Local Allergic Rhinitis: A Different Rhinitis Endotype? Literature Overview
Abstract
:Featured Application
Abstract
1. Introduction
1.1. Definition of Rhinitis
1.2. Different Types of Chronic Rhinitis and LAR Definition
2. Epidemiological Chronology of LAR
3. Physiopathology of LAR
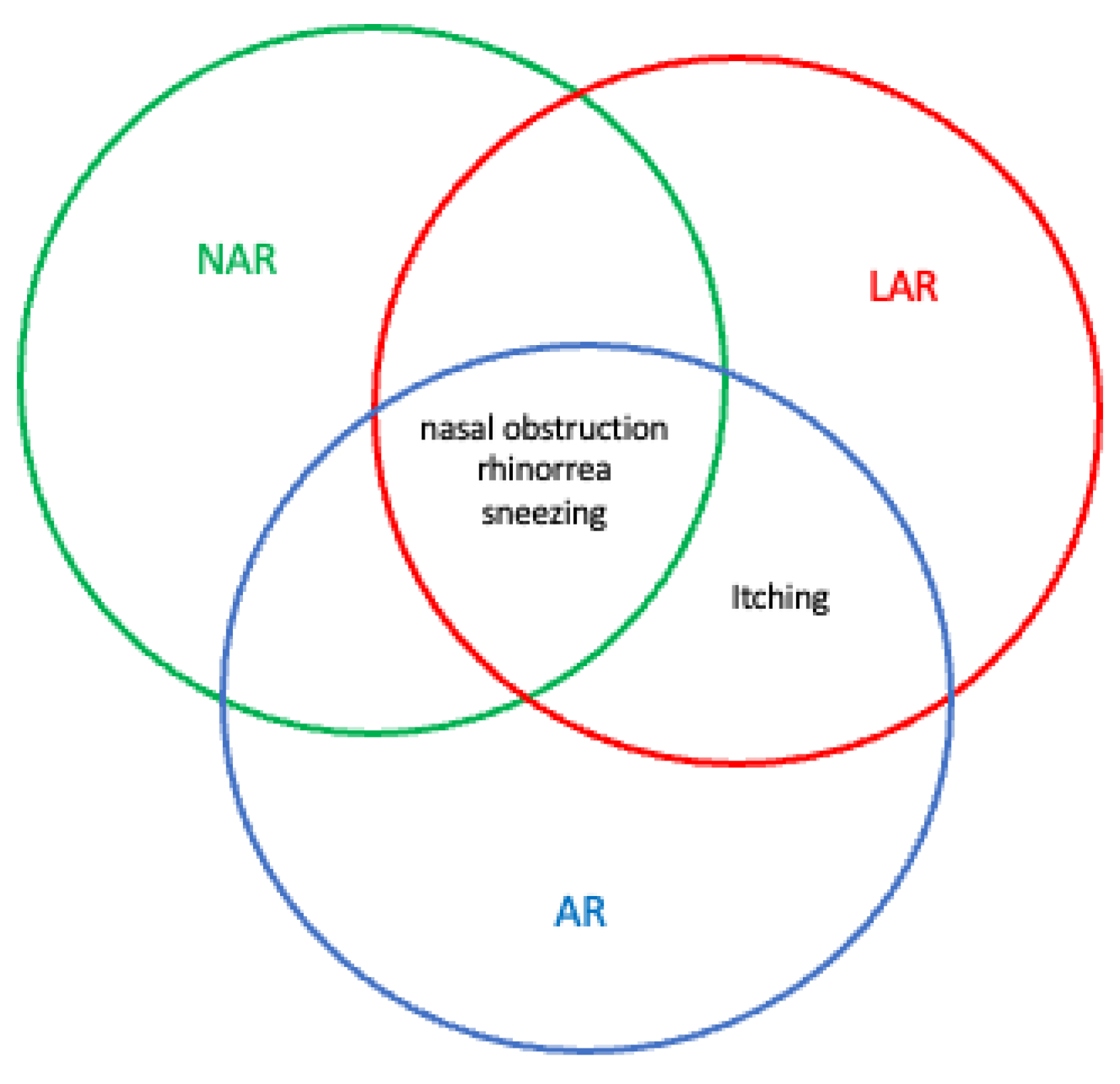
4. Clinical Manifestations of LAR
5. Diagnostic Work Up
6. NAC Technique
7. Clinical Management
8. Rhinitis Endotypes
8.1. LR vs. AR
8.2. LAR vs. NAR
8.3. LAR and Asthma
8.4. LAR and Conjunctivitis
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savouré, M.; Bousquet, J.; Jaakkola, J.J.K.; Jaakkola, M.S.; Jacquemin, B.; Nadif, R. Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution. Clin. Transl. Allergy 2022, 12, e12130. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Guibas, G.V. Rhinitis subtypes, endotypes, and definitions. Immunol. Allergy Clin. N. Am. 2016, 36, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Ariza, A.; Testera-Montes, A.; Rondón, C.; Campo, P. Allergen Immunotherapy for Local Respiratory Allergy. Curr. Allergy Asthma Rep. 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yan, B.; Wang, Y.; Wu, D.; Zhang, L.; Wang, C. Diagnosis and management of nonallergic rhinitis with eosinophilia syndrome using cystatin SN together with symptoms. World Allergy Organ J. 2020, 13, 100134. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Freedman, P.M.; Boswell, R.N. Nonallergic rhinitis with eosinophilia (NARES syndrome). Clinical and immunogic presentation. J. Allergy Clin. Immunol. 1981, 67, 253–262. [Google Scholar] [CrossRef]
- Rondón, C.; Bogas, G.; Barrionuevo, E.; Blanca, M.; Torres, M.J.; Campo, P. Nonallergic rhinitis and lower airway disease. Allergy 2017, 72, 24–34. [Google Scholar] [CrossRef]
- Vardouniotis, A.; Doulaptsi, M.; Aoi, N.; Karatzanis, A.; Kawauchi, H.; Prokopakis, E. Local Allergic Rhinitis Revisited. Curr. Allergy Asthma Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Herrera, R.; Blanca-Lopez, N.; Melendez, L.; Canto, G.; Torres, M.J.; Blanca, M. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. Allergy Clin. Immunol. 2011, 128, 1192–1197. [Google Scholar] [CrossRef]
- Arasi, S.; Pajno, G.B.; Lau, S.; Matricardi, P.M. Local allergic rhinitis: A critical reappraisal from a paediatric perspective. Pediatr. Allergy Immunol. 2016, 27, 569–573. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Pérez-Sánchez, N.; Bogas, G.; Campo, P.; Rondón, C. How to Diagnose and Treat Local Allergic Rhinitis: A Challenge for Clinicians. J. Clin. Med. 2019, 8, 1062. [Google Scholar] [CrossRef]
- Campo, P.; Eguiluz-Gracia, I.; Plaza-Seron, M.C.; Salas, M.; Rodriguez, M.J.; Perez-Sanchez, N.; Gonzalez, M.; Molina, A.; Mayorga, C.; Torres, M.J.; et al. Bronchial asthma triggered by house dust mites in patients with local allergic rhinitis. Allergy 2019, 74, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Campo, P.; Eguiluz-Gracia, I.; Bogas, G.; Salas, M.; Seron, C.P.; Perez, N.; Mayorga, C.; Torres, M.J.; Shamji, M.; Rondon, C. Local allergic rhinitis: Implications for management. Clin. Exp. Allergy 2019, 49, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamana, Y.; Fukuda, K.; Ko, R.; Uchio, E. Local allergic conjunctivitis: A phenotype of allergic conjunctivitis. Int. Ophthalmol. 2019, 39, 2539–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondón, C.; Campo, P.; Galindo, L.; Blanca-López, N.; Cassinello, M.S.; Rodriguez-Bada, J.L.; Torres, M.J.; Blanca, M. Prevalence and clinical relevance of local allergic rhinitis. Allergy 2012, 67, 1282–1288. [Google Scholar] [CrossRef]
- Hamizan, A.W.; Rimmer, J.; Alvarado, R.; Sewell, W.A.; Kalish, L.; Sacks, R.; Harvey, R. Positive allergen reaction in allergic and nonallergic rhinitis: A systematic review. Int. Forum Allergy Rhinol. 2017, 7, 868–877. [Google Scholar] [CrossRef]
- Hamizan, A.W.; Rimmer, J.; Husain, S.; Alvarado, R.; Tatersall, J.; Sewell, W.; Kalish, L.; Harvey, R.J. Local specific immunoglobulin E among patients with nonallergic rhinitis: A systematic review. Rhinology 2019, 57, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Krajewska-Wojtys, A.; Jarzab, J.; Gawlik, R.; Bozek, A. Local allergic rhinitis to pollens is underdiagnosed in young patients. Am. J. Rhinol. Allergy 2016, 30, 198–201. [Google Scholar] [CrossRef]
- Huggins, K.; Brostoff, J. Local production of specific IgE antibodies in allergic—Rhinitis patients with negative skin tests. Lancet 1975, 2, 148–150. [Google Scholar] [CrossRef]
- Samter, M.; Becker, E.L. Ragweed reagins in nasal secretion. Proc. Soc. Exp. Biol. Med. 1947, 65, 140–141. [Google Scholar] [CrossRef]
- Tse, K.S.; Wicher, K.; Arbesman, C.E. IgE antibodies in nasal secretions of ragweed—Allergic subjects. J. Allergy 1970, 46, 352–357. [Google Scholar] [CrossRef]
- Platts-Mills, T.A. Local production of IgG, IgA and IgE antibodies in grass pollen hay fever. J. Immunol. 1979, 22, 2218–2225. [Google Scholar]
- Sensi, L.G.; Piacentini, G.L.; Nobile, E.; Ghebregzabher, M.; Brunori, R.; Zanolla, L.; Boner, A.L.; Marcucci, F. Changes in nasal specific IgE to mites after periods of allergen exposure—Avoidance: A comparison with serum levels. Clin. Exp. Allergy 1994, 24, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan, A.; Vinke, J.G.; Severijnen, L.W.F.M.; Fokkens, W.J. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur. Respir. J. 2000, 15, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Cameron, L.; Hamid, Q.; Wright, E.; Nakamura, Y.; Christodoulopoulos, P.; Muro, S.; Frenkiel, S.; Lavigne, F.; Durham, S.; Gould, H. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J. Allergy Clin. Immunol. 2000, 106, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Jagger, C.; Kleinjan, A.; Carney, A.S.; Jenkins, D.; Jones, N.S. ’Entopy’: Localized mucosal allergic disease in the absence of systemic responses for atopy. Clin. Exp. Allergy 2003, 33, 1374–1379. [Google Scholar] [CrossRef]
- Rondón, C.; Fernández, J.; López, S.; Campo, P.; Doña, I.; Torres, M.J.; Mayorga, C.; Blanca, M. Nasal inflammatory mediators and specific- IgE production after nasal challenge with grass in local allergic rhinitis. J. Allergy Clin. Immunol. 2009, 124, 1005–1011. [Google Scholar] [CrossRef]
- Zhang, N.; Holtappels, G.; Gevaert, P.; Patou, J.; Dhaliwal, B.; Gould, H.; Bachert, C. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy 2011, 66, 141–148. [Google Scholar] [CrossRef]
- Durham, S.R.; Gould, H.; Thienes, C.; Jacobson, M.; Masuyama, K.; Rak, S.; Lowhagen, O.; Schotman, E.; Cameron, L.; Hamid, Q.A. Expression of epsilon germ-line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur. J. Immunol. 1997, 27, 2899–2906. [Google Scholar] [CrossRef]
- Powe, D.G.; Huskisson, R.S.; Carney, A.S.; Jenkins, D.; Jones, N.S. Evidence for an inflammatory pathophysiology in idiopathic rhinitis. Clin. Exp. Allergy 2001, 31, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Kormelink, T.G.; Sisson, M.; Blokhuis, B.J.; Kramer, M.F.; Jones, N.S.; Redegeld, F.A. Evidence for the involvement of free light chain immunoglobulins in allergic and non-allergic rhinitis. J. Allergy Clin. Immunol. 2010, 125, 139–145e1–145e3. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Zambonino, M.A.; Blanca-Lopez, N.; Torres, M.J.; Melendez, L.; Herrera, R.; Guéant-Rodriguez, R.-M.; Guéant, J.-L.; Canto, G.; et al. Follow-up study in local allergic rhinitis shows a consistent entity not evolving to systemic allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 1026–1031. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef]
- Tantilipikorn, P.; Siriboonkoom, P.; Sookrung, N.; Thianboonsong, A.; Suwanwech, T.; Pinkaew, B.; Asanasaen, P. Prevalence of local allergic rhinitis to Dermatophagoides pteronyssinus in chronic rhinitis with negative skin prick test. Asian Pac. J. Allergy Immunol. 2021, 39, 111–116. [Google Scholar] [CrossRef]
- Becker, S.; Rasp, J.; Eder, K.; Berghaus, A.; Kramer, M.; Gröger, M. Non-allergic rhinitis with eosinophilia syndrome is not associated with local production of specific IgE in nasal mucosa. Eur. Arch. Otorhinolaryngol. 2016, 273, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.-Y.; Kim, Y.H. Reversal of Olfactory Disturbance in Allergic Rhinitis Related to OMP Suppression by Intranasal Budesonide Treatment. Allergy Asthma Immunol. Res. 2020, 12, 110–124. [Google Scholar] [CrossRef]
- Altıntoprak, N.; Kar, M.; Muluk, N.B.; Oktemer, T.; Ipci, K.; Birdane, L.; Aricigil, M.; Senturk, M.; Bafaqeeh, S.A.; Cingi, C. Update on local allergic rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 105–109. [Google Scholar] [CrossRef]
- Hellings, P.W.; Klimek, L.; Cingi, C.; Agache, I.; Akdis, C.; Bachert, C.; Bousquet, J.; Demoly, P.; Gevaert, P.; Hox, V.; et al. Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2017, 72, 1657–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguiluz-Gracia, I.; Testera-Montes, A.; Gonzalez-Visiedo, M.; Perez-Sanchez, N.; Ariza-Veguillas, A.; Salas, M.; Moreno-Aguilar, C.; Campo, P.; Torres, M.J.; Rondon, C. Safety and reproducibility of nasal allergen challenge. Allergy 2019, 74, 1125–1134. [Google Scholar] [CrossRef]
- Colavita, L.; Catalano, N.; Sposito, G.; Loddo, S.; Galletti, B.; Salpietro, C.; Galletti, F.; Cuppari, C. Local Allergic Rhinitis in Pediatric Patients: Is IgE Dosage in Nasal Lavage Fluid a Useful Diagnostic Method in Children? Int. J. Mol. Cell. Med. 2017, 6, 174–182. [Google Scholar] [CrossRef]
- Rondón, C.; Romero, J.J.; López, S.; Antúnez, C.; Martín-Casañez, E.; Torres, M.J.; Mayorga, C.; R-Pena, R.; Blanca, M. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J. Allergy Clin. Immunol. 2007, 119, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Doña, I.; Lopez, S.; Campo, P.; Romero, J.J.; Torres, M.J.; Mayorga, C.; Blanca, M. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy 2008, 63, 1352e1358. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Fernandez-Santamaria, R.; Testera-Montes, A.; Ariza, A.; Campo, P.; Prieto, A.; Perez-Sanchez, N.; Salas, M.; Mayorga, C.; Torres, M.J.; et al. Coexistence of nasal reactivity to allergens with and without IgE sensitization in patients with allergic rhinitis. Allergy 2020, 75, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Campo, P.; Rondón, C.; Barrionuevo, E.; Blanca-López, N.; Torres, M.J.; Herrera, R.; Galindo, L.; Mayorga, C.; Blanca, M. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J. Allergy Clin. Immunol. 2013, 132, 975–976.e1–5. [Google Scholar] [CrossRef] [PubMed]
- Campo, P.; Rondón, C.; Gould, H.J.; Barrionuevo, E.; Gevaert, P.; Blanca, M. Local IgE in non-allergic rhinitis. Clin. Exp. Allergy. 2015, 45, 872–881. [Google Scholar] [CrossRef]
- Ferreira, R.D.; Ornelas, C.; Silva, S.; Morgado, R.; Pereira, D.; Escaleira, D.; Moreira, S.; Valença, J.; Pedro, E.; Ferreira, M.B.; et al. Contribution of in vivo and in vitro testing for the diagnosis of local allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 2019, 29, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Nomura, A.; Matsubara, A.; Hisada, T.; Tamada, Y.; Mikami, T.; Ishida, M. Effect of gut microbial composition and diversity on major inhaled allergen sensitization and onset of allergic rhinitis. Allergol. Int. 2022, 15, S1323-8930(22)00074-0. [Google Scholar] [CrossRef]
- Liu, J.; Zhen, Z.; Chen, A.; Guo, C.; Shi, K.; Wang, H.; Xu, K.; Yao, Y.; Wang, H.; Liao, B.; et al. Endoplasmic reticulum stress promotes local immunoglobulin E production in allergic rhinitis. Laryngoscope Investig. Otolaryngol. 2021, 6, 1256–1266. [Google Scholar] [CrossRef]
- Augé, J.; Vent, J.; Agache, I.; Airaksinen, L.; Mozo, P.C.; Chaker, A.; Cingi, C.; Durham, S.; Fokkens, W.; Gevaert, P.; et al. EAACI position paper on the standardization of nasal allergen challenges. Allergy 2018, 73, 1597–1608. [Google Scholar] [CrossRef]
- Wojas, O.; Samoliński, B.K.; Krzych-Fałta, E. Local allergic rhinitis: Nasal allergen provocation testing as a good tool in the differential diagnosis. Int. J. Occup. Med. Environ. Health 2020, 33, 241–246. [Google Scholar] [CrossRef]
- Cantone, E.; Gallo, S.; Torretta, S.; Detoraki, A.; Cavaliere, C.; Di Nola, C.; Spirito, L.; Di Cesare, T.; Settimi, S.; Furno, D.; et al. The Role of Allergen-Specific Immunotherapy in ENT Diseases: A Systematic Review. J. Pers. Med. 2022, 12, 946. [Google Scholar] [CrossRef]
- Bozek, A.; Galuszka, B.; Gawlik, R.; Misiolek, M.; Scierski, W.; Grzanka, A.; Canonica, G.W. Allergen immunotherapy against house dust mites in patients with local allergic rhinitis and asthma. J. Asthm. 2022, 59, 1850–1858. [Google Scholar] [CrossRef]
- Hoang, M.; Samuthpongtorn, J.; Chitsuthipakorn, W.; Seresirikachorn, K.; Snidvongs, K. Allergen-specific immunotherapy for local allergic rhinitis: A systematic review and meta-analysis. Rhinology 2022, 60, 11–19. [Google Scholar] [CrossRef]
- Bozek, A.; Kozłowska, R.; Misiołek, M.; Ścierski, W.; Gawlik, R. Omalizumab added to allergen immunotherapy increased the effect of therapy in patients with severe local allergic rhinitis. Hum. Vaccin. Immunother. 2022, 11, 2097818. [Google Scholar] [CrossRef]
- Bozek, A.; Fiolka, J.Z.; Wojtys, A.K.; Galuszka, B.; Cudak, A. Potential Differences between Local and Systemic Allergic Rhinitis Induced by Birch Pollen. Int. Arch. Allergy Immunol. 2020, 181, 831–838. [Google Scholar] [CrossRef]
- Testera-Montes, A.; Jurado, R.; Salas, M.; Eguiluz-Gracia, I.; Mayorga, C. Diagnostic Tools in Allergic Rhinitis. Front. Allergy 2021, 2, 721851. [Google Scholar] [CrossRef]
- Smurthwaite, L.; Durham, S.R. Local IgE synthesis in allergic rhinitis and asthma. Curr. Allergy Asthma Rep. 2002, 2, 231–238. [Google Scholar] [CrossRef]
- Kato, Y.; Akasaki, S.; Muto-Haenuki, Y.; Fujieda, S.; Matsushita, K.; Yoshimoto, T. Nasal sensitization with ragweed pollen induces local allergic-rhinitis-like symptoms in mice. PLoS ONE 2014, 9, e103540. [Google Scholar] [CrossRef] [Green Version]
- Gelardi, M.; Guglielmi, A.V.; Iannuzzi, L.; Quaranta, V.N.; Quaranta, N.; Landi, M.; Correale, M.; Sonnante, A.; Rossini, M.; Mariggiò, M.A.; et al. Local allergic rhinitis: Entopy or spontaneous response? World Allergy Organ. J. 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckrich, J.; Hinkel, J.; Fischl, A.; Herrmann, E.; Holtappels, G.; Bachert, C.; Zielen, S. Nasal IgE in subjects with allergic and non-allergic rhinitis. World Allergy Organ. J. 2020, 13, 100129. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Kawata, R. Diagnosis and Treatment of Local Allergic Rhinitis. Pathogens 2022, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Eguiluz-Gracia, I.; Yazicioglu, M.; Campo, P. Pediatric Local Allergic Rhinitis. Turk. J. Pediatr. 2020, 62, 701–710. [Google Scholar] [CrossRef] [PubMed]
| Chronic Rhinitis | SPT 1 | Serum IgE | Nasal IgE | NAC 2 | BAT 3 | Reference |
|---|---|---|---|---|---|---|
| AR | + | +(−) | +(−) | + | + | [36,37] |
| NAR | − | − | − | − | − | [36,37] |
| LAR | − | − | +(−) | + | +(−) | [36,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantone, E.; Detoraki, A.; De Corso, E. Local Allergic Rhinitis: A Different Rhinitis Endotype? Literature Overview. Appl. Sci. 2022, 12, 11141. https://doi.org/10.3390/app122111141
Cantone E, Detoraki A, De Corso E. Local Allergic Rhinitis: A Different Rhinitis Endotype? Literature Overview. Applied Sciences. 2022; 12(21):11141. https://doi.org/10.3390/app122111141
Chicago/Turabian StyleCantone, Elena, Aikaterini Detoraki, and Eugenio De Corso. 2022. "Local Allergic Rhinitis: A Different Rhinitis Endotype? Literature Overview" Applied Sciences 12, no. 21: 11141. https://doi.org/10.3390/app122111141






