Lab-on-Chip Culturing System for Fungi—Towards Nanosatellite Missions
Abstract
:1. Introduction
2. Materials and Methods
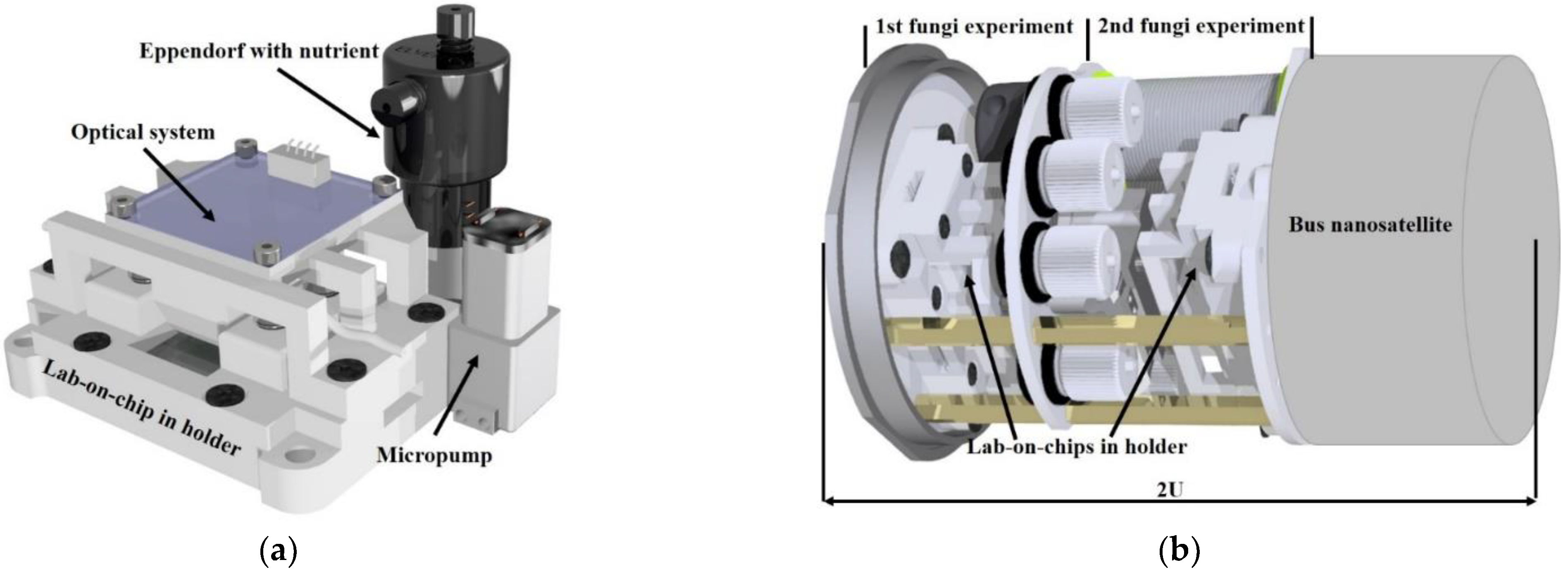
2.1. Biomedical Experimentation in Space—Tools
2.2. Object of the Study—F. culmorum
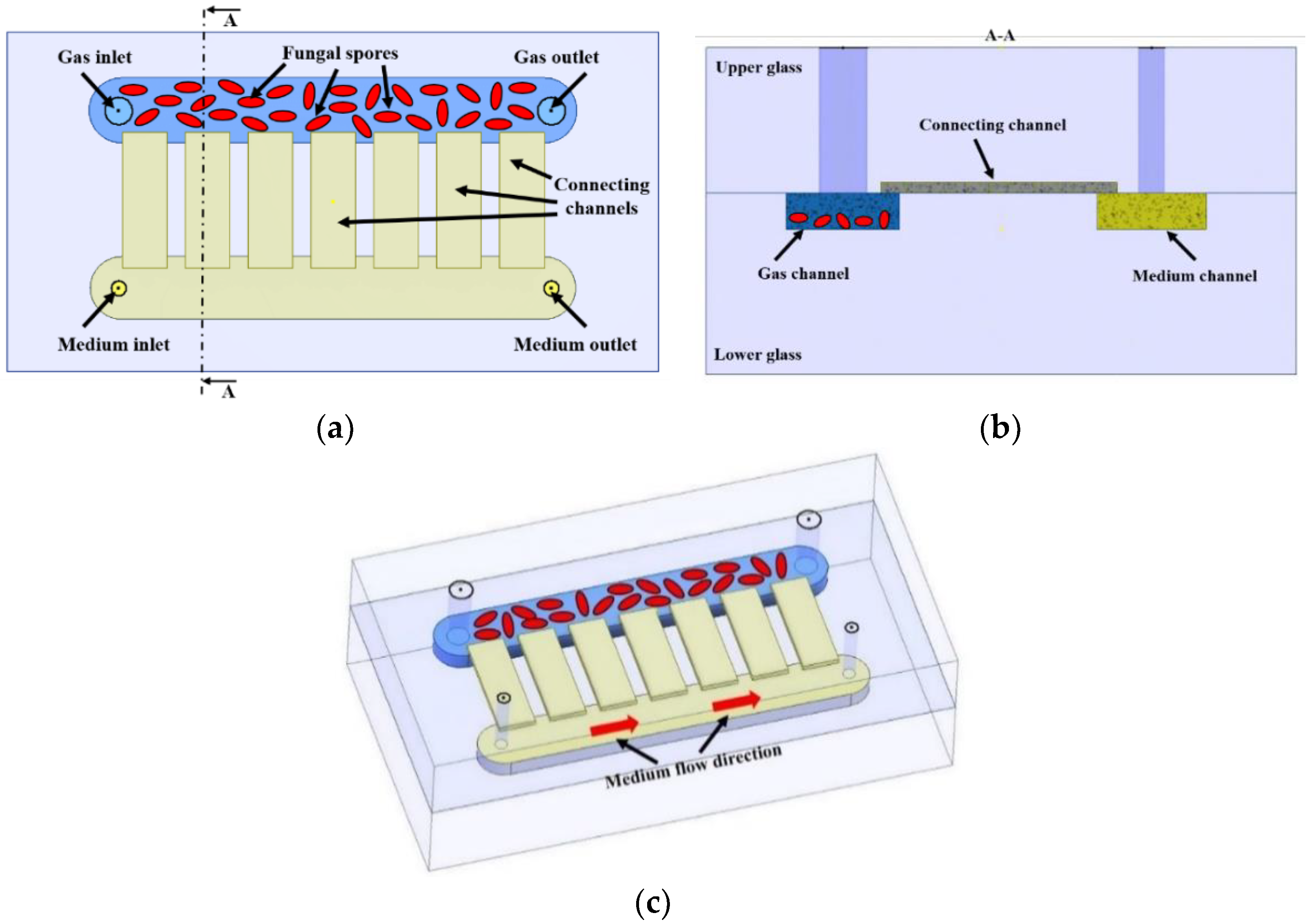
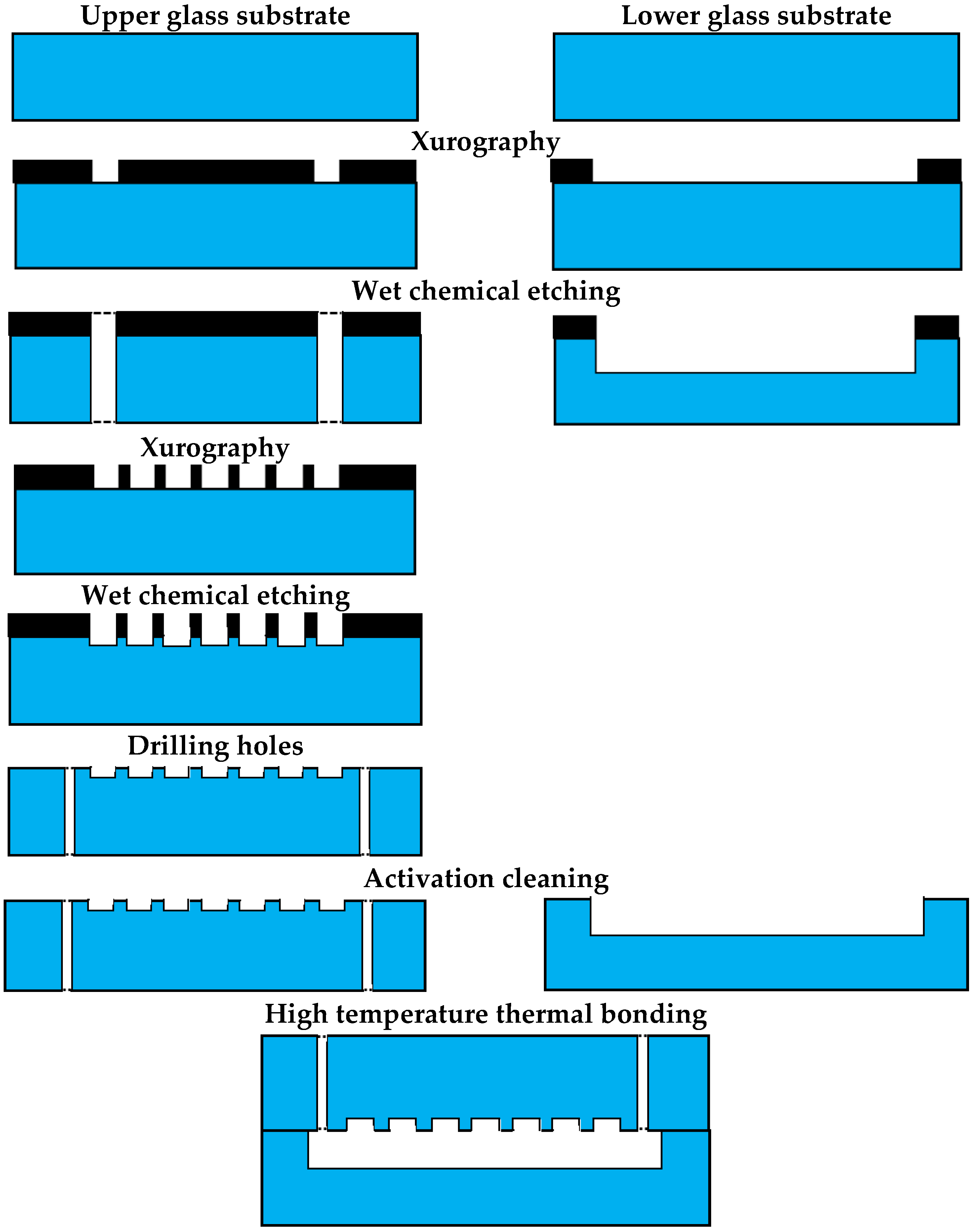
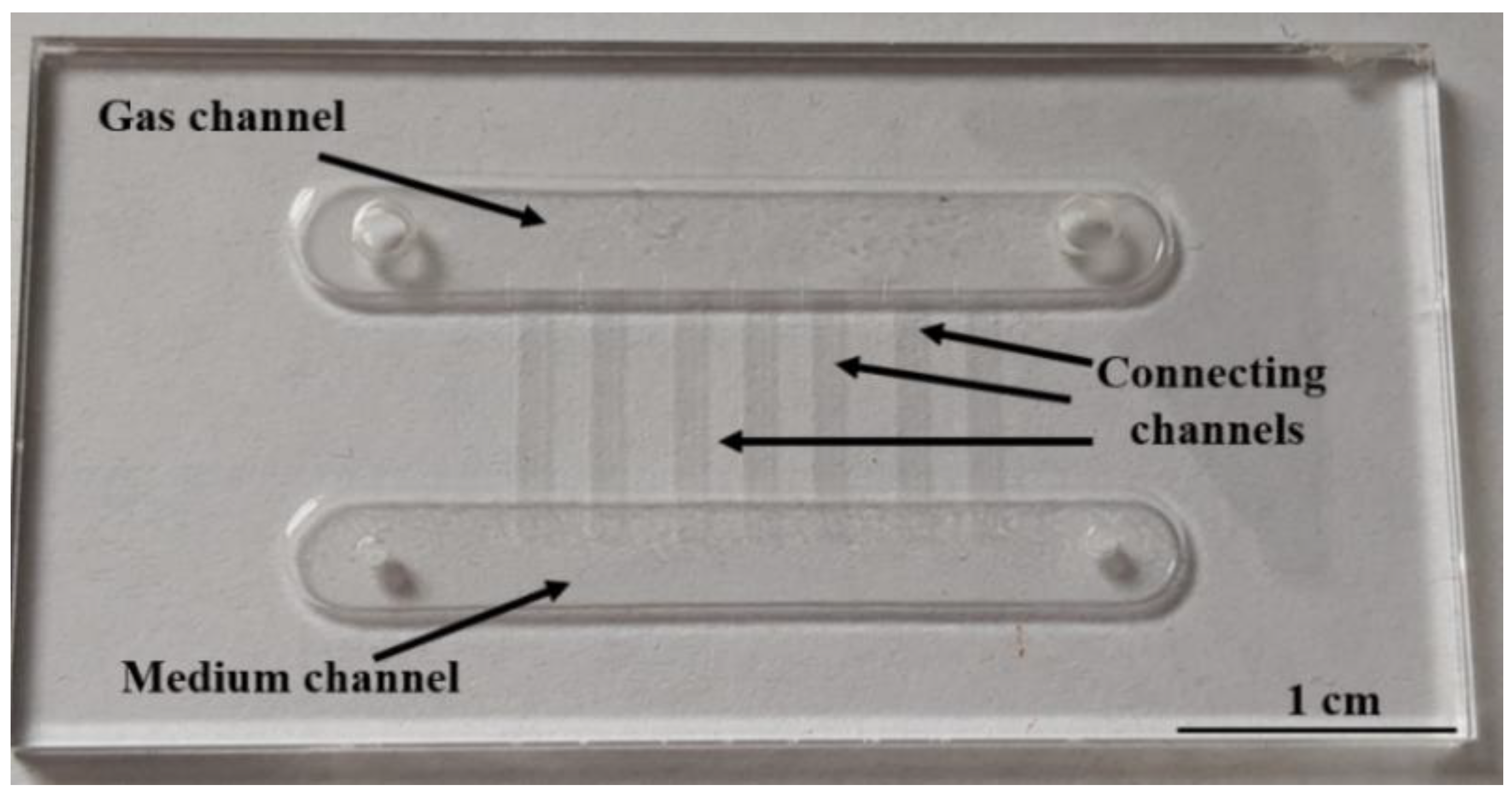
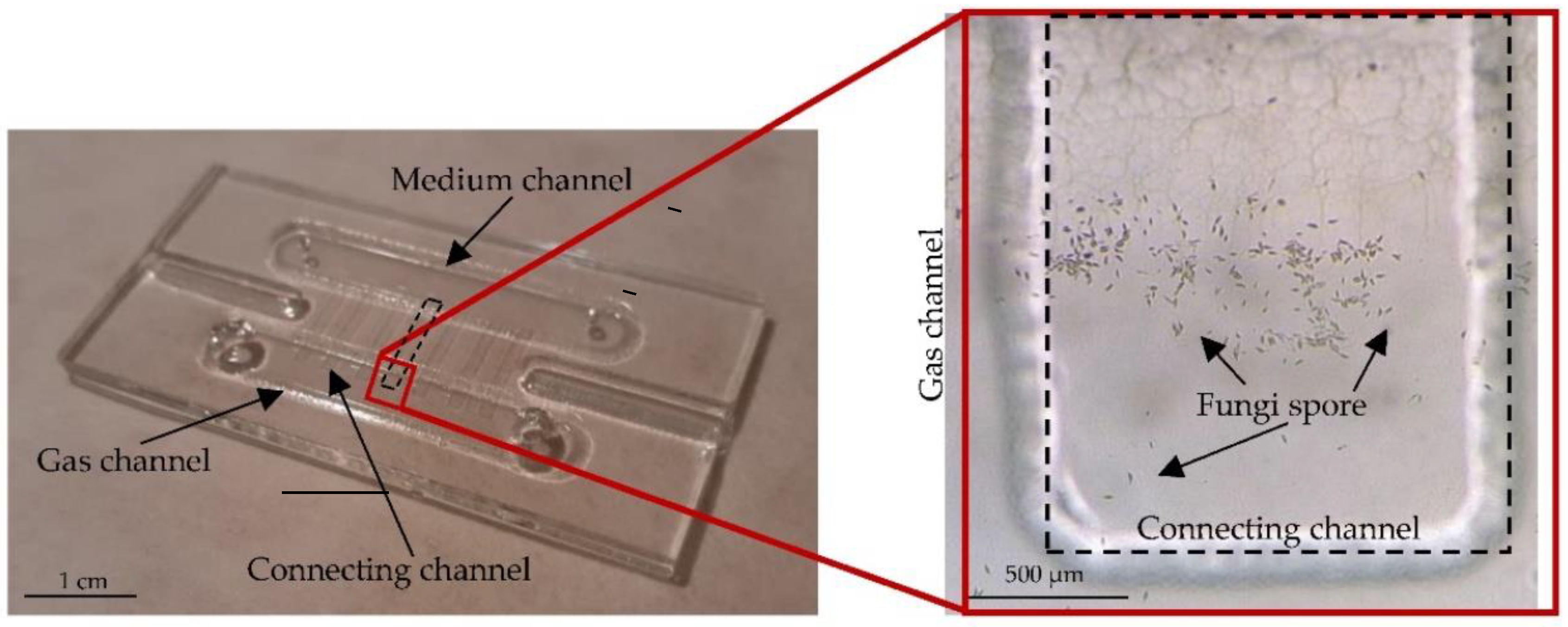
2.3. LOC—Construction and Technology
2.4. Microgravity Simulation with RWV
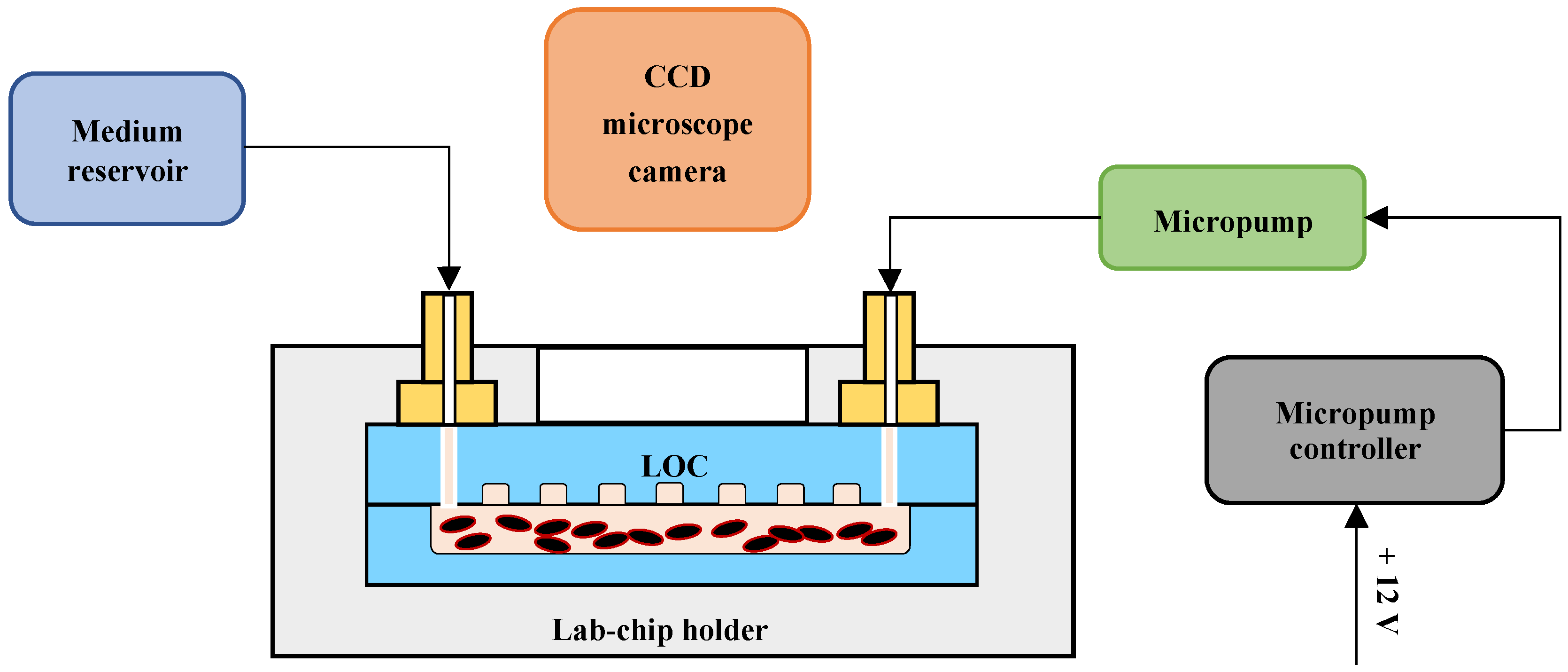
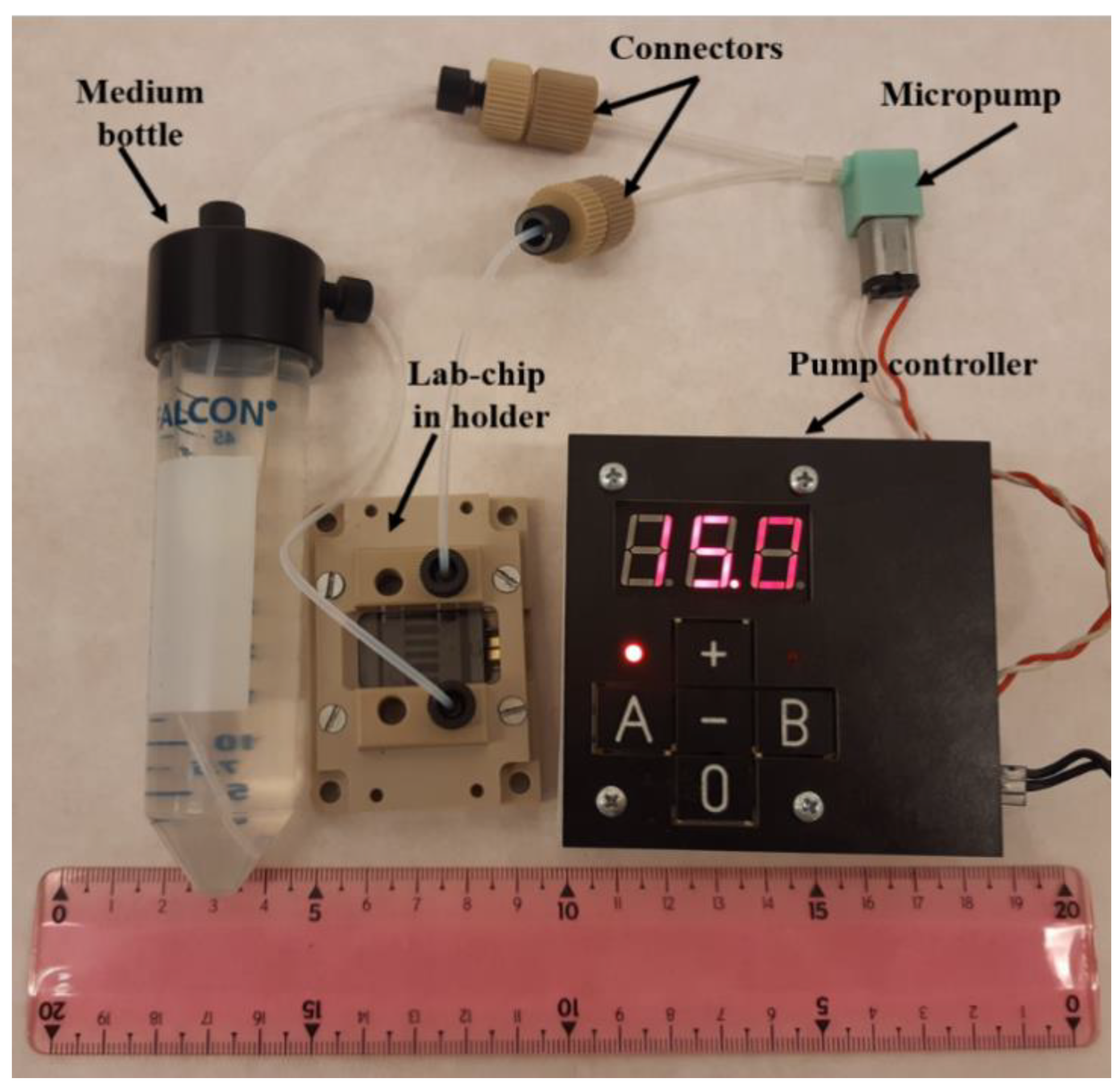
2.5. Culturing System
3. Results and Discussion
3.1. Fungi Cultivation in Simulated Microgravity
3.2. Fungi Cultivation with Culturing System
3.3. Fungi Cultivation in Space—Concept
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hol, F.J.H.; Dekker, C. Zooming in to see the bigger picture: Microfluidic and nanofabrication tools to study bacteria. Science 2014, 346, 6208. [Google Scholar] [CrossRef]
- Podwin, A.; Lizanets, D.; Przystupski, D.; Kubicki, W.; Śniadek, P.; Kulbacka, J.; Wymysłowski, A.; Walczak, R.; Dziuban, J.A. Lab-on-Chip Platform for Culturing and Dynamic Evaluation of Cells Development. Micromachines 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, D.R.; van Heeren, H.; Guha, S.; Herbertson, L.; Tzannis, A.P.; Ducrée, J.; Bissig, H.; Becker, H. Accelerating innovation and commercialization through standardization of microfluidic-based medical devices. Lab Chip 2020, 21, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Narayanamurthy, V.; Jeroish, Z.E.; Bhuvaneshwari, K.S.; Bayat, P.; Premkumar, R.; Samsuri, F.; Yusoff, M.M. Advances in passively driven microfluidics and lab-on-chip devices: A comprehensive literature review and patent analysis. RSC Adv. 2020, 10, 11652–11680. [Google Scholar] [CrossRef]
- Walsh, D.I.; Kong, D.S.; Murthy, S.K.; Carr, P.A. Enabling Microfluidics: From Clean Rooms to Makerspaces. Trends Biotechnol. 2017, 35, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Walczak, R.; Śniadek, P.; Dziuban, J.A.; Kluger, J.; Chełmońska-Soyta, A. Supravital fluorometric apoptosis detection in a single mouse embryo using lab-on-a-chip. Lab Chip 2011, 11, 3263–3268. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The Role of Microfluidics for Organ on Chip Simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lu, Y.-T.; Chang, C.-M.; Liu, C.-H. Finger-powered cell-sorting microsystem chip for cancer-study applications. Sens. Actuators B Chem. 2022, 370, 132430. [Google Scholar] [CrossRef]
- Feng, J.J.; Hedtrich, S. A similarity scaling approach for organ-on-chip devices. Lab Chip 2022, 22, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Davín, A.A.; Tannier, E.; Williams, T.A.; Boussau, B.; Daubin, V.; Szöllősi, G.J. Gene transfers can date the tree of life. Nat. Ecol. Evol. 2018, 2, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Kim, S.-H. A genome Tree of Life for the Fungi kingdom. Proc. Natl. Acad. Sci. USA 2017, 114, 9391–9396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyanarayana, T.; Deshmukh, S.K.; Johri, B.N. Developments in Fungal Biology and Applied Mycology; Springer Nature Singapore Pte Ltd.: Singapore, 2017. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Deshmukh, S.K.; Deshpande, M.V. Advancing Frontiers in Mycology & Mycotechnology: Basic and Applied Aspects of Fungi; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar] [CrossRef]
- Hamid, A.; Zheng, T.; Yu, X. Fungi Contamination of Drinking Water. Rev. Environ. Contam. Toxicol. 2014, 228, 121–139. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, K.; Grant, W.P.; Green, L.E.; Hunter, S.; Jeger, M.J.; Lowe, P.; Medley, G.F.; Mills, P.; Phillipson, J.; Poppy, G.M.; et al. Infectious diseases of animals and plants: An interdisciplinary approach. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011, 366, 1933–1942. [Google Scholar] [CrossRef] [Green Version]
- Held, M.; Lee, A.P.; Edwards, C.; Nicolau, D.V. Microfluidics structures for probing the dynamic behaviour of filamentous fungi. Microelectron. Eng. 2010, 87, 786–789. [Google Scholar] [CrossRef]
- Stanley, C.E.; Grossmann, G.; Casadevall, X.; de-Mello, A.J. Soil-on-a-Chip: Microfluidic platforms for environmental organismal studies. Lab Chip 2016, 16, 228–241. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van Der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Rai, M.; Agarkar, G. Plant–fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428–438. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in microfluidics for environmental analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef]
- Stanley, C.E.; van der Heijden, M.G.A. Microbiome-on-a-Chip: New Frontiers in Plant-Microbiota Research. Trends Microbiol. 2017, 25, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Millet, L.J.; Aufrecht, J.; Labbé, J.; Uehling, J.; Vilgalys, R.; Estes, M.L.; Guennoc, C.M.; Deveau, A.; Olsson, S.; Bonito, G.; et al. Increasing access to microfluidics for studying fungi and other branched biological structures. Fungal Biol. Biotechnol. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Nandagopal, M.S.G.; Yegneswaran, P.P.; Singhal, H.R.; Mani, N.K. Inkjet printing of paraffin on paper allows low-cost point-of-care diagnostics for pathogenic fungi. Cellulose 2020, 27, 7691–7701. [Google Scholar] [CrossRef]
- Burgwyn Fuchs, B.; Eatemadpour, S.; Martel-Foley, J.M.; Stott, S.; Toner, M.; Mylonakis, E. Rapid Isolation and Concentration of Pathogenic Fungi Using Inertial Focusing on a Chip-Based Platform. Front. Cell. Infect. Microbiol. 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Kordzadeh-Kermani, V.; Madadelahi, M.; Ashrafizadeh, S.N.; Kulinsky, L.; Martinez-Chapa, S.O.; Madou, M.J. Electrified lab on disc systems: A comprehensive review on electrokinetic applications. Biosens. Bioelectron. 2022, 214, 114381. [Google Scholar] [CrossRef]
- Ur Rahman, H.; Yue, X.; Yu, Q.; Zhang, W.; Zhang, Q.; Li, P. Current PCR-based methods for the detection of mycotoxigenic fungi in complex food and feed matrices. World Mycotoxin J. 2020, 136, 139–150. [Google Scholar] [CrossRef]
- Karouia, F.; Peyvan, K.; Pohorille, A. Toward biotechnology in space: High-throughput instruments for in situ biological research beyond Earth. Biotechnol. Adv. 2017, 35, 905–932. [Google Scholar] [CrossRef]
- De Middeleer, G.; Leys, N.; Sas, B.; De Saeger, S. Fungi and Mycotoxins in Space—A Review. Astrobiology 2019, 19, 915–926. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Ricco, A.; Squires, D.; Kitts, C.; Agasid, E.; Bramall, N.; Bryson, K.; Chittenden, J.; Conley, C.; Cook, A.; et al. The O/OREOS mission—Astrobiology in low Earth orbit. Acta Astronaut. 2014, 93, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Low, L.A.; Giulianotti, M.A. Tissue Chips in Space: Modeling Human Diseases in Microgravity. Pharm. Res. 2020, 37, 8. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Melnik, D.; Kopp, S.; Sahana, J.; Infanger, M.; Lützenberg, R.; Relja, B.; Wehland, M.; Grimm, D.; Krüger, M. Breast Cancer Cells in Microgravity: New Aspects for Cancer Research. Int. J. Mol. Sci. 2020, 21, 7345. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Melnik, D.; Kopp, S.; Buken, C.; Sahana, J.; Bauer, J.; Wehland, M.; Hemmersbach, R.; Corydon, T.J.; Infanger, M.; et al. Fighting Thyroid Cancer with Microgravity Research. Int. J. Mol. Sci. 2019, 20, 2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W. Spaceflight Promotes Biofilm Formation by Pseudomonas aeruginosa. PLoS ONE 2013, 8, e62437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutte, G.; Monje, O.; Hatfield, R.; Paul, A.; Ferl, R.; Simone, C. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 2006, 224, 1038–1049. [Google Scholar] [CrossRef]
- Sathasivam, M.; Hosamani, R.; Swamy, B.; Kumaran, G.S. Plant responses to real and simulated microgravity. Life Sci. Space Res. 2020, 28, 74–86. [Google Scholar] [CrossRef]
- Lewis, B.; Hanel, R.; Bhattacharya, S.; Ricco, A.J.; Agasid, E.; Reiss-Bubbenheim, D.; Straume, T.; Parra, M.; Boone, T.; Maria, S.S.; et al. BioSentinel: Monitoring DNA Damage Repair Beyond Low Earth Orbit on a 6U Nanosatellite. In Proceedings of the 28th Annual AIAA/USU Conference on Small Satellites, Moffett Field, CA, USA, 18 March 2014; pp. 1–10. [Google Scholar]
- Zea, L. Microbiological Experiments Onboard CubeSats—A Review and Prospects. In Proceedings of the 1st Latin American IAA CubeSat Workshop, Brasília, Brazil, 8–11 December 2014; pp. 1–13. [Google Scholar]
- Bizzarri, M.; Monici, M.; Loon, J. How Microgravity Affects the Biology of Living Systems. BioMed Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Guo, S.; Li, B.-B.; Jiang, N.; Li, A.; Yan, H.-F.; Yang, H.-M.; Zhou, J.-L.; Li, C.-L.; Cui, Y. Effect of Weightlessness on the 3D Structure Formation and Physiologic Function of Human Cancer Cells. BioMed Res. Int. 2019, 2019, 4894083. [Google Scholar] [CrossRef]
- Mylabathula, P.L.; Li, L.; Bigley, A.B.; Markofski, M.M.; Crucian, B.E.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Rezvani, K.; Simpson, R.J. Simulated microgravity disarms human NK-cells and inhibits anti-tumor cytotoxicity in vitro. Acta Astronaut. 2020, 174, 32–40. [Google Scholar] [CrossRef]
- Krüger, M.; Kopp, S.; Wehland, M.; Bauer, J.; Baatout, S.; Moreels, M.; Egli, M.; Corydon, T.J.; Infanger, M.; Grimm, D. Growing blood vessels in space: Preparation studies of the SPHEROIDS project using related ground-based studies. Acta Astronaut. 2019, 159, 267–272. [Google Scholar] [CrossRef]
- Scott, T.J.; Vonortas, N.S. Microgravity protein crystallization for drug development: A bold example of public sector entrepreneurship. J. Technol. Transf. 2019, 46, 1442–1461. [Google Scholar] [CrossRef]
- Poghosyan, A.; Golkar, A. CubeSat evolution: Analyzing CubeSat capabilities for conducting science missions. Prog. Aerosp. Sci. 2017, 88, 59–83. [Google Scholar] [CrossRef]
- Zhu, T.; Moussa, E.M.; Witting, M.; Zhou, D.; Sinha, K.; Hirth, M.; Gastens, M.; Shang, S.; Nere, N.; Somashekar, S.C.; et al. Predictive models of lyophilization process for development, scale-up/tech transfer and manufacturing. Eur. J. Pharm. Biopharm. 2018, 128, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Quan, G.B.; Liu, X.Z.; Ma, E.P.; Liu, A.; Jin, P.; Cao, W. Improved preservation of human red blood cells by lyophilization. Cryobiology 2005, 51, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species; CRC Press: Washington, WA, USA, 2011. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; Gams, W., Ed.; IHW-Verlag: Freising, Germany, 2007; p. 672. [Google Scholar] [CrossRef]
- Hongbin, Y.; Guangya, Z.; Siong, C.F.; Shouhua, W.; Feiwen, L. Novel polydimethylsiloxane (PDMS) based microchannel fabrication method for lab-on-a-chip application. Sens. Actuators B 2009, 137, 754–761. [Google Scholar] [CrossRef]
- Regehr, K.J.; Domenech, M.; Koepsel, J.T.; Carver, K.C.; Ellison-Zelski, S.J.; Murphy, W.L.; Schuler, L.A.; Alarid, E.T.; Beebe, D.J. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip 2009, 9, 2132–2139. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podwin, A.; Kubicki, W.; Dziuban, J. Study of the behavior of Euglena viridis, Euglena gracilis and Lepadella patella cultured in all-glass microaquarium. Biomed. Microdevices 2017, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Podwin, A.; Janisz, T.; Patejuk, K.; Szyszka, P.; Walczak, R.; Dziuban, J. Towards microfluidics for mycology—Material and technological studies on LOCs as new tools ensuring investigation of microscopic fungi and soil organisms. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, e136212. [Google Scholar] [CrossRef]
- Schott Products Catalog. Available online: https://www.schott.com/english/index.html (accessed on 2 January 2022).
- Przystupski, D.; Górska, A.; Michel, O.; Podwin, A.; Sniadek, P.; Łapczynski, R.; Saczko, J.; Kulbacka, J. Testing Lab-on-a-Chip Technology for Culturing Human Melanoma Cells under Simulated Microgravity. Cancers 2021, 13, 402. [Google Scholar] [CrossRef]
- Garstecki, P.; Ziolkowski, J.; Kanp, P. Microfluidischer Chip with an Unvented Gas Cavity in a Microfluidic Chip. Patent No. WO 2019/185885 A1, 3 October 2019. [Google Scholar]
- Brennan, M.D.; Rexius-Hall, M.L.; Elgass, L.J.; Eddington, D.T. Oxygen control with microfluidics. Lab Chip 2014, 14, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Stybayeva, G.; Revzin, A. Fabrication of composite microfluidic devices for local control of oxygen tension in cell cultures. Lab Chip 2018, 19, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Śniadek, P.; Kubicki, W.; Podwin, A. Mikroprzepływowe Laboratorium Hodowlane. Patent No. 437619, 20 April 2021. [Google Scholar]
- Dartoomi, H.; Khatibi, M.; Ashrafizadeh, S.N. Importance of nanochannels shape on blue energy generation in soft nanochannels. Electrochim. Acta 2022, 431, 141175. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Khatibi, M.; Ashrafizadeh, S.N.; Mondal, P.K. Blue energy generation by the temperature-dependent properties in funnel-shaped soft nanochannels. Phys. Chem. Chem. Phys. 2022, 24, 20303–20317. [Google Scholar] [CrossRef]
- Grzebyk, T.; Szmajda, T.; Szyszka, P.; Górecka-Drzazga, A.; Dziuban, J. Glow-discharge ion source for MEMS mass spectrometer. Vacuum 2020, 171, 109008. [Google Scholar] [CrossRef]
- Hoshyargar, V.; Khorami, A.; Ashrafizadeh, S.N.; Sadeghi, A. Solute dispersion by electroosmotic flow through soft microchannels. Sens. Actuators B Chem. 2018, 255, 3585–3600. [Google Scholar] [CrossRef]
- Hoshyargar, V.; Talebi, M.; Ashrafizadeh, S.N.; Sadeghi, A. Hydrodynamic dispersion by electroosmotic flow of viscoelastic fluids within a slit microchannel. Microfluid. Nanofluidics 2018, 22, 4. [Google Scholar] [CrossRef]
- Hammond, T.; Hammond, J. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol.-Ren. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A. Organotypic 3D cell culture models: Using the rotating wall vessel to study host–pathogen interactions. Nat. Rev. 2010, 8, 791–801. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Roberts, M.; Castro, S.; Oubre, C.; Makimura, K.; Leys, N.; Grohmann, E.; Sugita, T.; Ichijo, T.; Nasu, M. Microbial Monitoring of Crewed Habitats in Space—Current Status and Future Perspectives. Microbes Environ. 2014, 29, 250–260. [Google Scholar] [CrossRef]

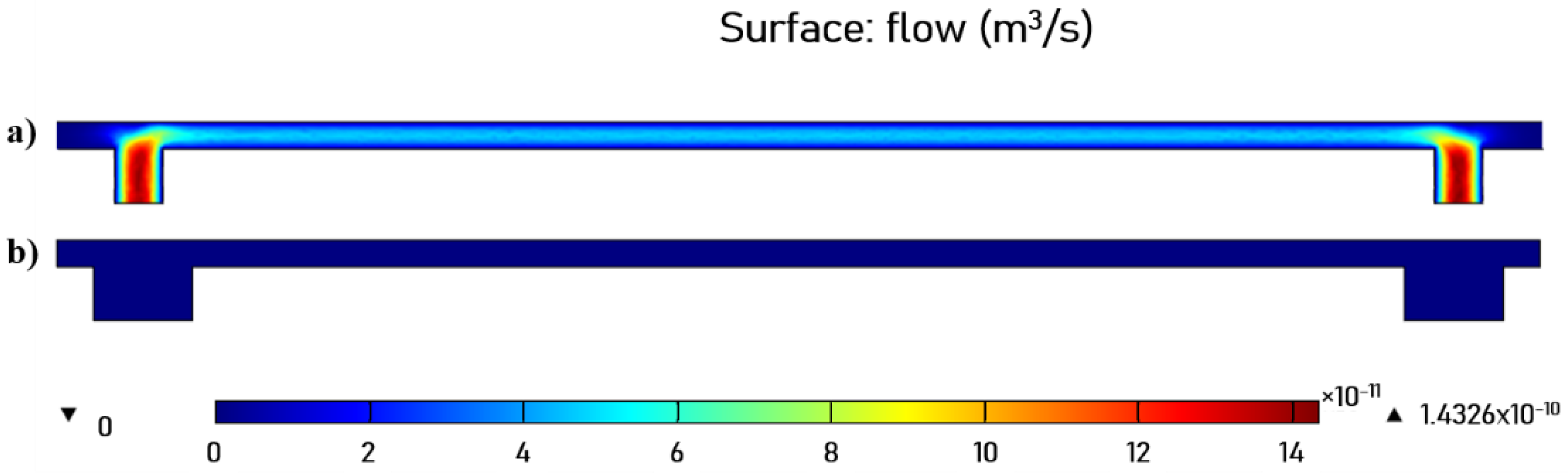

| Number of Mesh Elements | Flow in Liquid Channel [m3/s] | Flow in Gas Channel [m3/s] |
|---|---|---|
| Coarse—304757 | 4.89441 × 10−9 | 1.56306 × 10−13 |
| Normal—626127 | 4.85098 × 10−9 | 1.55514 × 10−13 |
| Fine—1332789 | 4.98822 × 10−9 | 1.57639 × 10−13 |
| LOC—RWV | [%] of Coverage | LOC—no RWV | [%] of Coverage | Comments | |
|---|---|---|---|---|---|
| Day 0 | 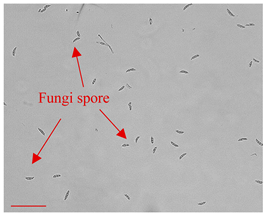 | 5 | 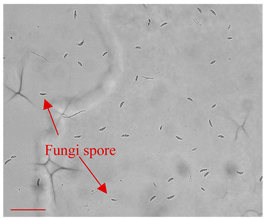 | 5 | Dried fungi spores introduced to the LOC. |
| Day 3 | 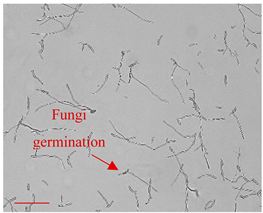 | 20 |  | 15 | The beginning of the fungi germination process. |
| Day 7 | 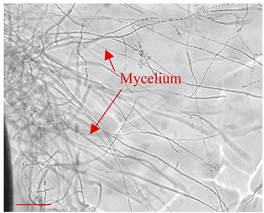 | 45 | 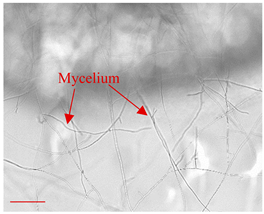 | 20 | Expansion of the fungus hyphae. |
| Day 14 | 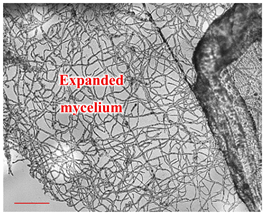 | 90 | 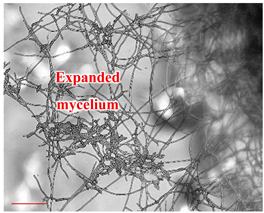 | 54 | Formation of the mycelium. |
| LOC Platform | [%] of Coverage | Petri Dish (Control) | [%] of Coverage | Comments | |
|---|---|---|---|---|---|
| Day 0 | 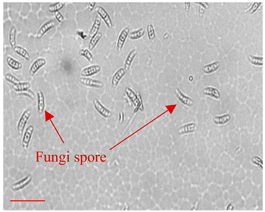 | 5 | 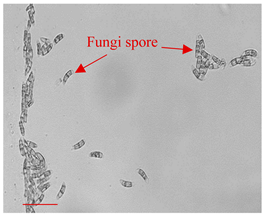 | 5 | Dried fungi spores of appropriate shape and size introduced to the LOC. |
| Day 3 | 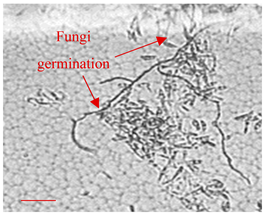 | 20 | 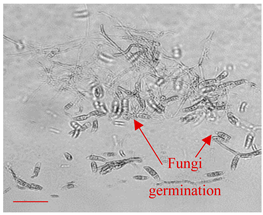 | 15 | A start of fungi germination process (time consistent with literature data [49]). |
| Day 7 | 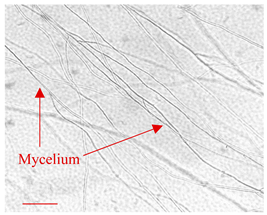 | 30 | 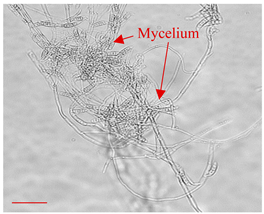 | 27 | Mycelium of fungi under development. Slightly better growth on-chip than in Petri dish. |
| Day 14 | 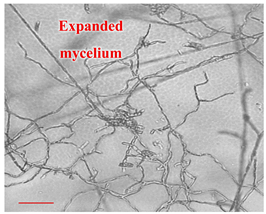 | 45 | 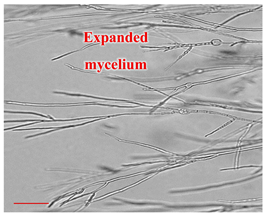 | 38 | Appropriate mycelium growth. Long and branched structures of fungal hyphae visible. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakos, A.; Śniadek, P.; Jurga, M.; Białas, M.; Kaczmarek-Pieńczewska, A.; Matkowski, K.; Walczak, R.; Dziuban, J. Lab-on-Chip Culturing System for Fungi—Towards Nanosatellite Missions. Appl. Sci. 2022, 12, 10627. https://doi.org/10.3390/app122010627
Krakos A, Śniadek P, Jurga M, Białas M, Kaczmarek-Pieńczewska A, Matkowski K, Walczak R, Dziuban J. Lab-on-Chip Culturing System for Fungi—Towards Nanosatellite Missions. Applied Sciences. 2022; 12(20):10627. https://doi.org/10.3390/app122010627
Chicago/Turabian StyleKrakos (Podwin), Agnieszka, Patrycja Śniadek, Marta Jurga, Marcin Białas, Agata Kaczmarek-Pieńczewska, Krzysztof Matkowski, Rafał Walczak, and Jan Dziuban. 2022. "Lab-on-Chip Culturing System for Fungi—Towards Nanosatellite Missions" Applied Sciences 12, no. 20: 10627. https://doi.org/10.3390/app122010627
APA StyleKrakos, A., Śniadek, P., Jurga, M., Białas, M., Kaczmarek-Pieńczewska, A., Matkowski, K., Walczak, R., & Dziuban, J. (2022). Lab-on-Chip Culturing System for Fungi—Towards Nanosatellite Missions. Applied Sciences, 12(20), 10627. https://doi.org/10.3390/app122010627






