Abstract
Boiogito (BO), a Japanese traditional herbal medicine, has been reported to prevent knee osteoarthritis (KOA) development in in vivo studies. In the early stage of KOA, osteoclasts proliferate in the subchondral bone. This study aimed to investigate the preventive effect of BO on osteoclast proliferation, which remains unclear, in a KOA-induced rat model. KOA was induced in 12-week-old male Wistar rats using surgical destabilization of the medial meniscus (DMM). BO was mixed with powdered chow, applying 1%, 3%, and 5% of the total feed, and administered to KOA-induced rats. The rats were divided into 6 groups: control, sham, DMM, DMM + BO 1%, DMM + BO 3%, and DMM + BO 5%. Rotarod tests were performed each week to assess the locomotor function, and the right knees were harvested 28 days after surgery for histological analysis. Oral administration of BO significantly inhibited the decrease in the latency to fall off in the rotarod test, which was aggravated in the DMM group. Furthermore, KOA development was significantly prevented in the BO-administrated groups as assessed by the Osteoarthritis Research Society International score. The number of multinucleated activated osteoclasts in the subchondral bone was decreased in the BO-treated groups, which was increased in the DMM group. Therefore, oral administration of BO may reduce articular cartilage degeneration, osteoclast differentiation and proliferation in the KOA patients.
1. Introduction
Osteoarthritis (KOA) is a chronic joint disease that presents with swelling, pain, restrained range of motion, and gait disturbance due to deformity of the joint structure and articular inflammation [1]. The knee joint is composed of articular cartilage, subchondral bone, meniscus, anterior/posterior cruciate ligaments, and synovium. Synovitis, osteosclerosis in the subchondral bone, osteophyte formation, or chondrocyte degeneration, are involved in KOA development and progression [2]. KOA is more common in people over the age of 40, with approximately 300 million patients with KOA worldwide [3], and the number of patients is still increasing [4]. The economic burden of KOA in the U.S. is estimated to be as much as $34 billion annually in healthcare expenditures [5], and there is a need to change treatment strategies for KOA from an economic perspective and to extend healthy life expectancy.
The current treatment strategies for KOA can be categorized into conservative treatment and surgical treatment [3]. Conservative treatment, including locomotor exercise, medication, and orthotics, is used to reduce pain and functional disability associated with KOA [6], while surgical treatment, including periprosthetic osteotomy and arthroplasty, is used for patients who are refractory to conservative treatment, as severe KOA causes significant impairment in daily living activities. The postoperative clinical outcomes of the surgical treatment are good, but the number of surgeries increases every year, and if this number continues to increase, not only will the medical economic burden increases, but also will the number of revision surgeries due to implant loosening and infection [7]. Thus, it is very important to develop therapeutic strategies in conservative treatment. According to the Osteoarthritis Research Society International (OARSI) treatment guidelines published in 2019 [6], exercise therapy has proved to be effective in improving KOA-related symptoms, and mind-body exercises to improve lower limb-trunk balance, such as yoga and Tai Chi, were newly introduced with a high recommendation. As for drug therapy, non-steroidal anti-inflammatory drugs and corticosteroids have been described; they are not effective for long-term treatment, and they also may increase the catabolic enzyme for articular cartilage from synovial cells [8].
Recently, several researchers have focused on the use of herbal medicine as a therapeutic strategy for inhibiting KOA progression [9]. Curcumin, a substance in turmeric, is expected to activate chondrocytes, increase cartilage matrix production, and promote cartilage tissue repair [10]. In animal experiments, curcumin has been shown to inhibit KOA progression in KOA-induced mouse models [11]. Oleanolic acid contained in olive oil has been suggested to promote autophagy in cartilage cells and suppress cellular senescence, resulting in the repair and maintenance of cartilage tissue [12]. Furthermore, Osteoking, a Chinese herbal medicine, has been suggested to suppress the KOA progression by decreasing the expression of matrix metalloprotase-13 (MMP-13) and increasing the activation of Smad2, which is involved in chondrocyte proliferation, in the cartilage tissue in a KOA-induced rat model [13]. These studies suggest that herbal medicine is a therapeutic option for treating KOA. Boiogito (BO), a traditional herbal medicine (called Kampo medicine in Japan), has been used to treat swelling and pain associated with KOA. BO (Tsumura & Co. (TJ-20; Lot No. 2190020010), Tokyo, Japan) contains a dry extract of the mixed drug substance consisting of Sinomenium stem 5.0 g, Astragalus root 5.0 g, Atractylodes lancea rhizome 3.0 g, jujube 3.0 g, Glycyrrhiza 1.5 g, and ginger 1.0 g. These herbs were mixed and extracted with purified water at 95.1 °C for 1 h, and the soluble extract was then approved by the Japanese Ministry of Health, Labour and Welfare [14]. In previous studies, we have reported that BO inhibited KOA progression in a rat model [15] and further demonstrated its analgesic effect on KOA [16]. Although many studies have reported the effect of various natural ingredients on the inhibition of KOA progression, no report has suggested the inhibition of KOA progression by a clinically-used drug. Therefore, we decided to conduct further research on BO.
In the early stage of KOA, multinucleated osteoclasts proliferate in the subchondral bone, and abnormal bone metabolism is observed before the cartilage tissue begins to degenerate [17]. With KOA progression, subchondral bone shows bone cyst formation, abnormal osteosclerosis, and osteophyte formation [18]. It has been reported that chondrocytes also release the receptor activator of the nuclear factor kappa-B ligand, which promotes osteoclast differentiation and proliferation in the subchondral bone [19]. Based on these reports, it is suggested that suppressing osteoclast differentiation and proliferation in the subchondral bone in the early stages of KOA plays a very crucial role in inhibiting subsequent KOA progression.
Some of the BO ingredients could inhibit osteoclast differentiation and proliferation. Magnoflorine in Sinomenium stem has been reported to inhibit osteoclast differentiation [20]. Furthermore, isoflavone in Astragalus root has a female hormone-like effect and has the potential to inhibit osteoclast proliferation [21]. These findings indicate that BO suppresses osteoclast differentiation and proliferation in the subchondral bone when administered to KOA-induced rats. To prove the inhibitory effect of BO on the KOA progression, it is necessary to examine the effect of inhibiting osteoclast differentiation and proliferation in the subchondral bone, in addition to inhibiting cartilage tissue degeneration. Thus, this study aimed to investigate the inhibitory effects of BO on osteoclast proliferation in the subchondral bone in a KOA rat model.
2. Materials and Methods
2.1. Animals
Twelve-week-old male Wistar rats (300–350 g) were used in this study. Rats were kept in cages of 3–4 animals per cage under room temperature of 24–26 °C, humidity of 40–50%, and a light/dark cycle of 12 h/12 h. Powdered chow (CE-2, CLEA Japan, Tokyo, Japan) and water were provided ad libitum. This study was conducted after approval by the Ethics Committee for Animal Experiments at the Showa University Animal Laboratory (Approval No.: 03053).
2.2. KOA Induction in a Rat Model
The destabilization of the medial meniscus (DMM) model [22] was employed as the KOA-induced rat model. According to previous reports, various studies have been conducted in rats in terms of similarity to the pathogenesis of KOA in humans [23,24]. We followed the method described in our previous report [15] to create the DMM rat model. Briefly, under general anesthesia with isoflurane inhalation, a longitudinal incision was made in the right knee joint, and the medial joint capsule was dissected to expose the medial meniscotibial ligament (MMTL). Transecting the MMTL results in the loss of stability and hoop function of the medial meniscus [24]. The medial capsule and skin were repaired by suturing with 6-0 Vicryl (Ethicon Inc., Somerville, NJ, USA).
2.3. Experimental Protocol
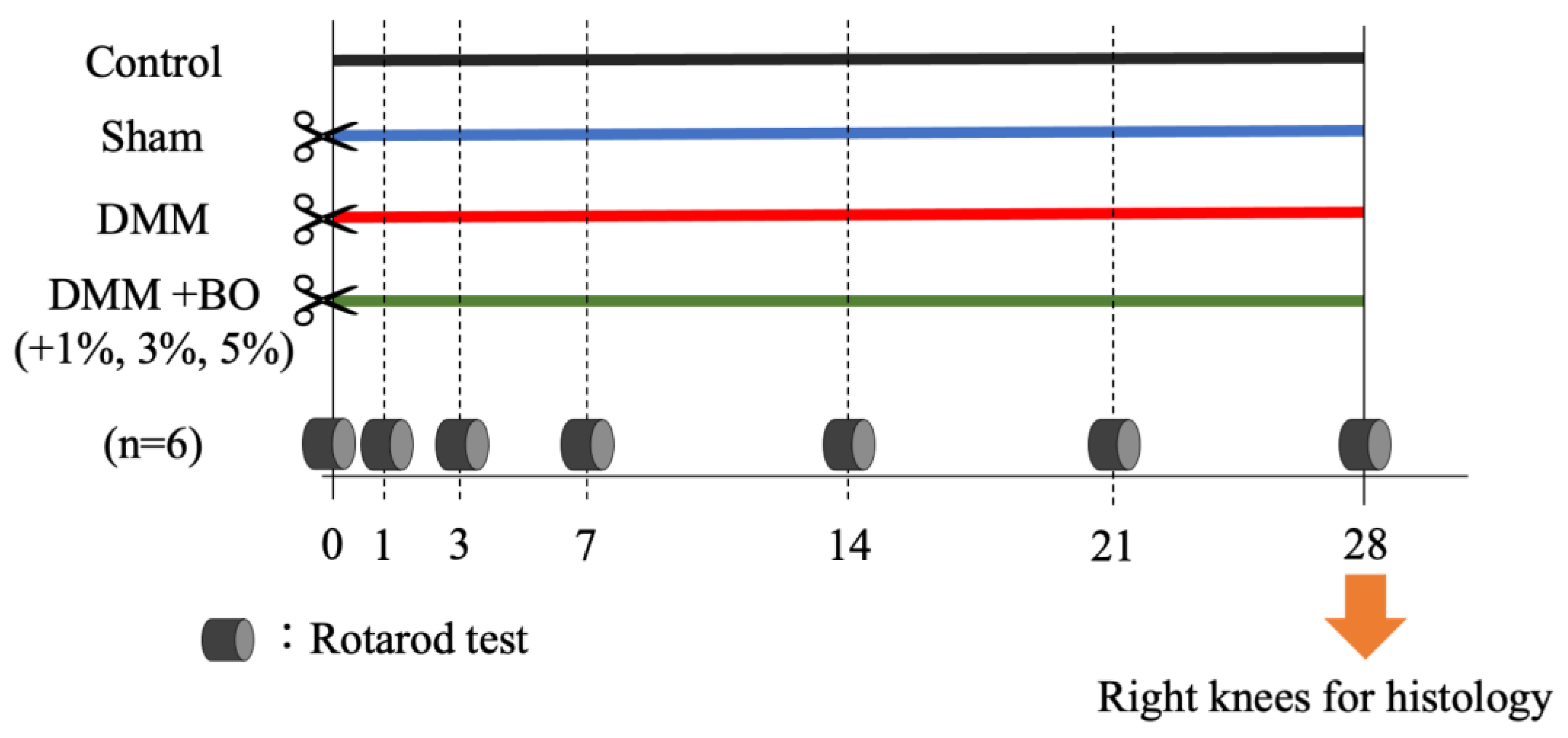
In the present study, BO was mixed with powdered chow (CE-2, CLEA Japan) and administered to rats, and 1%, 3%, and 5% of the total diet were applied to determine whether varying concentrations of BO had different therapeutic effects. Therefore, the rats were divided into 6 groups: control, sham, DMM, DMM + BO 1%, DMM + BO 3%, and DMM + BO 5% (n = 6 each). The rats were treated for 4 weeks postoperatively, and knee joints were harvested; the rotarod test was weekly performed to assess mobility and pain in rodents [25] (Figure 1). Rats were set on an accelerating rotarod apparatus (LE8305, Panlab Harvard Apparatus, Barcelona, Spain) with a diameter of 60 mm and a lane width of 75 mm, which gradually accelerates, and the latency to fall off is measured. In a joint disease model such as the one used in this study, the longer the latency to fall, the higher the locomotor function and the less pain can be estimated. In this study, the rotarod test was performed prior to DMM surgery, and 7, 14, 21, and 28 days after the DMM surgery. The rats were acclimated to the measurement apparatus two days before the start of the experiment and allowed to ride on the rotarod at 4 rpm for 180 s [25]. On each measurement day, after confirming acclimatization to the measurement device, the average of the three latencies to fall off was calculated.

Figure 1.
Experimental protocol of this study. Twelve-week-old male Wistar rats were divided into 6 groups: Control, Sham, DMM, DMM + BO 1%, DMM + BO 3%, DMM + BO 5% (n = 6 for each). DMM was performed in the right knee in the general inhalation anesthesia. Rotarod tests were performed weekly, and then the right knees were harvested for histological analysis. BO; Boiogito, DMM; Destabilization of the medial meniscus.
2.4. Histological Analysis
Rats were anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg; Somnopentyl, Kyoritsu Seiyaku, Tokyo, Japan) and then perfused with phosphate-buffered saline at pH 7.4. The knee joint was fixed by perfusion fixation with 4% paraformaldehyde. Knee joint slices for tissue staining were prepared according to OARSI recommendations [26]. Samples were fixed in 4% paraformaldehyde for 3 days, then demineralized in EDTA-2Na for 3 weeks, and the knee joint was divided in half at the center of the anteroposterior diameter of the tibia. After these samples were embedded in paraffin, slices of the knee joint were cut at a thickness of 4 μm using a Retratome (REM-700; Yamato Kohki Industrial Co., Ltd., Saitama, Japan). Slices were made every 200 μm anteriorly or posteriorly, and affixed to glass slides and then dried. Samples adhered to glass slides were stained with toluidine blue, and viewed using an Olympus BX 53 microscope (Olympus, Tokyo, Japan). The OARSI scoring system [26] is composed of cartilage degeneration (0–15 points), subchondral bone destruction (0–5 points), and osteophyte formation (0–4 points). Therefore, the total score ranged from 0–24 points, in which a lower score indicated less joint degeneration.
2.5. Measurement of the Number of Osteoclasts in the Subchondral Bone
Abnormal bone turnover in the subchondral bone is known to occur in the early stages of KOA [17], and therefore, the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts in the subchondral bone in the sliced samples was counted using the TRAP staining kit. (Fujifilm-Wako pure chemical, Osaka, Japan). TRAP-positive osteoclasts can be identified as multinucleated giant cells. The high number of these cells suggests that bone resorption in the subchondral bone is progressive, indicating that the initial pathology of KOA is developing [27].
2.6. Statistical Analysis
Data are represented as the mean ± SD of multiple repeats of the same experiment for the data (n = 6). Statistical analysis was performed in JMP® Pro version 16.0 software (SAS Inc., Cary, NC, USA) by one-way analysis of variance (ANOVA) and Dunnett test in the rotarod test, and by Tukey’s test in the histological analysis, following the Shapiro–Wilk normality test. A correlation analysis was performed between the OARSI score and the number of TRAP-positive osteoclasts in the subchondral bone; p-values < 0.05 were indicated statistically significant differences.
3. Results
3.1. Rotarod Test
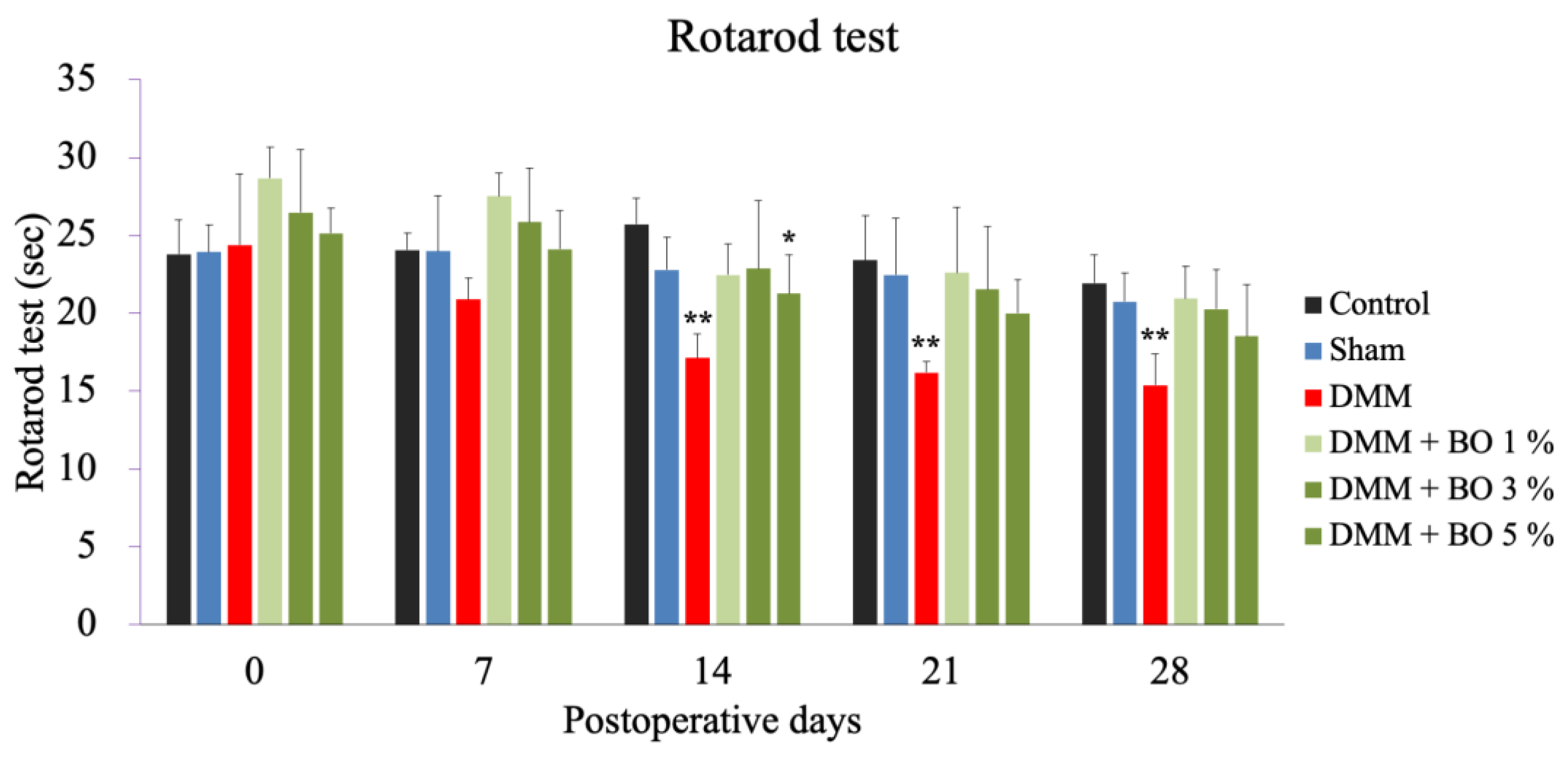
The DMM group showed a significant decrease in running time on postoperative days 14, 21, and 28 compared to the control group. The DMM + BO 5% group showed a significant decrease in running time on postoperative day 14 compared to the control group, while the DMM + BO 1% and DMM + BO 3% groups showed no significant difference on all measurement days compared to the control group (Figure 2).
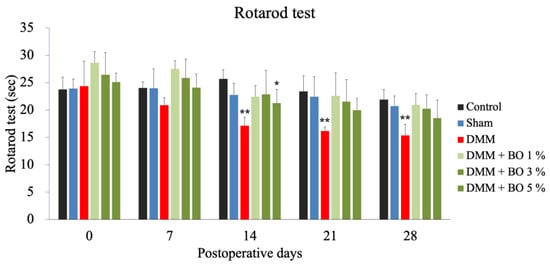
Figure 2.
Rotarod test as a locomotive function. Rotarod test was performed prior to surgery, and 7, 14, 21, and 28 days after DMM surgery. * p < 0.05, ** p < 0.01 vs. Control by Dunnett test. BO; Boiogito, DMM; Destabilization of the medial meniscus.
3.2. Histological Examination
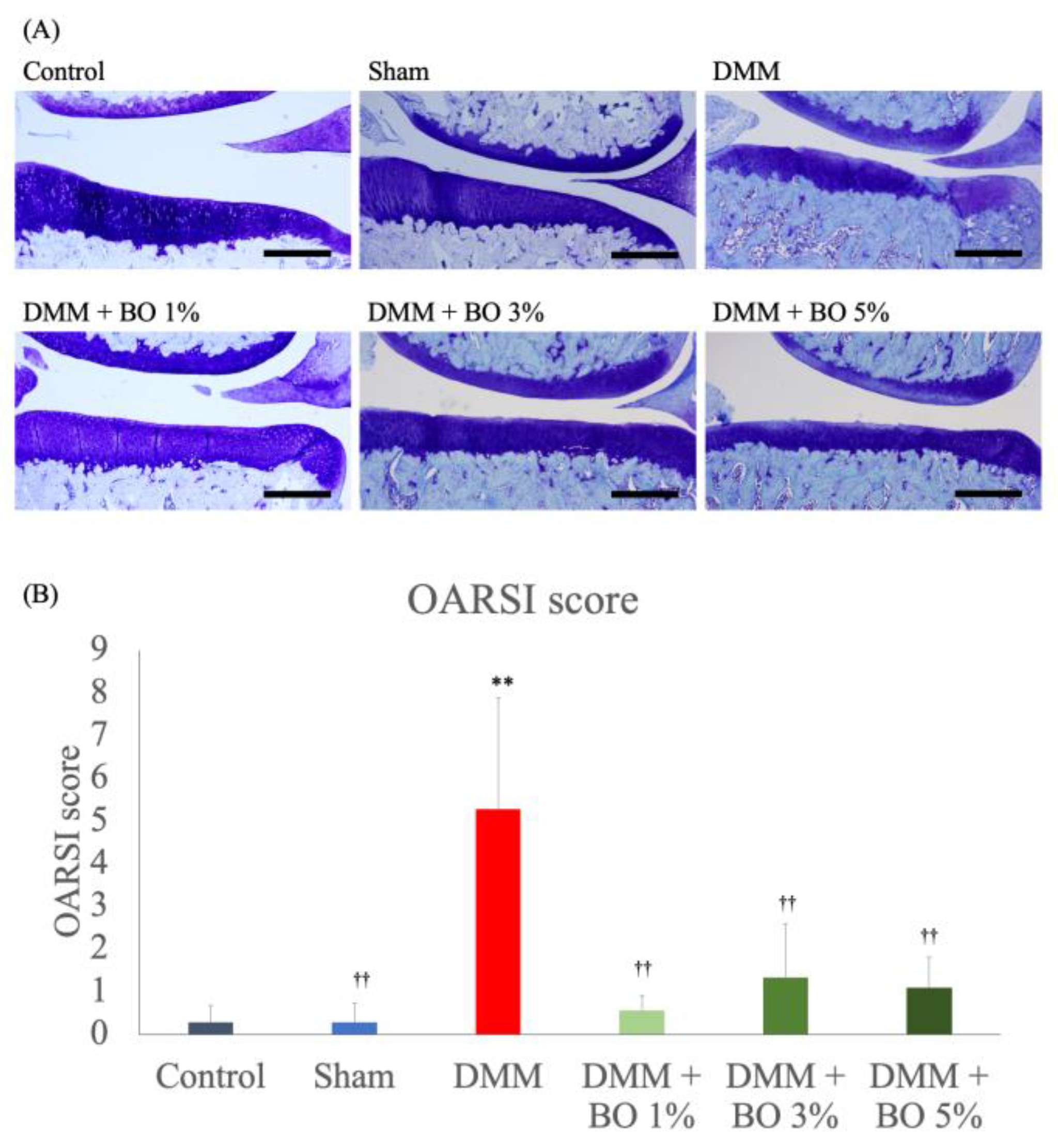
Toluidine blue staining of the rat knee joint slices revealed articular cartilage degeneration, subchondral bone destruction, and osteophyte formation in the DMM group (Figure 3A). The average OARSI scores were 0.28 ± 0.39 in the control group, 0.28 ± 0.44 in the sham group, 5.3 ± 2.61 in the DMM group, 0.56 ± 0.34 in the DMM + BO 1% group, 1.33 ± 1.25 in the DMM + BO 3% group, and 1.08 ± 0.71 in the DMM + BO 5% group. The DMM group had a significantly higher score than the control group (p < 0.01). In addition, administration of BO to the DMM rats decreased the OARSI score at any concentrations (p < 0.01 vs. DMM + BO 1%, p < 0.05 vs. DMM + BO 3%, DMM + BO 5%). In this study, the DMM + BO 1% group showed the lowest score in the BO-administrated groups, but there was no difference between groups in terms of BO concentration (Figure 3B).
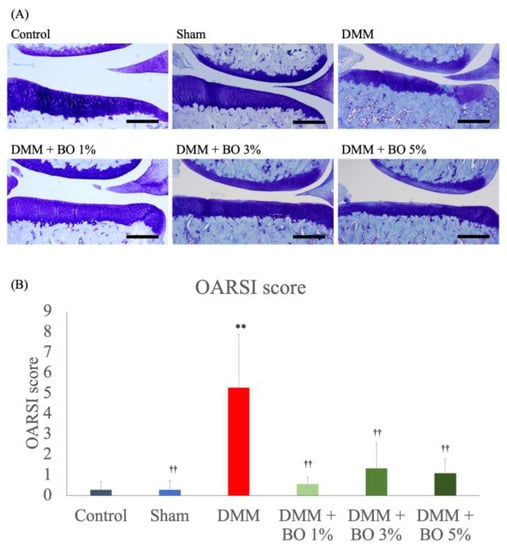
Figure 3.
Histological analysis for the development of KOA assessed with OARSI score. (A) Representative pictures of the toluidine blue–stained right knee samples in each group. Magnification: ×40. Scale bars = 200 μm. (B) At least 3 slices of the paraffin–embedded knee samples were prepared, stained by toluidine blue, and pictured with Olympus BX 53 microscope (Olympus, Tokyo, Japan). Articular cartilage degeneration (0–15 points), subchondral bone damage (0–5 points), and osteophyte formation (0–4 points) were measured and total score of the three parameters (0–24 points) was identified as the OARSI score of each rat. ** p < 0.01 vs. Control, †† p < 0.01 vs. DMM by Tukey-Kramer test. BO; Boiogito, DMM; Destabilization of the medial meniscus, KOA: knee osteoarthritis, OARSI; Osteoarthritis Research Society International.
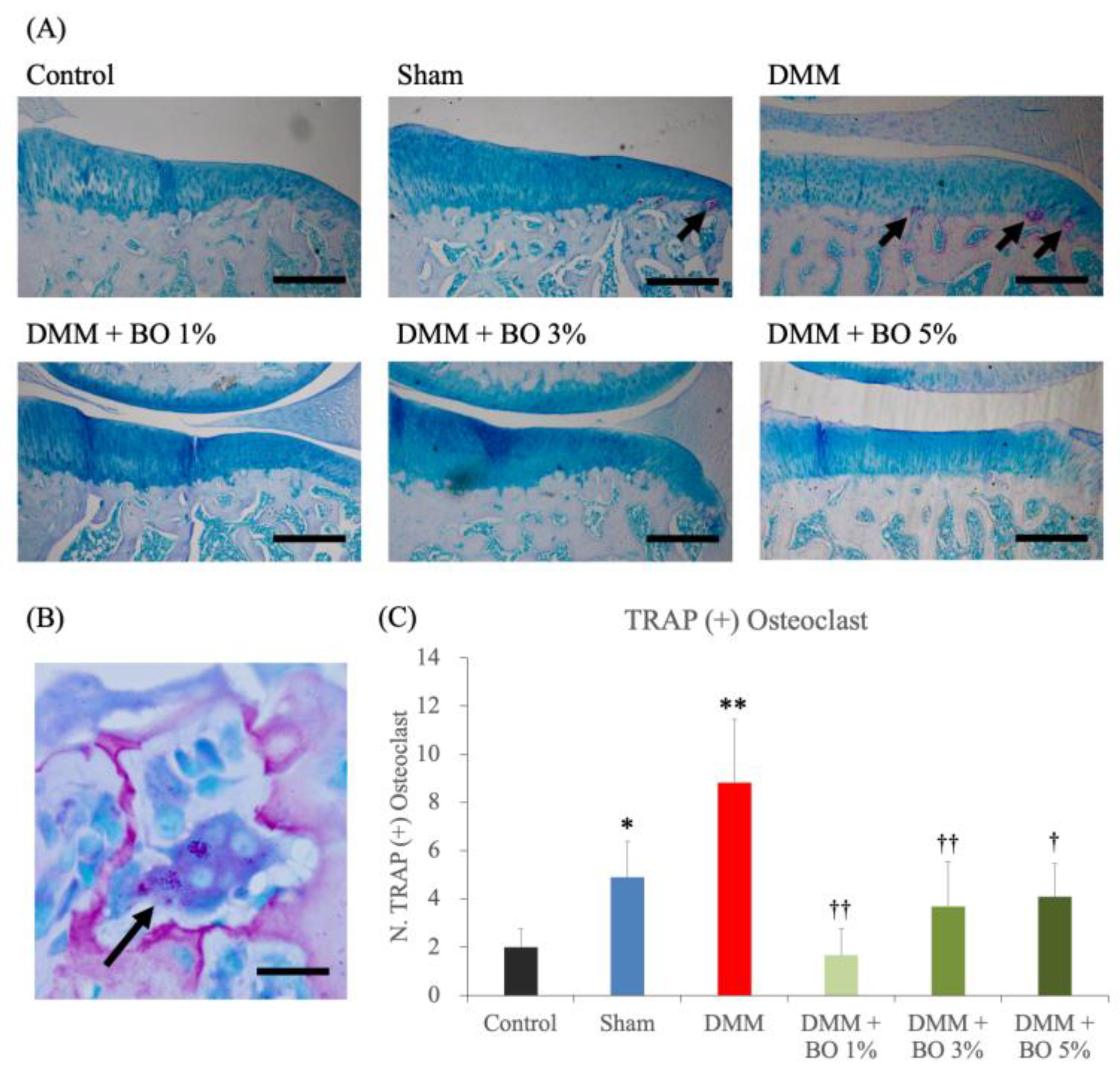
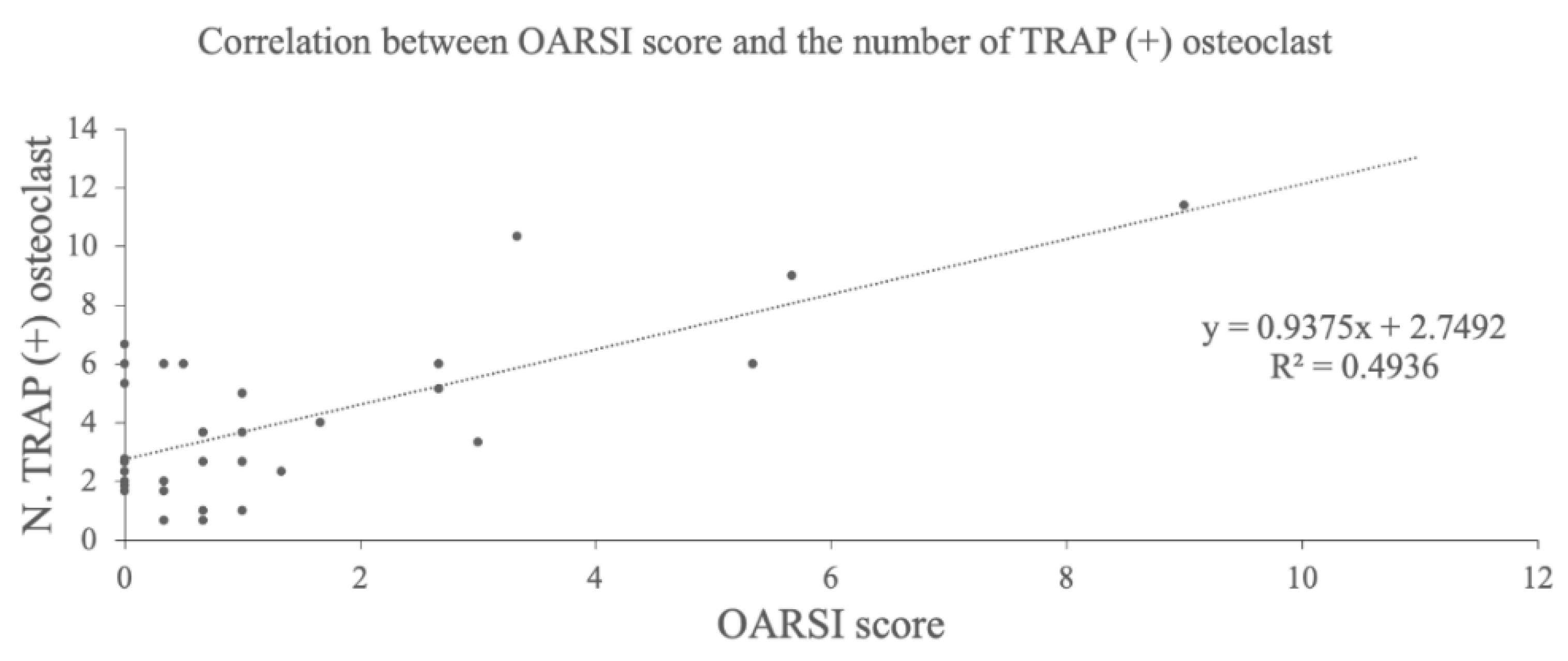
TRAP staining of the medial tibia revealed osteophytes and multinucleated TRAP-positive osteoclasts in the subchondral bone in the DMM group (Figure 4A,B). The average number of these cells were 2.0 ± 0.8 in the control group, 4.9 ± 1.5 in the sham group, 8.8 ± 2.6 in the DMM group, 1.7 ± 1.1 in the DMM + BO 1% group, 3.7 ± 1.9 in the DMM + BO 3% group, and 4.1 ± 1.4 in the DMM + BO 5% group. The number of osteoclasts was significantly increased in the DMM group compared to the control group, which was significantly decreased when BO was administered at any concentrations. In this study, the DMM + BO 1% group had the lowest cell count in the BO oral group, but there was no difference between groups by BO concentration (Figure 4C). Interestingly, the number of TRAP-positive osteoclasts and the OARSI score were strongly correlated (r = 0.70, p < 0.001) (Figure 5).
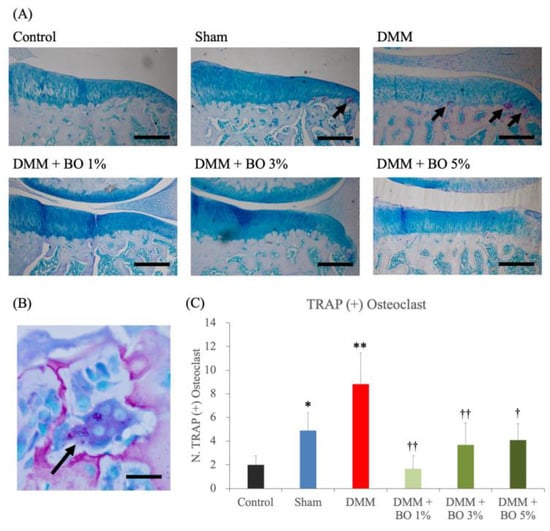
Figure 4.
Histological analysis for the number of TRAP-positive osteoclasts. (A) Representative pictures of TRAP staining for the right knees. Magnification: ×40. Scale bars = 200 μm. (B) Multinucleated TRAP-positive osteoclast (arrows). Magnification: ×1000. Scale bars = 10 μm. (C) The number of TRAP positive osteoclasts in the subchondral bone in the right knee. * p < 0.05, ** p < 0.01 vs. Control, † p < 0.05, †† p < 0.01 vs. DMM by Tukey–Kramer test. BO; Boiogito, DMM; Destabilization of the medial meniscus, TRAP; Tartrate-resistant acid phosphatase.
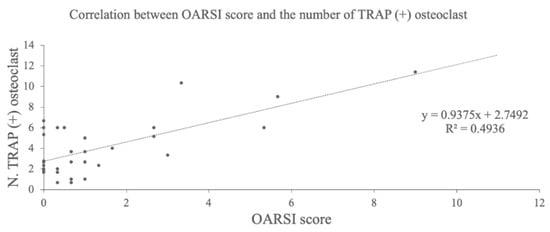
Figure 5.
Correlation analysis between OARSI score and the number of TRAP-positive osteoclasts. BO; Boiogito, DMM; Destabilization of the medial meniscus, OARSI; Osteoarthritis Research Society International, TRAP; Tartrate-resistant acid phosphatase.
4. Discussion
Osteoarthritis of the knee is very common worldwide, and there is great concern that the number of patients will continue to increase along with the increase in life expectancy associated with the development of medical care [3,4]. It is important to establish new treatments for inhibiting the progression of KOA in order to improve people’s quality of life and extend their healthy life expectancy. In addition, it is also important to reduce the burden of ever-rising medical costs [5]. A key aspect of treatment for KOA is to alleviate pain, swelling, dysfunction of the knee joint, and other symptoms associated with the disease [6]. In experimental studies, the rotarod test has become a popular method of assessing motor function and musculoskeletal pain [25], and in the present study, locomotor function was aggravated in the DMM rats, which was suppressed in the BO administrated group.
Our previous studies showed that BO has an analgesic effect, and that the inhibitory effect of the phosphorylation of ERK in layer I or II of the dorsal horn of the spinal cord is involved in one part of its mechanism. In the present study, high-dose and low-dose groups were also established in order to examine whether differences in BO doses would affect the therapeutic outcome. As a result, similar analgesic effects of BO were observed by the rotarod test. Furthermore, it is very interesting to note that a non-inferiority of the small dose of BO was demonstrated. Thereby, it was suggested that the therapeutic effect of oral administration of BO could be expected to be sufficient even at relatively small doses in the clinical situation.
In KOA, it has been reported that abnormal bone turnover, such as osteoclast differentiation and proliferation in the subchondral bone, occur prior to cartilage degeneration in the early stages of the disease [17]. The primary causes of this are increased loading due to weight gain, knee injury, and other factors. It has been reported that meniscal dysfunction due to meniscal injury or DMM results in approximately twice the normal load being applied to cartilage and subchondral bone [23]. The secondary causes include the exacerbation of synovitis, in which inflammatory cytokines secreted by immune cells in the synovial membrane, and humoral factors, such as nitric oxide, induce a reaction that stimulates bone resorption [28]. Furthermore, it has been reported that the receptor activator of the nuclear factor-kappa B ligand (RANKL) is released from degraded chondrocytes and promotes osteoclast differentiation and proliferation [19]. In the present study, the number of TRAP-positive osteoclasts in the subchondral bone was also increased. In addition, OARSI score was significantly increased in the DMM rats compared to the control group. Interestingly, KOA is a disease that develops and progresses not only in the articular cartilage but also in the subchondral bone. The increases in TRAP-positive osteoclasts and the OARSI score were inhibited by the administration of BO. As mentioned above, magnoflorine [20] contained in Sinomenium stem, and isoflavones [21] contained in Astragalus root may be involved in this inhibition. Specifically, magnoflorine suppressed MAPK and NF-κB signaling to prevent inflammatory osteolysis and RANKL-mediated osteoclast genesis [20]. Therefore, similar mechanisms may also be involved by BO administration. Further exploration into the components of BO may lead to the discovery of bioactive substances that can be utilized as novel therapeutic options to suppress osteoclast activity in the treatment for abnormal bone turnover. Further studies are needed in the future, including examining these detailed mechanisms of action.
The limitation of this study is that CT imaging of the knee joints in the DMM rats was not available due to the limitation of the experimental facilities. The fact that the DMM rats show osteoclast differentiation and proliferation of osteoclasts in the subchondral bone suggests that some changes in the subchondral bone microstructure may be occurring. The results of this study are based on fixed-point observations of the DMM rats at 4 weeks after KOA induction, and thus do not capture time-course changes, which will be an issue for future studies.
In conclusion, we found that osteoclasts in the subchondral bone of the knee joint were differentiated and proliferated in the early phase of osteoarthritis in a rat model of KOA. These results showed that oral administration of BO reduced the degeneration of articular cartilage and the number of TRAP-positive osteoclasts, and that even a lower dose of BO than that of the previous report was sufficient to improve the pathological condition. These results strongly suggest that BO may be an effective therapeutic agent which can inhibit the progression of KOA.
Author Contributions
Conceptualization: T.K. and T.O.; Methodology: T.O., H.I. and N.A.; Validation: T.O.; Formal analysis: T.O.; Investigation: T.K., H.T., M.M. and K.I.; Resources: H.I.; Data curation: H.I.; Writing—Original Draft: T.K.; Writing–Review & Editing: T.O., H.I., N.A., H.T., K.I, K.K. and M.S.; Visualization: T.O.; Supervision: K.K. and M.S.; Project administration: M.S.; Funding acquisition: T.O. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI (Grant no. JP22K15695).
Institutional Review Board Statement
This research was approved by the Institutional Ethics Committee for Care and Use of Animals of Showa University (certificate number: 03053, date of approval: 1 April 2021).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The dry powdered extract of BO was supplied by Tsumura & Co. (TJ-20; Lot No. 2190020010, Tokyo, Japan).
References
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Spitaels, D.; Mamouris, P.; Vaes, B.; Smeets, M.; Luyten, F.; Hermens, R.; Vankrunkelsven, P. Epidemiology of knee osteoarthritis in general practice: A registry-based study. BMJ Open 2020, 10, e031734. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Su, W.; Bedenbaugh, A.V.; Oruc, A. Health care resource utilization and burden of disease in a U.S. Medicare population with a principal diagnosis of osteoarthritis of the knee. J. Med. Econ. 2020, 23, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2020, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- Yorifuji, M.; Sawaji, Y.; Endo, K.; Kosaka, T.; Yamamoto, K. Limited efficacy of COX-2 inhibitors on nerve growth factor and metalloproteinases expressions in human synovial fibroblasts. J. Orthop. Sci. 2016, 21, 381–388. [Google Scholar] [CrossRef]
- Mobasheri, A. Intersection of Inflammation and Herbal Medicine in the Treatment of Osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 604–616. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Deng, M.; Li, Y.; Li, B.; Li, J.; Ma, Y.; Chen, Z.; Wang, G.; Liu, S. Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-κB/HIF-2α axis. Klin. Wochenschr. 2020, 98, 1479–1491. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef]
- Bao, J.; Yan, W.; Xu, K.; Chen, M.; Chen, Z.; Ran, J.; Xiong, Y.; Wu, L.; Seth, R.K. Oleanolic Acid Decreases IL-1β-Induced Activation of Fibroblast-Like Synoviocytes via the SIRT3-NF-κB Axis in Osteoarthritis. Oxidative Med. Cell. Longev. 2020, 2020, 7517219. [Google Scholar] [CrossRef]
- Ling, H.; Zeng, Q.; Ge, Q.; Chen, J.; Yuan, W.; Xu, R.; Shi, Z.; Xia, H.; Hu, S.; Jin, H.; et al. Osteoking Decelerates Cartilage Degeneration in DMM-Induced Osteoarthritic Mice Model Through TGF-β/smad-dependent Manner. Front. Pharmacol. 2021, 12, 678810. [Google Scholar] [CrossRef]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. Japanese Pharmacopoeia, 17th ed.; Yakuji Nippo: Tokyo, Japan, 2016. [Google Scholar]
- Oike, J.; Okumo, T.; Ikemoto, H.; Kunieda, Y.; Nakai, S.; Takemura, H.; Takagi, H.; Kanzaki, K.; Sunagawa, M. Preventive Effect of the Japanese Traditional Herbal Medicine Boiogito on Posttraumatic Osteoarthritis in Rats. Medicines 2020, 7, 74. [Google Scholar] [CrossRef]
- Kunieda, Y.; Okumo, T.; Ikemoto, H.; Adachi, N.; Tanaka, M.; Kimura, T.; Yusa, K.; Kanzaki, K.; Sunagawa, M. Analgesic Effect of Boiogito, a Japanese Traditional Kampo Medicine, on Post-Traumatic Knee Osteoarthritis through Inhibition of ERK1/2 Phosphorylation in the Dorsal Horn of the Spinal Cord. Appl. Sci. 2021, 11, 8421. [Google Scholar] [CrossRef]
- Fang, H.; Huang, L.; Welch, I.; Norley, C.; Holdsworth, D.W.; Beier, F.; Cai, D. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci. Rep. 2018, 8, 2855. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Martínez-Calatrava, M.J.; Prieto-Potín, I.; A Roman-Blas, J.; Tardio, L.; Largo, R.; Herrero-Beaumont, G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res. Ther. 2012, 14, R149. [Google Scholar] [CrossRef]
- Sun, Z.; Zeng, J.; Wang, W.; Jia, X.; Wu, Q.; Yu, D.; Mao, Y. Magnoflorine Suppresses MAPK and NF-κB Signaling to Prevent Inflammatory Osteolysis Induced by Titanium Particles In Vivo and Osteoclastogenesis via RANKL In Vitro. Front. Pharmacol. 2020, 11, 389. [Google Scholar] [CrossRef]
- Kong, X.; Wang, F.; Niu, Y.; Wu, X.; Pan, Y. A comparative study on the effect of promoting the osteogenic function of osteoblasts using isoflavones from Radix Astragalus. Phytotherapy Res. 2018, 32, 115–124. [Google Scholar] [CrossRef]
- Glasson, S.; Blanchet, T.; Morris, E. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Allaire, R.; Muriuki, M.; Gilbertson, L.; Harner, C.D. Biomechanical Consequences of a Tear of the Posterior Root of the Medial Meniscus. J. Bone Jt. Surg. 2008, 90, 1922–1931. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Kawabata, K.; Koga, H.; Nakagawa, Y.; Saito, R.; Udo, M.; Yanagisawa, K.; Ohara, T.; Mochizuki, T.; et al. Centralization of extruded medial meniscus delays cartilage degeneration in rats. J. Orthop. Sci. 2017, 22, 542–548. [Google Scholar] [CrossRef]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of Behavioral Tests Assessing General Locomotion, Muscular Strength, and Coordination in Mice. J. Vis. Exp. 2018, 131, 55491. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.; Glasson, S.; Carlson, C. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Iijima, H.; Ito, A.; Nagai, M.; Tajino, J.; Yamaguchi, S.; Kiyan, W.; Nakahata, A.; Zhang, J.; Wang, T.; Aoyama, T.; et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthr. Cartil. 2017, 25, 964–975. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).