Mixed-Layer Illite-Smectite Illitization under Supercritical CO2 Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blue Marl as Sealing Rock
2.2. Methods and Characterization Procedures
3. Results
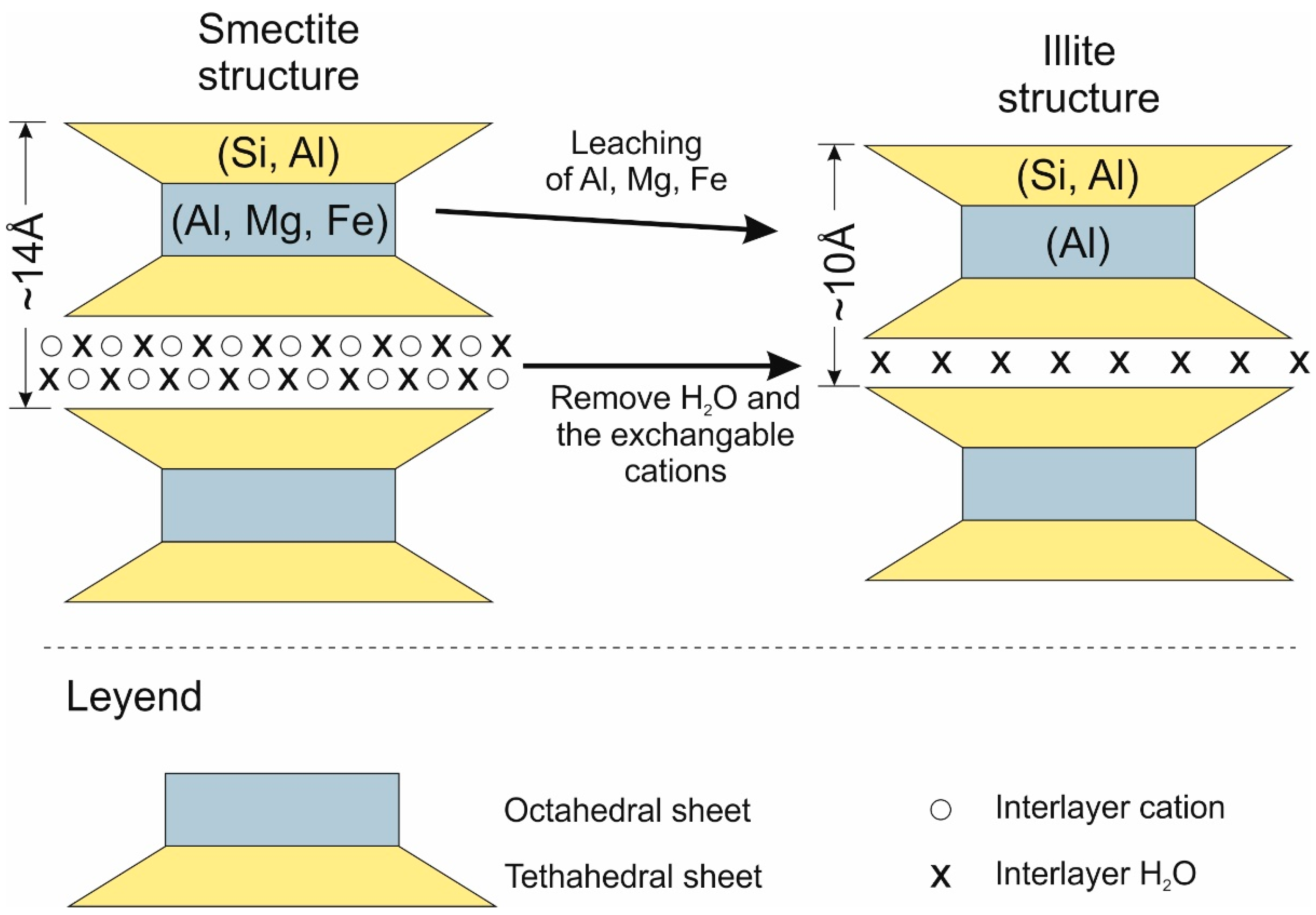
- Leaching of Al, Mg, and Fe from the octahedral and tetrahedral sheets.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nityashree, N.; Price, C.A.H.; Pastor-Perez, L.; Manohara, G.V.; Garcia, S.; Maroto-Valer, M.M.; Reina, T.R. Carbon stabilised saponite supported transition metal-alloy catalysts for chemical CO2 utilisation via reverse water-gas shift reaction. Appl. Catal. B Environ. 2020, 261, 118241. [Google Scholar] [CrossRef]
- Rogelj, J.; Geden, O.; Cowie, A.; Reisinger, A. Three ways to improve net-zero emissions targets. Nature 2021, 591, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, J.; Schaeffer, M.; Meinshausen, M.; Knutti, R.; Alcamo, J.; Riahi, K.; Hare, W. Zero emission targets as long-term global goals for climate protection. Environ. Res. Lett. 2015, 10, 105007. [Google Scholar] [CrossRef]
- United Nations Summary of the Paris Agreement. In Adoption of the Paris Agreement; United Nations Framework Convention on Climate Change (UNFCCC): Paris, France, 2015.
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef] [Green Version]
- Becattini, V.; Gabrielli, P.; Mazzotti, M. Role of carbon capture, storage, and utilization to enable a Net-Zero-CO2-emissions aviation sector. Ind. Eng. Chem. Res. 2021, 60, 6848–6862. [Google Scholar] [CrossRef]
- Ajoma, E.; Saira; Sungkachart, T.; Ge, J.; Le-Hussain, F. Water-saturated CO2 injection to improve oil recovery and CO2 storage. Appl. Energy 2020, 266, 114853. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L.; IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Working Group III; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2005; Volume 2, ISBN 9780521866439. [Google Scholar]
- Busch, A.; Amann, A.; Bertier, P.; Waschbusch, M.; Krooss, B.M. The significance of caprock sealing integrity for CO2 storage. In Proceedings of the SPE International Conference on CO2 Capture, Storage and Utilization, New Orleans, LA, USA, 10–12 November 2010; pp. 300–307. [Google Scholar]
- Fatah, A.; Bennour, Z.; Ben Mahmud, H.; Gholami, R.; Hossain, M.M. A review on the influence of CO2/shale interaction on shale properties: Implications of CCS in shales. Energies 2020, 13, 3200. [Google Scholar] [CrossRef]
- Harvey, O.R.; Qafoku, N.P.; Cantrell, K.J.; Lee, G.; Amonette, J.E.; Brown, C.F. Geochemical implications of gas leakage associated with geologic CO2 storage—A qualitative review. Environ. Sci. Technol. 2013, 47, 23–36. [Google Scholar] [CrossRef]
- Olabode, A.; Radonjic, M. Shale Caprock/Acidic Brine Interaction in Underground CO2 Storage. J. Energy Resour. Technol. 2014, 136, 1–6. [Google Scholar] [CrossRef]
- Scherer, G.W.; Celia, M.A.; Prevost, J.H.; Bachu, S.; Bruant, R.G.; Duguid, A.; Fuller, R.; Gasda, S.E.; Radonjic, M.; Vicjit-Vadakna, W. Leakage of CO2 through abandoned wells: Role of corrosion of cement. In The CO2 Capture and Storage Project (CCP) for Carbon Dioxide Storage in Deep Geologic Formations for Climate Change Mitigation; Benson, S.M., Ed.; Elsevier: London, UK, 2004; pp. 827–848. [Google Scholar]
- Tian, H.; Xu, T.; Zhu, H.; Yang, C.; Ding, F. Heterogeneity in mineral composition and its impact on the sealing capacity of caprock for a CO2 geological storage site. Comput. Geosci. 2019, 125, 30–42. [Google Scholar] [CrossRef]
- Zhang, M.; Bachu, S. Review of integrity of existing wells in relation to CO2 geological storage: What do we know? Int. J. Greenh. Gas Control 2011, 5, 826–840. [Google Scholar] [CrossRef]
- Jia, B.; Chen, Z.; Xian, C. Investigations of CO2 storage capacity and flow behavior in shale formation. J. Pet. Sci. Eng. 2022, 208, 109659. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Johnson, J.W.; Nitao, J.J.; Knauss, K.G. Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms and sequestration partitioning. Geol. Soc. Spec. Publ. 2004, 233, 107–128. [Google Scholar] [CrossRef] [Green Version]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation. Literature Review; Report from the Energy research Centre of the Netherlands; Energy research Centre of the Netherlands: Pettern, The Netherlands, 2003. [Google Scholar]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Santamarina, J.C. CO2 breakthrough—Caprock sealing efficiency and integrity for carbon geological storage. Int. J. Greenh. Gas Control 2017, 66, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Gaus, I.; Azaroual, M.; Czernichowski-Lauriol, I. Reactive transport modelling of the impact of CO2 injection on the clayey cap rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Change in cap rock porosity triggered by pressure and temperature dependent CO2–water–rock interactions in CO2 storage systems. Petroleum 2017, 3, 96–108. [Google Scholar] [CrossRef]
- Tian, H.; Xu, T.; Li, Y.; Yang, Z.; Wang, F. Evolution of sealing efficiency for CO2 geological storage due to mineral alteration within a hydrogeologically heterogeneous caprock. Appl. Geochem. 2015, 63, 380–397. [Google Scholar] [CrossRef]
- Tian, H.; Pan, F.; Xu, T.; McPherson, B.J.; Yue, G.; Mandalaparty, P. Impacts of hydrological heterogeneities on caprock mineral alteration and containment of CO2 in geological storage sites. Int. J. Greenh. Gas Control 2014, 24, 30–42. [Google Scholar] [CrossRef]
- Jia, B.; Xian, C.G. Permeability measurement of the fracture-matrix system with 3D embedded discrete fracture model. Pet. Sci. 2022, 19, 1757–1765. [Google Scholar] [CrossRef]
- Little, M.G.; Jackson, R.B. Potential impacts of leakage from deep CO2 geosequestration on overlying freshwater aquifers. Environ. Sci. Technol. 2010, 44, 9225–9232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Partin, J.W.; Hovorka, S.D.; Wong, C. Potential risks to freshwater resources as a result of leakage from CO2 geological storage: A batch-reaction experiment. Environ. Earth Sci. 2010, 60, 335–348. [Google Scholar] [CrossRef]
- Lyu, Q.; Tan, J.; Li, L.; Ju, Y.; Busch, A.; Wood, D.A.; Ranjith, P.G.; Middleton, R.; Shu, B.; Hu, C.; et al. The role of supercritical carbon dioxide for recovery of shale gas and sequestration in gas shale reservoirs. Energy Environ. Sci. 2021, 14, 4203–4227. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Shen, Z. A feasibility analysis on shale gas exploitation with supercritical carbon dioxide. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1426–1435. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Martín, D.; Aparicio, P.; Galán, E. Mineral carbonation of ceramic brick at low pressure and room temperature. A simulation study for a superficial CO2 store using a common clay as sealing material. Appl. Clay Sci. 2018, 161. [Google Scholar] [CrossRef]
- Martín, D.; Aparicio, P.; Galán, E. Time evolution of the mineral carbonation of ceramic bricks in a simulated pilot plant using a common clay as sealing material at superficial conditions. Appl. Clay Sci. 2019, 180, 5191. [Google Scholar] [CrossRef]
- Galán, E.; Aparicio, P. Experimental study on the role of clays as sealing materials in the geological storage of carbon dioxide. Appl. Clay Sci. 2014, 87, 22–27. [Google Scholar] [CrossRef]
- Bradley, W.F. Molecular associations between montmorillonite and some polyfunctional organic liquids1. J. Am. Chem. Soc. 1945, 67, 975–981. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification, 1st ed.; The Mineralogical Society of Great Britain and Ireland: London, UK, 1980; Volume 5, ISBN 0903056089. [Google Scholar]
- Moore, D.M.; Reynolds Jr, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press (OUP): New York, NY, USA, 1997; ISBN 019505170X. [Google Scholar]
- Środoń, J. Identification and Quantitative Analysis of Clay Minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherland, 2013; Volume 5, pp. 25–49. ISBN 9780080982595. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghi, R.K.; Chapoy, A.; Peirera, L.M.C.; Yang, J.; Tohidi, B. pH of CO2 saturated water and CO2 saturated brines: Experimental measurements and modelling. Int. J. Greenh. Gas Control 2017, 66, 190–203. [Google Scholar] [CrossRef]
- Jeon, P.R.; Kim, D.W.; Lee, C.H. Dissolution and reaction in a CO2-brine-clay mineral particle system under geological CO2 sequestration from subcritical to supercritical conditions. Chem. Eng. J. 2018, 347, 1–11. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Ni, X.; Li, Q.; Chen, W. Dissolution kinetics of Si and Al from montmorillonite in carbonic acid solution. Int. J. Coal Sci. Technol. 2014, 1, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Pentrák, M.; Madejová, J.; Komadel, P. Effect of chemical composition and swelling on acid dissolution of 2: 1 clay minerals. Philos. Mag. 2010, 90, 2387–2397. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mineral carbonation from metal wastes: Effect of solid to liquid ratio on the efficiency and characterization of carbonated products. Appl. Energy 2014, 113, 515–532. [Google Scholar] [CrossRef] [Green Version]
- Steudel, A.; Batenburg, L.F.; Fischer, H.R.; Weidler, P.G.; Emmerich, K. Alteration of swelling clay minerals by acid activation. Appl. Clay Sci. 2009, 44, 105–115. [Google Scholar] [CrossRef]
- Pawar, R.J.; Bromhal, G.S.; Carey, J.W.; Foxall, W.; Korre, A.; Ringrose, P.S.; Tucker, O.; Watson, M.N.; White, J.A. Recent advances in risk assessment and risk management of geologic CO2 storage. Int. J. Greenh. Gas Control 2015, 40, 292–311. [Google Scholar] [CrossRef]
- Glassley, W.E.; Simmons, A.M.; Kercher, J.R. Mineralogical heterogeneity in fractured, porous media and its representation in reactive transport models. Appl. Geochem. 2002, 17, 699–708. [Google Scholar] [CrossRef]
- Lai, P.; Moulton, K.; Krevor, S. Pore-scale heterogeneity in the mineral distribution and reactive surface area of porous rocks. Chem. Geol. 2015, 411, 260–273. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, Y.; Liu, J.; Gao, J.; Ji, Z.; Guo, X.; Liu, J.; Yuan, J. Trash to treasure: Seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination 2017, 407, 85–92. [Google Scholar] [CrossRef]
- Kohler, E.; Parra, T.; Vidal, O. Clayey cap-rock behavior in H2O-CO2 media at low pressure and temperature conditions: An experimental approach. Clays Clay Miner. 2009, 57, 616–637. [Google Scholar] [CrossRef]
- Credoz, A.; Bildstein, O.; Jullien, M.; Raynal, J.; Trotignon, L.; Pokrovsky, O. Mixed-layer illite-smectite reactivity in acidified solutions: Implications for clayey caprock stability in CO2 geological storage. Appl. Clay Sci. 2011, 53, 402–408. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Zhu, C.; Xiao, Y. Coupled reactive flow and transport modeling of CO2 sequestration in the Mt. Simon sandstone formation, Midwest U.S.A. Int. J. Greenh. Gas Control 2011, 5, 294–307. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson, G.K.W.; Law, A.C.K.; Biddle, D.; Golding, S.D. Reactivity of micas and cap-rock in wet supercritical CO2 with SO2 and O2 at CO2 storage conditions. Appl. Geochem. 2016, 72, 59–76. [Google Scholar] [CrossRef] [Green Version]
- Aljamaan, H.; Holmes, R.; Vishal, V.; Haghpanah, R.; Wilcox, J.; Kovscek, A.R. CO2 Storage and Flow Capacity Measurements on Idealized Shales from Dynamic Breakthrough Experiments. Energy Fuels 2017, 31, 1193–1207. [Google Scholar] [CrossRef]
- Rani, S.; Padmanabhan, E.; Bakshi, T.; Prusty, B.K.; Pal, S.K. CO2 sorption and rate characteristics in micropores of shales. J. Nat. Gas Sci. Eng. 2019, 68, 102903. [Google Scholar] [CrossRef]






| Original | 120 h | 240 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals | wt% | ±σ | LOQ | LOD | wt% | ±σ | LOQ | LOD | wt% | ±σ | LOQ | LOD |
| Calcite | 35.4 | 0.9 | 1.2 | 0.6 | 38.0 | 1.4 | 1.9 | 0.9 | 39.3 | 1.8 | 2.4 | 1.2 |
| Dolomite | 3.3 | 0.6 | 0.8 | 0.4 | 5.3 | 0.8 | 1.1 | 0.6 | 6.3 | 0.9 | 1.2 | 0.6 |
| Quartz | 14.0 | 0.5 | 0.6 | 0.3 | 11.8 | 0.6 | 0.8 | 0.4 | 11.5 | 0.6 | 0.8 | 0.4 |
| Plagioclase (albite) | 3.9 | 0.5 | 0.6 | 0.3 | 3.0 | 0.5 | 0.7 | 0.3 | 2.9 | 0.5 | 0.6 | 0.3 |
| Pyrite | 0.9 | 0.2 | 0.3 | 0.2 | 0.4 | 0.5 | 0.7 | 0.3 | 0.6 | 0.5 | 0.7 | 0.3 |
| Siderite | N.D. | -- | -- | -- | 1.1 | 0.6 | 0.8 | 0.4 | 1.1 | 0.4 | 0.5 | 0.2 |
| Illite | 22.3 | 1.6 | 2.1 | 1.0 | 24.6 | 3.0 | 4.0 | 2.0 | 25.2 | 3.9 | 5.2 | 2.6 |
| Kaolinite | 5.2 | 0.8 | 1.1 | 0.6 | 5.4 | 0.8 | 1.1 | 0.6 | 6.0 | 1.0 | 1.3 | 0.7 |
| Smectite | 10.3 | 1.8 | 2.4 | 1.2 | 8.2 | 1.9 | 2.5 | 1.3 | 5.7 | 1.7 | 2.5 | 1.2 |
| I/Sm | 4.5 | 1.0 | 1.3 | 0.6 | 2.2 | 0.6 | 0.8 | 0.4 | 1.4 | 1.1 | 1.5 | 0.7 |
| Element (mg/L) | Original | 120 h | 240 h |
|---|---|---|---|
| Al | <0.002 | ≤0.015 | ≤0.015 |
| Ca | 7.83 ± 0.16 | 46.1 ± 0.4 | 33.2 ± 0.9 |
| Fe | 0.004 ± 0.001 | 0.39 ± 0.07 | 0.67 ± 0.04 |
| K | 21.3 ± 0.9 | 10.1 ± 0.6 | 11.1 ± 0.6 |
| Mg | 21.1 ± 0.3 | 53.1 ± 0.5 | 72.1 ± 0.5 |
| Na | 19.2 ± 0.7 | 38.8 ± 0.5 | 41.5 ± 0.3 |
| Si | 2.50 ± 0.11 | 2.19 ± 0.09 | 3.46 ± 0.09 |
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | |
|---|---|---|---|
| Original | 36.11 | 0.070 | 7.79 |
| 120 h | 37.88 | 0.073 | 7.68 |
| 240 h | 38.10 | 0.077 | 6.99 |
| Original | 120 h | 240 h | ||
|---|---|---|---|---|
| BET Surface Area (m2/g) | 14.5298 | 15.1392 | 15.4480 | |
| Total Volume in Pores (cm3/g) | ≤1.066 nm | 0.00233 | 0.00259 | 0.00287 |
| Area in Pores (m2/g) | >1.066 nm | 8.953 | 8.769 | 8.787 |
| Total Area in Pores (m2/g) | ≥0.367 nm | 16.758 | 17.176 | 18.363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, D.; Aparicio, P.; García, S.; Maroto-Valer, M.M. Mixed-Layer Illite-Smectite Illitization under Supercritical CO2 Conditions. Appl. Sci. 2022, 12, 11477. https://doi.org/10.3390/app122211477
Martín D, Aparicio P, García S, Maroto-Valer MM. Mixed-Layer Illite-Smectite Illitization under Supercritical CO2 Conditions. Applied Sciences. 2022; 12(22):11477. https://doi.org/10.3390/app122211477
Chicago/Turabian StyleMartín, Domingo, Patricia Aparicio, Susana García, and María Mercedes Maroto-Valer. 2022. "Mixed-Layer Illite-Smectite Illitization under Supercritical CO2 Conditions" Applied Sciences 12, no. 22: 11477. https://doi.org/10.3390/app122211477








