Abstract
Previous studies investigating cardiovascular indicators in people with deficit schizophrenia (D-SCZ) have provided mixed findings. None of these studies controlled for the effects of lifestyle characteristics. We aimed to compare cardiometabolic parameters between patients with deficit schizophrenia (D-SCZ), those with nondeficit schizophrenia (ND-SCZ) and healthy control subjects (HCs) while taking into consideration lifestyle characteristics. A total of 168 participants were assessed. The following parameters were determined from serum samples: total cholesterol, low- and high-density lipoproteins, triglycerides, insulin resistance index (HOMA-IR) and C-reactive protein (CRP). The levels of CRP, but not other parameters, were significantly higher in patients with D-SCZ compared with those with ND-SCZ and HCs after adjustment for body mass index, adherence to Mediterranean diet, level of physical activity, nicotine dependence and dosage of antipsychotics. Higher levels of CRP were associated with lower attention in patients with schizophrenia as well as lower immediate memory and global cognition in healthy controls. Moreover, a significant positive relationship between the concentration of CRP and severity of negative symptoms was observed. These findings suggest that individuals with D-SCZ show subclinical inflammation that cannot be simply explained by an unhealthy lifestyle. Elevated CRP levels might be associated with cognitive impairment in schizophrenia.
1. Introduction
There is a general consensus that somatic comorbidity largely accounts for reduced life expectancy in people with schizophrenia [1]. It has been demonstrated that cardiovascular diseases are among the leading causes of mortality in this clinical population [2]. Studies show that about 30% of individuals with schizophrenia develop metabolic syndrome, which is a clustering of visceral obesity, atherogenic dyslipidemia, insulin resistance and hypertension [3]. To date, a variety of factors contributing to this phenomenon have been identified. These mainly include lifestyle factors (unhealthy dietary patterns, low physical activity and cigarette smoking) and the effects of psychopharmacological treatment [4]. However, there is robust evidence that subclinical metabolic abnormalities, such as lipid profile disturbances [5], impaired glucose homeostasis [6] and subclinical inflammation, occur at the onset of psychosis [7,8]. This suggests that other mechanisms, intrinsic to the pathophysiology of psychosis, might impact cardiovascular risk among people with schizophrenia [9].
Accumulating evidence indicates high heterogeneity of schizophrenia in terms of its clinical manifestation and outcomes. In 1988, Carpenter et al. proposed to dissect the deficit schizophrenia subtype (D-SCZ), which manifests as primary and enduring negative symptoms [10]. Subsequently, it has been observed that patients with D-SCZ show unfavorable clinical and functional outcomes, which are likely attributable to greater cognitive deficits as well as neurostructural and neurofunctional alterations [11,12,13]. Some studies indicate that patients with D-SCZ might be at higher cardiovascular risk. For instance, Arango et al. [14] found that patients with D-SCZ are at greater risk of fatal outcomes during coronary heart disease and that they are more likely to develop obesity than those with nondeficit schizophrenia (ND-SCZ). In turn, another study performed on a smaller sample observed no significant differences in cardiovascular indicators between individuals with D-SCZ and those with ND-SCZ [15]. There are also studies showing elevated concentrations of inflammatory cytokines and C-reactive protein (CRP) in patients with D-SCZ in comparison with patients diagnosed with ND-SCZ and healthy controls [16,17].
The mechanisms underlying the association of elevated cardiovascular risk and D-SCZ remain unknown. Notably, previous studies investigating these associations have not controlled for the effects of several lifestyle characteristics. It has been found that patients with schizophrenia have unhealthy dietary habits that might predispose them to develop obesity, type 2 diabetes and cardiovascular complications [18,19]. Moreover, individuals with schizophrenia show less physical activity and more sedentary behavior [20]. Some studies also indicate that unhealthy dietary habits and low levels of physical activity might be associated with higher levels of negative symptoms [21,22]. Following these considerations, we aimed to compare the lipid profile, glucose homeostasis parameters and CRP levels between patients with D-SCZ, patients with ND-SCZ and healthy controls after adjustment for the effects of lifestyle characteristics. Furthermore, we tested the hypothesis that cardiovascular alterations are related to psychopathological manifestations and cognitive performance in patients with schizophrenia.
2. Materials and Methods
2.1. Recruitment Procedures
We recruited 118 individuals with schizophrenia and 60 healthy controls as the convenience sample. Among them, 42 participants met the criteria for D-SCZ, while 76 individuals had a diagnosis of ND-SCZ. The inclusion criteria for the group of individuals with schizophrenia were: (1) age: 18–65 years; (2) a diagnosis of schizophrenia based on the DSM-IV criteria and that was confirmed using the Operational Criteria for Psychotic Illness (OPCRIT) checklist [23]; (3) constant antipsychotic treatment during ≥6 preceding months and (4) remission of psychotic and disorganization symptoms over the preceding 6 months, as determined using the Positive and Negative Syndrome Scale (PANSS) [24]. The DSM-IV criteria were used due to unavailability of standardized tools validated for the Polish population to confirm a diagnosis according to the DSM-V criteria. Patients were excluded if they met any of the following criteria: (1) age < 18 or >65 years; (2) comorbid substance dependence (except for nicotine and caffeine dependence); (3) a history of intellectual disability; (4) unstable physical health impairments and (5) current clinical symptoms of infectious disease. The daily dosage of antipsychotics is presented as chlorpromazine equivalents (CPZeq).
Healthy controls had no first- or second-degree relatives with psychotic or mood disorders. All of them were screened using the Mini-International Neuropsychiatric Interview (M.I.N.I.) [25].
2.2. Clinical Assessment
The scales used to assess clinical manifestations were: (1) PANSS [26]; (2) the Social and Occupational Functioning Assessment Scale [27] and (3) the Calgary Depression Scale for Schizophrenia [28].
The Schedule for the Deficit Schizophrenia (SDS) was used to diagnose D-SCZ [29]. On the basis of the SDS, D-SCZ can be diagnosed in the presence of at least two primary, enduring and moderately severe negative symptoms. The SDS is the gold standard instrument to diagnose D-SCZ and has good inter-rater reliability [30].
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess cognition [31]. The RBANS is composed of 12 tasks that assess 5 domains of cognitive performance.
2.3. Lifestyle Characteristics
The Food Frequency Questionnaire 6 (FFQ-6) was used to assess dietary habits over the preceding 12 months [32]. In the present study, dietary habits were conceptualized as the level of adherence to the Mediterranean diet according to the aMED score (for a detailed description of scoring procedures, see [33,34,35]). The aMED score ranges from 0 to 8. Greater scores represent better adherence.
The Polish version of the International Physical Activity Questionnaire Short-Form (IPAQ-SF) was administered to analyze physical activity [36,37]. The IPAQ-SF records vigorous, moderate and walking physical activity. The amount of physical activity is calculated in the metabolic equivalent task (MET) minutes per week. Specifically, it is calculated by multiplying the reported minutes per week by the MET energy expenditure estimate assigned to the specific subtype of physical activity (8.0 METs for vigorous physical activity, 4.0 METs for moderate physical activity and 3.3 METs for walking) [38].
The use of nicotine was evaluated using the Fagerstrom Test for Nicotine Dependence (FTND) [39]. This is a standard questionnaire that was developed to record physical addiction to nicotine (the total score ranges from 0 to 10; higher scores indicate greater physical addiction to nicotine).
2.4. The Analysis of Metabolic Parameters in Serum Samples
Fasting blood samples were obtained in the morning (7:00–9:00 a.m.). Biochemical parameters determined from serum samples included: (1) glucose; (2) insulin; (3) total cholesterol; (4) low-density lipoproteins (LDL); (5) high-density lipoproteins (HDL); (6) triglycerides and (7) high-sensitivity CRP. An immunoturbidometric assay was used to determine the level of high-sensitivity CRP (Cobas8000c/701, Roche). Colorimetric methods were applied to analyze the concentrations of total cholesterol, lipoproteins and triglycerides. In turn, the electrochemiluminescence assay and spectrophotometric methods were used to assess the concentrations of insulin and glucose, respectively. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated as follows: insulin × glucose (mg/dL)/405.
2.5. Statistics
Differences in the distribution of categorical data were evaluated using χ2 tests. Either the Kruskal–Wallis test or one-way analysis of variance (ANOVA), depending on the distribution of data (examined by the Kolmogorov–Smirnov test), was implemented to compare individuals with D-SCZ-D, ND-SCZ and healthy controls in terms of continuous variables. The Games–Howell test and Bonferroni correction were used as post hoc tests for the results of one-way ANOVA and the Kruskal–Wallis test, respectively. Next, significant bivariate differences in biochemical parameters were evaluated using the analysis of co-variance (ANCOVA). The Spearman rank correlation coefficients were calculated to analyze correlations of biochemical parameters. Subsequently, significant bivariate correlations were assessed using linear regression analyses. Before conducting ANCOVA and linear regression analyses, dependent variables were naturally log-transformed in cases where the data distribution appeared to be non-normal. Covariates were selected from variables with significant between-group differences. Analyses of biochemical parameters were performed after excluding individuals with CRP levels greater than 10 mg/L, as this might indicate clinically relevant inflammation. Results were interpreted as significant if the p-value was <0.05. Data analyses were conducted in the SPSS software, version 28.
3. Results
The groups of participants did not significantly differ in terms of age or sex (Table 1). Individuals with D-SCZ had significantly higher body mass index (BMI) in comparison with healthy controls. The aMED score and level of total physical activity were significantly lower in patients with D-SCZ in comparison with healthy controls. The FTND score was significantly higher in both subgroups of individuals with schizophrenia in comparison with healthy controls. As expected, the level of negative symptoms and impairment in social functioning were significantly higher in subjects with D-SCZ than in patients with ND-SCZ. The CPZeq was significantly higher in patients with D-SCZ than in those with ND-SCZ. Specific subgroups of participants significantly differed with respect to all RBANS scores.

Table 1.
Sample characteristics.
Distinct subgroups of participants significantly differed with respect to HOMA-IR as well as CRP, HDL and triglyceride levels in the unadjusted analyses (Table 2). Both groups of patients (D-SCZ and ND-SCZ) had significantly higher HOMA-IR and triglyceride levels in comparison with healthy controls. Similarly, HDL levels were significantly lower in both groups of patients in comparison with healthy controls. However, there were no significant differences between patients with D-SCZ and those with ND-SCZ with respect to HOMA-IR or the levels of triglycerides and HDL. Importantly, CRP levels were significantly increased in patients with D-SCZ in comparison with those with ND-SCZ and healthy controls. The differences in CRP levels between patients with ND-SCZ and healthy controls were not significant.

Table 2.
Between-group differences in biochemical parameters.
Due to significant between-group differences in BMI, FTND score, aMED score, total physical activity and CPZeq, these variables were further added as covariates to the ANCOVA when testing for differences in specific metabolic parameters. The ANCOVA revealed a significant effect of group (D-SCZ vs. ND-SCZ vs. healthy controls) on the levels of CRP, but not on HOMA-IR or triglyceride and HDL levels (Table 3).

Table 3.
Results of the analysis of covariance (ANCOVA) testing for between-group differences in biochemical parameters after adjustment for potential confounders.
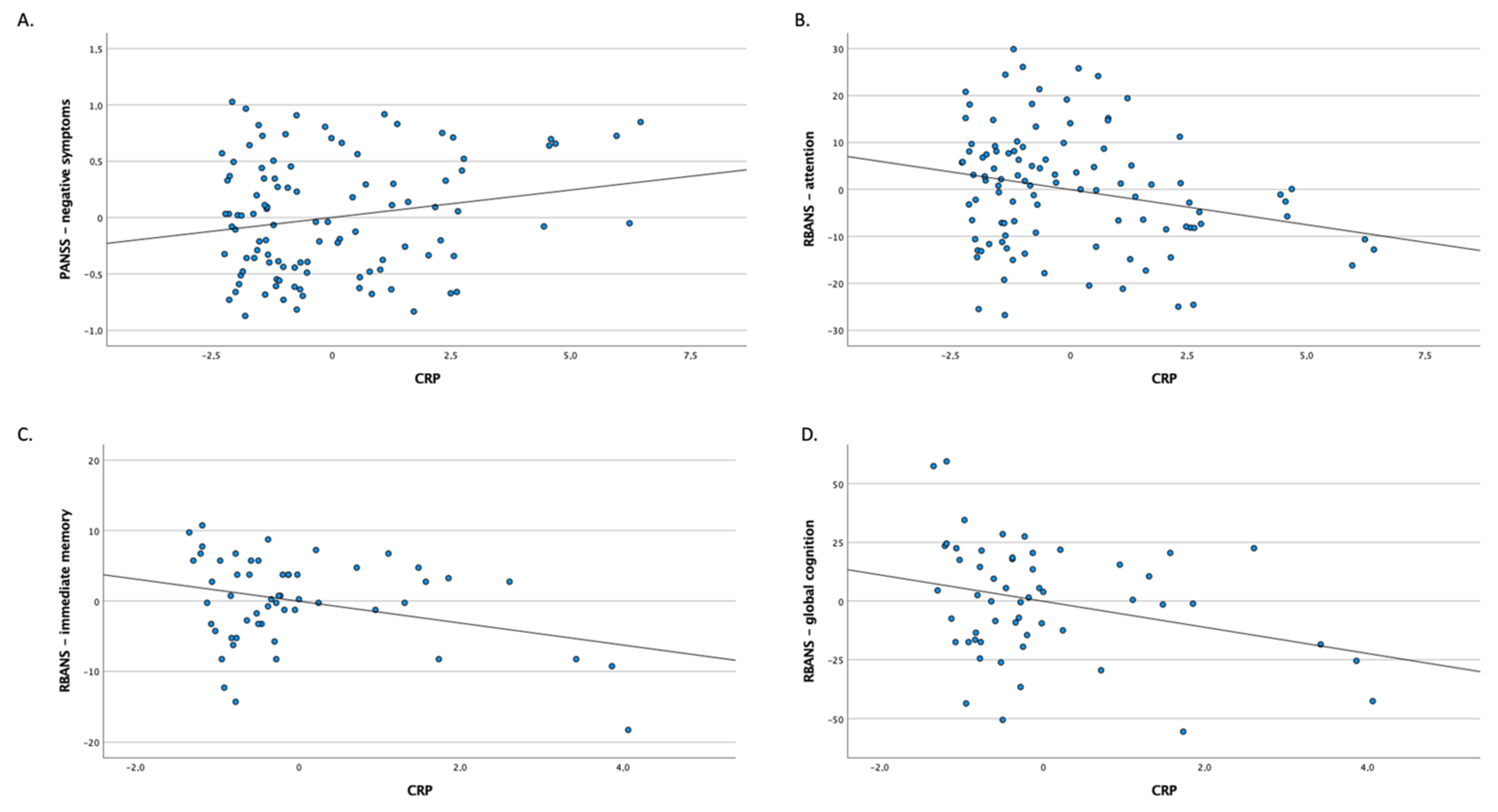
The bivariate correlations of CRP levels with clinical characteristics are presented in Table 4 and Figure 1. Higher CRP levels were significantly correlated with greater severity of negative symptoms and lower RBANS scores of attention in patients with schizophrenia in adjusted and unadjusted analyses. In healthy controls, higher CRP levels were significantly associated with lower RBANS scores of immediate memory and global cognition.

Table 4.
Bivariate correlations of CRP levels with clinical manifestation and cognitive performance.
Figure 1.
Partial regression plots showing correlation of CRP levels in the whole sample of patients with schizophrenia (A,B) and healthy controls (C,D).
4. Discussion
Our observations from the present study indicate that patients with D-SCZ, as opposed to those with ND-SCZ, show elevated levels of CRP, even after adjustment for lifestyle characteristics. Importantly, no significant differences between subgroups of patients and healthy controls were found with respect to lipid profile (the levels of HDL and triglycerides) or glucose metabolism alterations (HOMA-IR) after controlling for BMI, lifestyle characteristics and dosage of antipsychotics. These observations suggest that metabolic disturbances in patients with D-SCZ can be attributed to environmental exposures and lifestyle. Moreover, higher CRP levels were correlated with worse attention and higher levels of negative symptoms in patients with schizophrenia. In healthy controls, higher concentrations of CRP were correlated with lower scores of immediate memory and global cognition. There were no significant differences between subgroups of participants in terms of lipid profile or HOMA-IR after adjustment for lifestyle characteristics.
It was observed that subclinical inflammation is associated with greater cardiovascular risk in people with schizophrenia [40]. Importantly, studies investigating immune-inflammatory markers have consistently shown subclinical inflammation in subjects with D-SCZ. For instance, Goldsmith et al. [16] found elevated levels of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) in patients with D-SCZ compared with those with ND-SCZ and healthy controls. Another study demonstrated the association between elevated CRP levels in patients with D-SCZ and lower levels of global cognition [17]. Similarly, Maes et al. [41] found increased concentrations of IL-4, IL-6 and TNF-α in patients with D-SCZ compared with those with ND-SCZ and healthy controls. Recent systematic reviews and meta-analyses also show that subclinical inflammation might be associated with cognitive impairment in schizophrenia [42,43]. Bora et al. [42] found that increased CRP levels correlated with impairments, albeit with small effect size estimates, across all cognitive domains except for fluency. Importantly, the RBANS attention score is calculated from the subscores of two tasks: digit span and digit coding. Each task might not only measure attention, but also working memory and processing speed. Similarly, a more recent study by North et al. [44], which also used the RBANS to measure cognition, revealed that higher concentrations of CRP were associated with lower attention scores and greater volume reductions in brain regions related to attention.
Our findings are similar to those obtained by Arango et al., who found a significantly higher prevalence of obesity and a greater 10-year risk of coronary heart disease in patients with schizophrenia (n = 1452) [14]. Notably, the authors did not observe significant differences in the distribution of dyslipidemia, hyperglycemia or hypertension between patients with D-SCZ and those with ND-SCZ. Although the authors recorded several characteristics of dietary habits, they did not control for their effects in the data analyses. Another study performed on a smaller sample of individuals with schizophrenia (n = 99) did not reveal any significant differences in cardiometabolic risk indicators between patients with D-SCZ and ND-SCZ [15]. However, neither study used the SDS, which is the gold standard instrument to diagnose D-SCZ. Moreover, the authors did not include healthy controls or adjust the findings for the effects of lifestyle characteristics. In both studies, the proxy measure of D-SCZ, based on the PANSS, was used. Although proxy measures of D-SCZ have good sensitivity and specificity, there are some concerns regarding face validity [30]. Moreover, the poor temporal stability of proxy measures was reported at a 1-year follow-up [45].
Although the present study has important strengths (e.g., the inclusion of several lifestyle characteristics and cardiovascular risk indicators), certain limitations need to be considered. First, the sample size was not large, which means false positive and false negative findings could not be excluded. Second, we used the convenience sample of outpatients, and representativeness could therefore not be evaluated. Moreover, we limited adjustment for medication effects to the total dosage of antipsychotics on the day of enrollment and did not analyze other characteristics of pharmacotherapy (e.g., lifetime exposure to antipsychotics, the use of specific antipsychotics or other psychiatric medications). Additionally, recording quality of sleep may increase insights into lifestyle characteristics. Therefore, our results may not be generalizable to the whole population of patients with schizophrenia. Another limitation is that we did not adopt a longitudinal design, and conclusions regarding causality could therefore not be established. Finally, the use of self-reports to record lifestyle characteristics can also be perceived as a limitation due to a risk of recall bias.
In summary, the findings for our study indicate that patients with D-SCZ, as opposed to those with ND-SCZ, might show elevated CRP levels, even when lifestyle characteristics are controlled. However, additional studies are still required to identify the mechanisms of subclinical inflammation in patients with D-SCZ. Moreover, the two groups of patients did not differ in terms of lipid profile or glucose homeostasis. From a clinical perspective, these findings indicate that both groups of patients require monitoring of cardiovascular risk.
Author Contributions
Conceptualization, A.C. and B.M.; methodology, B.M.; formal analysis, B.M.; investigation, A.C., P.P., J.S., A.S., E.T. and Ł.Ł.; data curation, B.M.; writing—original draft preparation, A.C.; writing—review & editing, B.M., P.P. and J.S.; visualization, B.M.; supervision, B.M.; project administration, A.C. and P.P.; funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the OPUS grant awarded by the National Science Centre (grant number: 2018/31/B/NZ5/00527).
Institutional Review Board Statement
The study was carried out according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee at Wroclaw Medical University (Wroclaw, Poland, approval number: 512/2019) and Pomeranian Medical University (Szczecin, Poland, approval number: KB-0012/130/2019). All participants signed written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data obtained in the study are available upon reasonable request sent to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piotrowski, P.; Gondek, T.M.; Królicka-Deręgowska, A.; Misiak, B.; Adamowski, T.; Kiejna, A. Causes of mortality in schizophrenia: An updated review of European studies. Psychiatr. Danub. 2017, 29, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ringen, P.A.; Engh, J.A.; Birkenaes, A.B.; Dieset, I.; Andreassen, O.A. Increased mortality in schizophrenia due to cardiovascular disease—A non-systematic review of epidemiology, possible causes and interventions. Front. Psychiatry 2014, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; de Hert, M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—A systematic review and meta-analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef]
- Misiak, B.; Frydecka, D.; Piotrowski, P.; Kiejna, A. The multidimensional nature of metabolic syndrome in schizophrenia: Lessons from studies of one-carbon metabolism and DNA methylation. Epigenomics 2013, 5, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Stańczykiewicz, B.; Łaczmański, Ł.; Frydecka, D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: A systematic review and meta-analysis. Schizophr. Res. 2017, 190, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; Beck, K.; Gobjila, C.; Donocik, J.G.; Jauhar, S.; Howes, O.D. Impaired glucose homeostasis in first-episode schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Bartoli, F.; Carrà, G.; Stańczykiewicz, B.; Gładka, A.; Frydecka, D.; Samochowiec, J.; Jarosz, K.; Hadryś, T.; Miller, B.J. Immune-inflammatory markers and psychosis risk: A systematic review and meta-analysis. Psychoneuroendocrinology 2021, 127, 105200. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Mizuki, Y.; Sakamoto, S.; Okahisa, Y.; Yada, Y.; Hashimoto, N.; Takaki, M.; Yamada, N. Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus. Int. J. Neuropsychopharmacol. 2021, 24, 367–382. [Google Scholar] [CrossRef]
- Carpenter, W.T.; Heinrichs, D.W.; Wagman, A.M.I. Deficit and nondeficit forms of schizophrenia: The concept. Am. J. Psychiatry 1988, 145, 578–583. [Google Scholar]
- Bora, E.; Binnur Akdede, B.; Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychol. Med. 2017, 47, 2401–2413. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Mucci, A.; Galderisi, S. Primary, Enduring Negative Symptoms: An Update on Research. Schizophr. Bull. 2017, 43, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Bobes, J.; Kirkpatrick, B.; Garcia-Garcia, M.; Rejas, J. Psychopathology, coronary heart disease and metabolic syndrome in schizophrenia spectrum patients with deficit versus non-deficit schizophrenia: Findings from the CLAMORS study. Eur. Neuropsychopharmacol. 2011, 21, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nurjono, M.; Tay, Y.H.; Lee, T.S.; Remington, G. A comparison of cardio-metabolic risk between the deficit and non-deficit subtypes of schizophrenia. Schizophr. Res. 2014, 153, 246–247. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Haroon, E.; Miller, A.H.; Strauss, G.P.; Buckley, P.F.; Miller, B.J. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr. Res. 2018, 199, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Qian, M.; Qu, W.; Tang, Q.; Yan, Y. Serum c-reactive protein in patients with deficit schizophrenia and the relationship with cognitive function. Neuropsychiatr. Dis. Treat. 2020, 16, 2891–2897. [Google Scholar] [CrossRef]
- Peet, M. Diet, diabetes and schizophrenia: Review and hypothesis. Br. J. Psychiatry Suppl. 2004, 47, S102–S105. [Google Scholar] [CrossRef]
- Dipasquale, S.; Pariante, C.M.; Dazzan, P.; Aguglia, E.; McGuire, P.; Mondelli, V. The dietary pattern of patients with schizophrenia: A systematic review. J. Psychiatr. Res. 2013, 47, 197–207. [Google Scholar] [CrossRef]
- Vancampfort, D.; Firth, J.; Schuch, F.B.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.B.; Gaughran, F.; de Hert, M.; et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry 2017, 16, 308–315. [Google Scholar] [CrossRef]
- Hahn, L.A.; Galletly, C.A.; Foley, D.L.; Mackinnon, A.; Watts, G.F.; Castle, D.J.; Waterreus, A.; Morgan, V.A. Inadequate fruit and vegetable intake in people with psychosis. Aust. N. Z. J. Psychiatry 2014, 48, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.S.; Speyer, H.; Nørgaard, H.C.B.; Karlsen, M.; Hjorthøj, C.; Krogh, J.; Mors, O.; Nordentoft, M.; Toft, U. Dietary patterns and physical activity in people with schizophrenia and increased waist circumference. Schizophr. Res. 2018, 199, 109–115. [Google Scholar] [CrossRef] [PubMed]
- McGuffin, P. A Polydiagnostic Application of Operational Criteria in Studies of Psychotic Illness. Arch. Gen. Psychiatry 1991, 48, 764. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Carpenter, W.T.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D. The Mini International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview. J. Clin. Psychiatry 1998, 59, 22. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Smith, G.N.; Ehmann, T.S.; Flynn, S.W.; MacEwan, G.W.; Tee, K.; Kopala, L.C.; Thornton, A.E.; Schenk, C.H.; Honer, W.G. The Assessment of Symptom Severity and Functional Impairment With DSM-IV Axis V. Psychiatr. Serv. 2011, 62, 411–417. [Google Scholar] [CrossRef]
- Addington, D.; Addington, J.; Maticka-Tyndale, E. Specificity of the Calgary Depression Scale for schizophrenics. Schizophr. Res. 1994, 11, 239–244. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Buchanan, R.W.; McKenny, P.D.; Alphs, L.D.; Carpenter, W.T. The schedule for the deficit syndrome: An instrument for research in schizophrenia. Psychiatry Res. 1989, 30, 119–123. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.Ø.; Glenthøj, L.B.; et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of a non-quantitative food frequency questionnaire (62-item FFQ-6) and PCA-driven dietary pattern identification in 13-21-year-old females. Nutrients 2019, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Krusinska, B.; Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Czerwinska, A.; Golota, J.J. Associations of mediterranean diet and a posteriori derived dietary patterns with breast and lung cancer risk: A case-control study. Nutrients 2018, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Czerwinska, A.; Golota, J.J. Adherence to prudent and mediterranean dietary patterns is inversely associated with lung cancer in moderate but not heavy male Polish smokers: A case-control study. Nutrients 2020, 12, 3788. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Bogudzińska, B.; Stańczykiewicz, B.; Piotrowski, P.; Bielawski, T.; Samochowiec, J.; Szczygieł, K.; Plichta, P.; Misiak, B. The Deficit Schizophrenia Subtype Is Associated with Low Adherence to the Mediterranean Diet: Findings from a Case-Control Study. J. Clin. Med. 2022, 11, 568. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Biernat, E.; Stupnicki, R.; Gajewski, A.K. International Physical Activity Questionnaire (IPAQ)—Polish version. Wych. Fiz. Sport 2007, 51, 47–54. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, J.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Pomerleau, C.S.; Majchrzak, M.J.; Pomerleau, O.F. Nicotine dependence and the Fagerstrom Tolerance Questionnaire: A brief review. J. Subst. Abuse 1989, 1, 471–477. [Google Scholar] [CrossRef]
- Dieset, I.; Hope, S.; Ueland, T.; Bjella, T.; Agartz, I.; Melle, I.; Aukrust, P.; Røssberg, J.I.; Andreassen, O.A. Cardiovascular risk factors during second generation antipsychotic treatment are associated with increased C-reactive protein. Schizophr. Res. 2012, 140, 169–174. [Google Scholar] [CrossRef]
- Maes, M.; Sirivichayakul, S.; Matsumoto, A.K.; Maes, A.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.G.; Barbosa, D.S.; Geffard, M.; et al. Increased Levels of Plasma Tumor Necrosis Factor-α Mediate Schizophrenia Symptom Dimensions and Neurocognitive Impairments and Are Inversely Associated with Natural IgM Directed to Malondialdehyde and Paraoxonase 1 Activity. Mol. Neurobiol. 2020, 57, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Bora, E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: A meta-analysis. Psychol. Med. 2019, 49, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Stańczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2018, 192, 16–29. [Google Scholar] [CrossRef] [PubMed]
- North, H.F.; Bruggemann, J.; Cropley, V.; Swaminathan, V.; Sundram, S.; Lenroot, R.; Pereira, A.M.; Zalesky, A.; Bousman, C.; Pantelis, C.; et al. Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Subotnik, K.L.; Nuechterlein, K.H.; Ventura, J.; Green, M.F.; Hwang, S.S. Prediction of the deficit syndrome from initial deficit symptoms in the early course of schizophrenia. Psychiatry Res. 1998, 80, 53–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).