; Liu, H. A Biocompatible, Stimuli-Responsive, and Injectable Hydrogel with Triple Dynamic Bonds. Molecules. 2020, 25, 3050. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tang, Q.; Hu, D.; Sun, X.; Li, Q.; Wu, J. Electric Field Sensitivity of Conducting Hydrogels with Interpenetrating Polymer Network Structure. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 177–183. [Google Scholar] [CrossRef]
- Fermani, M.; Platania, V.; Kavasi, R.M.; Karavasili, C.; Zgouro, P.; Fatouros, D.; Chatzinikolaidou, M.; Bouropoulos, N. 3D-Printed Scaffolds from Alginate/Methyl Cellulose/Trimethyl Chitosan/Silicate Glasses for Bone Tissue Engineering. Appl. Sci. 2021, 11, 8677. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.D.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D Printed Hydrogel Scaffolds: Influence of Hydrogel Composition and Printing Parameters. Appl. Sci. 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.J.; Klobukoski, V.; de Paula, J.I.S.; Riegel-Vidotti, I.C.; Vidotti, M. Assembly of Symmetric Supercapacitor Based on Alginate Hydrogel Electrolyte and Polyaniline Modified Electrodes. Electrochim. Acta 2022, 429, 140914. [Google Scholar] [CrossRef]
- Bahram, M.; Keshvari, F.; Mohseni, N. A Novel Hydrogel Based Microextraction of Analytes. J. Saudi Chem. Soc. 2016, 20, S624–S631. [Google Scholar] [CrossRef]
- Castilhos, N.D.B.; Sampaio, N.M.F.M.; da Silva, B.C.; Riegel-vidotti, I.C.; Grassi, M.T.; Silva, B.J.G. Physical-Chemical Characteristics and Potential Use of a Novel Alginate/Zein Hydrogel as the Sorption Phase for Polar Organic Compounds. Carbohydr. Polym. 2017, 174, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.M.F.M.; Castilhos, N.D.B.; da Silva, B.C.; Riegel-Vidotti, I.C.; Silva, B.J.G. Evaluation of Polyvinyl Alcohol/Pectin-Based Hydrogel Disks as Extraction Phase for Determination of Steroidal Hormones in Aqueous Samples by GC-MS/MS. Molecules 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiseppi-Elie, A. Electroconductive Hydrogels: Synthesis, Characterization and Biomedical Applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- De Alvarenga, G.; Hryniewicz, B.M.; Jasper, I.; Silva, R.J.; Klobukoski, V.; Costa, F.S.; Cervantes, T.N.M.; Amaral, C.D.B.; Schneider, J.T.; Bach-Toledo, L.; et al. Recent Trends of Micro and Nanostructured Conducting Polymers in Health and Environmental Applications. J. Electroanal. Chem. 2020, 879, 114754. [Google Scholar] [CrossRef]
- Bueno, V.B.; Takahashi, S.H.; Catalani, L.H.; De Torresi, S.I.C.; Petri, D.F.S. Biocompatible Xanthan/Polypyrrole Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2015, 52, 121–128. [Google Scholar] [CrossRef]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising Bio-Composites of Polypyrrole and Chitosan: Surface Protective and in Vitro Biocompatibility Performance on 316L SS Implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef]
- Basavaraja, C.; Jo, E.A.; Kim, B.S.; Kim, D.G.; Huh, D.S. Electrical Conduction Mechanism of Polypyrrole-Alginate Polymer Films. Macromol. Res. 2010, 18, 1037–1044. [Google Scholar] [CrossRef]
- Abu-Rabeah, K.; Marks, R.S. Impedance Study of the Hybrid Molecule Alginate-Pyrrole: Demonstration as Host Matrix for the Construction of a Highly Sensitive Amperometric Glucose Biosensor. Sens. Actuators B Chem. 2009, 136, 516–522. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Abu-Rabeah, K.; Cosnier, S.; Marks, R.S. Improved Enzyme Retention from an Electropolymerized Polypyrrole-Alginate Matrix in the Development of Biosensors. Electrochem. Commun. 2005, 7, 1277–1282. [Google Scholar] [CrossRef]
- Bunkoed, O.; Nurerk, P.; Wannapob, R.; Kanatharana, P. Polypyrrole-Coated Alginate/Magnetite Nanoparticles Composite Sorbent for the Extraction of Endocrine-Disrupting Compounds. J. Sep. Sci. 2016, 39, 3602–3609. [Google Scholar] [CrossRef]
- Montes, R.; Méndez, S.; Carro, N.; Cobas, J.; Alves, N.; Neuparth, T.; Santos, M.M.; Quintana, J.B.; Rodil, R. Screening of Contaminants of Emerging Concern in Surface Water and Wastewater Effluents, Assisted by the Persistency-Mobility-Toxicity Criteria. Molecules 2022, 27, 3915. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, A.C.; Soares, D.P.; Sampaio, N.M.F.M.; Silva, B.J.G.; Vidotti, M. Polypyrrole Nanotubes for Electrochemically Controlled Extraction of Atrazine, Caffeine and Progesterone. Microchim. Acta 2019, 186, 398. [Google Scholar] [CrossRef] [PubMed]
- Ripanda, A.S.; Rwiza, M.J.; Nyanza, E.C.; Njau, K.N.; Vuai, S.A.H.; Machunda, R.L. A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa. Appl. Sci. 2022, 12, 56. [Google Scholar] [CrossRef]
- Dugheri, S.; Marrubini, G.; Mucci, N.; Cappelli, G.; Bonari, A.; Pompilio, I.; Trevisani, L.; Arcangeli, G. A Review of Micro-Solid-Phase Extraction Techniques and Devices Applied in Sample Pretreatment Coupled with Chromatographic Analysis. Acta Chromatogr. 2021, 33, 99–111. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A Comprehensive Look at Solid-Phase Microextraction Technique: A Review of Reviews. Microchem. J. 2020, 152, 104319. [Google Scholar] [CrossRef]
- Omastova, M.; Trchova, M.; Kovarova, J.; Stejskal, J. Synthesis and Structural Study of Polypyrroles Prepared in the Presence of Surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Witek-krowiak, A. Novel Nanoparticles Modified Composite Eco-Adsorbents—A Deep Insight into Kinetics Modelling Using Numerical Surface Diffusion and Artificial Neural Network Models. Chem. Eng. Res. Des. 2016, 109, 1–17. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, J.; Zhang, L.; Wu, Q.; Yang, Y. Lanthanum Adsorption Using Iron Oxide Loaded Calcium Alginate Beads. Hydrometallurgy 2010, 101, 76–83. [Google Scholar] [CrossRef]
- Yu, C.; Wang, M.; Dong, X.; Shi, Z.; Zhang, X.; Lin, Q. Removal of Cu (II) from Aqueous Solution Using Fe3O4-Alginate Modified Biochar Microspheres. RSC Adv. 2017, 7, 53135–53144. [Google Scholar] [CrossRef] [Green Version]
- Fundueanu, G.; Nastruzzi, C.; Carpov, A.; Desbrieres, J.; Rinaudo, M. Physico-Chemical Characterization of Ca-Alginate Microparticles Produced with Different Methods. Biomaterials 1999, 20, 1427–1435. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive Drug Delivery Systems in Intestinal Inflammation: State-of-the-Art and Future Perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Braek, G.S.; Smidsrod, O. Alginic Acid Gels: The Effect of Alginate Chemical Composition and Molecular Weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and Diffusion Studies of Calcium Polysaccharide Gels Intended for Film Coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel Based on an Alginate—Ca2+/Chondroitin Sulfate Matrix as a Potential Colon-Specific Drug Delivery System. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Lopes, L.C.; Simas-Tosin, F.F.; Cipriani, T.R.; Marchesi, L.F.; Vidotti, M.; Riegel-Vidotti, I.C. Effect of Low and High Methoxyl Citrus Pectin on the Properties of Polypyrrole Based Electroactive Hydrogels. Carbohydr. Polym. 2017, 155, 11–18. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Bastos, A.C.; Tedim, J.; Ferreira, M.G.S.; Marino, C.E.B.; Riegel-Vidotti, I. On Demand Release of Cerium from an Alginate/Cerium Complex for Corrosion Protection of AISI1020 and AA2024 Substrates. J. Braz. Chem. Soc. 2022, 33, 987–996. [Google Scholar] [CrossRef]
- Kubiak, A.; Maćkiewicz, M.; Karbarz, M.; Biesaga, M. Application of Microgel as a Sorbent for Bisphenol Analysis in Liquid Food Samples. Appl. Sci. 2022, 12, 441. [Google Scholar] [CrossRef]
- Jaski, A.C.; Schmitz, F.; Horta, R.P.; Cadorin, L.; da Silva, B.J.G.; Andreaus, J.; Paes, M.C.D.; Riegel-Vidotti, I.C.; Zimmermann, L.M. Zein—A Plant-Based Material of Growing Importance: New Perspectives for Innovative Uses. Ind. Crops Prod. 2022, 186, 115250. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Gouda, M.H.; Elnouby, M.S.; Zahran, H.F.; Hashim, A.; El-Latif, M.M.A.; Santos, D.M.F. Novel Sodium Alginate/Polyvinylpyrrolidone/Tio2 Nanocomposite for Efficient Removal of Cationic Dye from Aqueous Solution. Appl. Sci. 2021, 11, 9186. [Google Scholar] [CrossRef]
- Basheer, C.; Suresh, V.; Renu, R.; Lee, H.K. Development and Application of Polymer-Coated Hollow Fiber Membrane Microextraction to the Determination of Organochlorine Pesticides in Water. J. Chromatogr. A 2004, 1033, 213–220. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, M.; Wu, J.; Wang, L.; He, H. Preparation of Fe3O4@PPy Magnetic Nanoparticles as Solid-Phase Extraction Sorbents for Preconcentration and Separation of Phthalic Acid Esters in Water by Gas Chromatography—Mass Spectrometry. J. Chromatogr. B 2016, 1011, 33–44. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacumasso, S.C.; de Alvarenga, G.; de Lazzari, A.C.; Sampaio, N.M.F.M.; Silva, B.J.G.; Marchesi, L.F.; Vidotti, M.; Riegel-Vidotti, I.C. Alginate/Polypyrrole Hydrogels as Potential Extraction Phase for Determination of Atrazine, Caffeine, and Progesterone in Aqueous Samples. Appl. Sci. 2022, 12, 10609. https://doi.org/10.3390/app122010609
Jacumasso SC, de Alvarenga G, de Lazzari AC, Sampaio NMFM, Silva BJG, Marchesi LF, Vidotti M, Riegel-Vidotti IC. Alginate/Polypyrrole Hydrogels as Potential Extraction Phase for Determination of Atrazine, Caffeine, and Progesterone in Aqueous Samples. Applied Sciences. 2022; 12(20):10609. https://doi.org/10.3390/app122010609
Chicago/Turabian StyleJacumasso, Sheila C., Gabriela de Alvarenga, Adriana C. de Lazzari, Naiara M. F. M. Sampaio, Bruno J. G. Silva, Luis F. Marchesi, Marcio Vidotti, and Izabel C. Riegel-Vidotti. 2022. "Alginate/Polypyrrole Hydrogels as Potential Extraction Phase for Determination of Atrazine, Caffeine, and Progesterone in Aqueous Samples" Applied Sciences 12, no. 20: 10609. https://doi.org/10.3390/app122010609
APA StyleJacumasso, S. C., de Alvarenga, G., de Lazzari, A. C., Sampaio, N. M. F. M., Silva, B. J. G., Marchesi, L. F., Vidotti, M., & Riegel-Vidotti, I. C. (2022). Alginate/Polypyrrole Hydrogels as Potential Extraction Phase for Determination of Atrazine, Caffeine, and Progesterone in Aqueous Samples. Applied Sciences, 12(20), 10609. https://doi.org/10.3390/app122010609







