Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic disorder characterized by peripheral eosinophilia, severe eosinophilic asthma, sinusitis, transient pulmonary infiltrates, and features of medium/small-vessel vasculitis. EGPA belongs to the group of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitides, although only 30 to 40% of patients display ANCA positivity, which is mainly of myeloperoxidase (MPO) specificity. Particularly, ANCA-positive patients typically show vasculitic features. Interleukin (IL)-5 has been demonstrated to play a crucial role in determining eosinophilic airway inflammation in EGPA patients. Specifically, maturation, activation, and survival of eosinophils especially depend on IL-5 availability. Therefore, blocking IL-5 biological activity may be a rewarding strategy for control of eosinophilic inflammation. Several monoclonal antibodies with the ability to interfere with the biological activity of IL-5 have been developed, namely, mepolizumab, reslizumab, and benralizumab. Here, we discuss the role of these drugs in the management of severe eosinophilic asthma in the context of EGPA and report the outcome of two EGPA patients with severe eosinophilic asthma treated at our outpatient clinic.
1. Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA, previously known as Churg–Strauss syndrome) belongs to the group of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides, which also include granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, and microscopic polyangiitis (MPA) [1,2]. In EGPA, ANCA specificity is mainly directed (~70% of cases) towards myeloperoxidase (MPO) and is typically associated with a perinuclear fluorescence pattern (p-ANCA) upon immunofluorescence staining of fixed neutrophils [3]. However, rates of ANCA positivity in EGPA are lower than those reported in the other two conditions of the group (GPA and MPA), being around 30–40% [1,2]. In addition, the clinical features of ANCA-positive patients significantly differ from those of ANCA-negative patients, with vasculitic involvement, in the form of glomerulonephritis, mononeuritis and/or alveolar hemorrhage, more frequent in the former, and cardiomyopathy more prevalent in the latter [1,2,4]. According to the 2012 revised Chapel Hill Consensus Conference (CHCC) definition of vasculitides, EGPA is an eosinophil-rich and necrotizing granulomatous inflammation often involving the respiratory tract, with necrotizing vasculitis predominantly affecting small to medium vessels [5,6] and is associated with asthma and eosinophilia. Since both vessel wall inflammation and eosinophilic infiltration are involved in the pathogenesis of organ damage, EGPA shares features of both systemic vasculitis and hypereosinophilic syndromes [7]. Clinically, the course of EGPA follows a sequential pattern, with a prodromal phase characterized by late-onset asthma and other allergic-like features, an eosinophilic phase with blood and tissue eosinophilia, and, eventually, a vasculitic phase, dominated by purpura, mononeuritis, glomerulonephritis, and/or alveolitis [1,2,8]. These phases may as well overlap. Patients may also complain of nonspecific constitutional symptoms, such as fever, fatigue, arthralgia, and weight loss. However, the time interval between onset of initial symptoms and the vasculitic phase may be rather long, thus delaying a correct diagnosis. More commonly, indeed, the onset of vasculitic manifestations (such as the appearance of mononeuritis multiplex, purpura, and glomerulonephritis), in a patient previously known to be affected by chronic rhinosinusitis, persistent moderate-to-severe asthma and eosinophilia, alerts the clinician to the diagnosis of EGPA [9,10,11].
2. Main Contributions
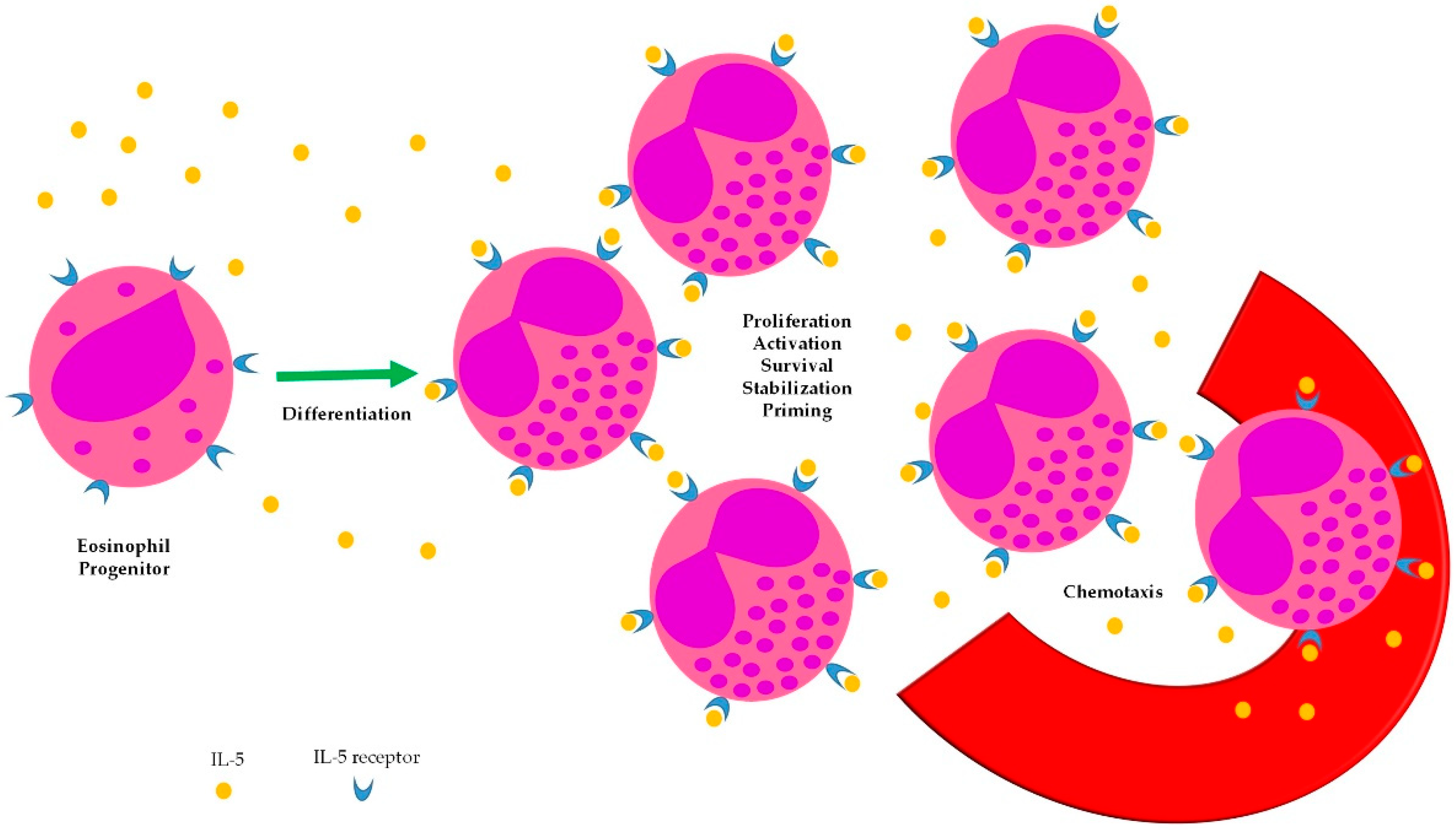
Scant evidence exists about the impact of interleukin (IL)-5-targeted therapies on the management of EGPA. Targeting IL-5 in EGPA is a reasonable therapeutic strategy, owing to the pathogenic role of eosinophils in this condition [7]. IL-5 has long been known to be a cytokine able to sustain several steps of eosinophil biology (Figure 1), however, monoclonal antibodies with neutralizing activity towards this soluble mediator have been developed only recently. The largest evidence from their use in EGPA comes from small series or case reports, whereas only one double-blind, randomized trial has been completed so far.
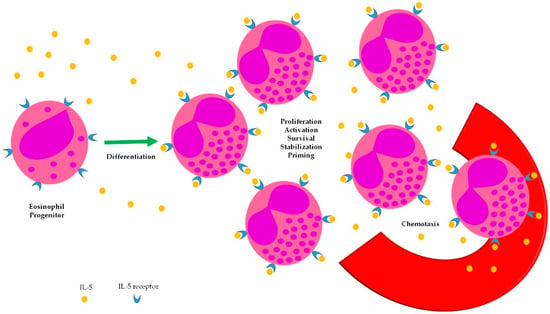
Figure 1.
Biologic effects of IL-5 on eosinophils.
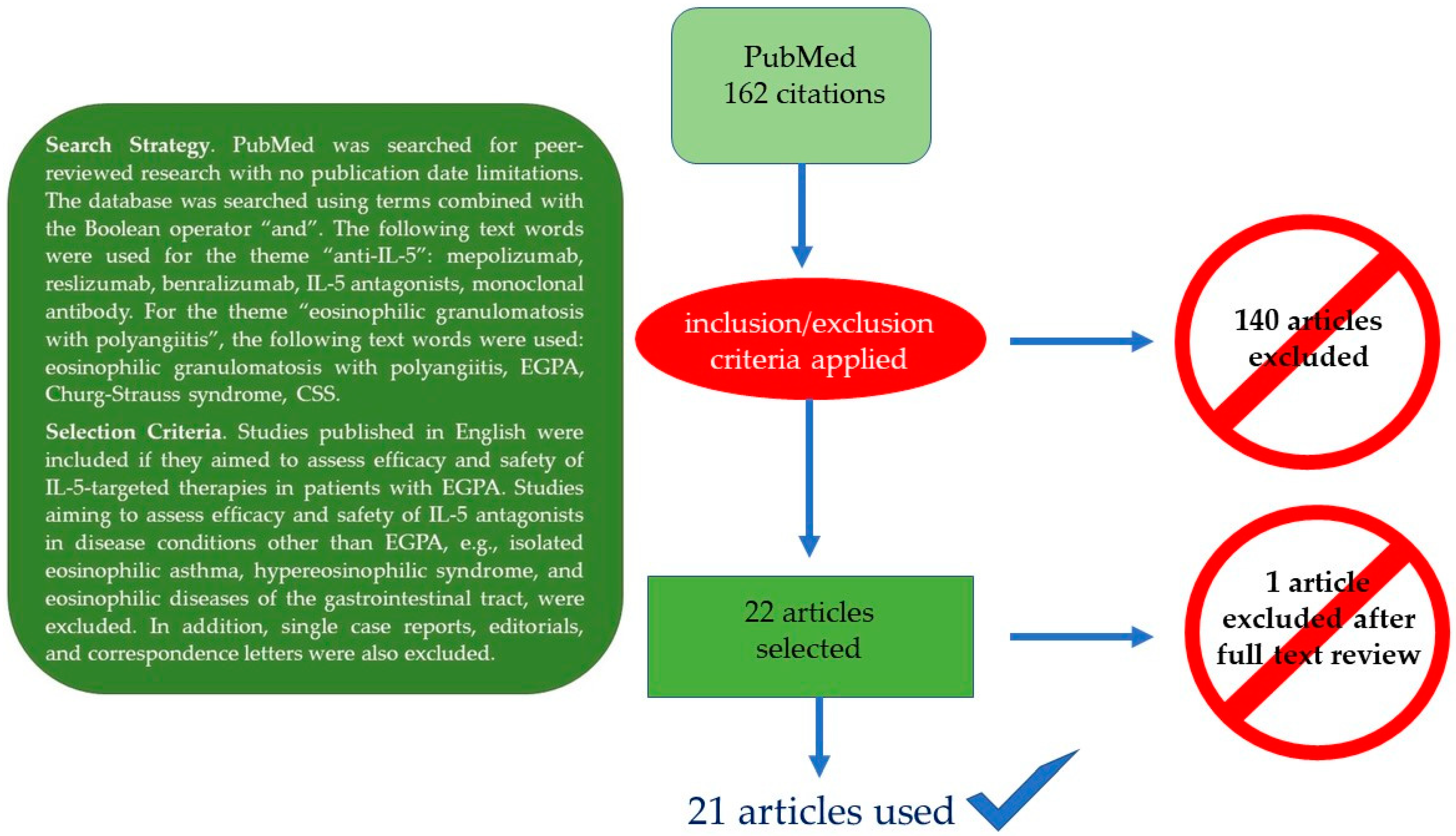
Here, we summarize the current knowledge on the role of IL-5-targeted therapies in EGPA with particular emphasis on the management of EGPA-associated eosinophilic asthma, which is responsible for a markedly impaired quality of life in nearly all patients. Moreover, we discuss possible future refinements of IL-5-targeted therapies and contribute to the field by describing the outcome of two EGPA patients treated at our institution. Figure 2 summarizes the PubMed-based literature search strategy and the selection criteria for the papers ultimately considered for the final review.
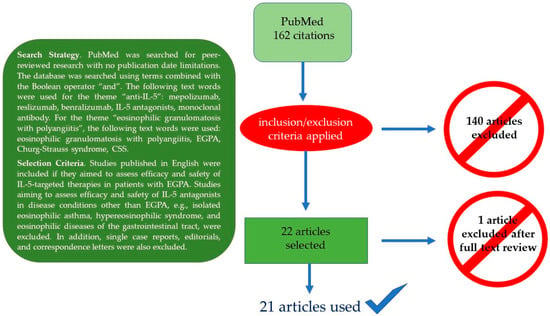
Figure 2.
Literature search strategy (box) and final search outcome.
3. Therapeutic Management of EGPA
3.1. Standard Therapy
The cornerstone of therapy for EGPA remission induction is represented by glucocorticoids. Immunosuppressants are usually added to the glucocorticoid regimen, particularly in case of life-threatening organ involvement [12], which may be assessed with the aid of the five factor score (FFS), a prognostic tool proposed in 1996 [13] and revised in 2011 [14] (Table 1). FFS has been shown to be useful for survival prediction in EGPA; however, the original 1996 items have been refined in 2011 based on a better knowledge of the disease outcome [13,14]. Using the original set of items (1996), mortality at 5 years was observed to be 11.9% in the absence of any prognostic factor; with 1 of the 5 factors present, mortality increased to 25.9%, while with 2 or more of the 5 factors present (FFS = 2, see explanation in Table 1), mortality was shown to loom over 45.95% of the patients. In the 2011 update, age ≥ 65 years was also recognized as an independent predictor of poor prognosis, while visceral involvement was retained because it was still found to heavily weigh on the outcome. Conversely, ear, nose, and throat symptoms were associated with a lower relative risk of death. According to the 2011 set of criteria, mortality at 5 years was as follows: 9%, 21%, and 40% with FFS = 0, 1, and 2, respectively. Hence, the FFS may help identify patients requiring more aggressive treatment.

Table 1.
Comparison of 1996 and 2011 five factor score (FFS) items.
Glucocorticoids are administered orally at a dose of 0.5–1.0 mg/kg of prednisone equivalent. EGPA patients with no unfavorable prognostic factors according to the FFS may obtain remission with glucocorticoid therapy only, albeit relapses may still occur [12]. However, in case of impending organ- or life-threatening disease (Table 1), intravenous (i.v.) boluses (e.g., methylprednisolone 500–1000 mg daily for 3–5 days) may be preferred over the oral route as initial therapy, followed by oral administration and progressive tapering, according to clinical response; cyclophosphamide or rituximab are usually added to the i.v. glucorticoid induction regimen [12]. Cyclophosphamide may be administered either orally (1.5–2.0 mg/kg for up to 6 months) or intravenously (15 mg/kg fortnightly for the first 3 infusions, then every three weeks for at least 3 more doses), with the oral route associated with a slightly greater risk of bladder toxicity and the i.v. route associated with a slightly greater risk of relapse. Cardiac involvement or ≥2 organ involvement may favor the use of cyclophosphamide because of an earlier onset of action with respect to rituximab [12]. Rituximab may be considered in ANCA-positive patients, in case of glomerulonephritis, or unsatisfactory response to cyclophosphamide [12]. Ease of administration with the protocol borrowed from rheumatoid arthritis (1 g, 2 weeks apart) [15] may favor rituximab over cyclophosphamide. Maintenance of remission is then attempted with methotrexate, azathioprine, or mycophenolate mofetil, while glucocorticoids are progressively tapered down [12]. However, many patients experience relapses, particularly during the tapering of glucocorticoids.
3.2. Anti-IL-5-Targeted Therapies
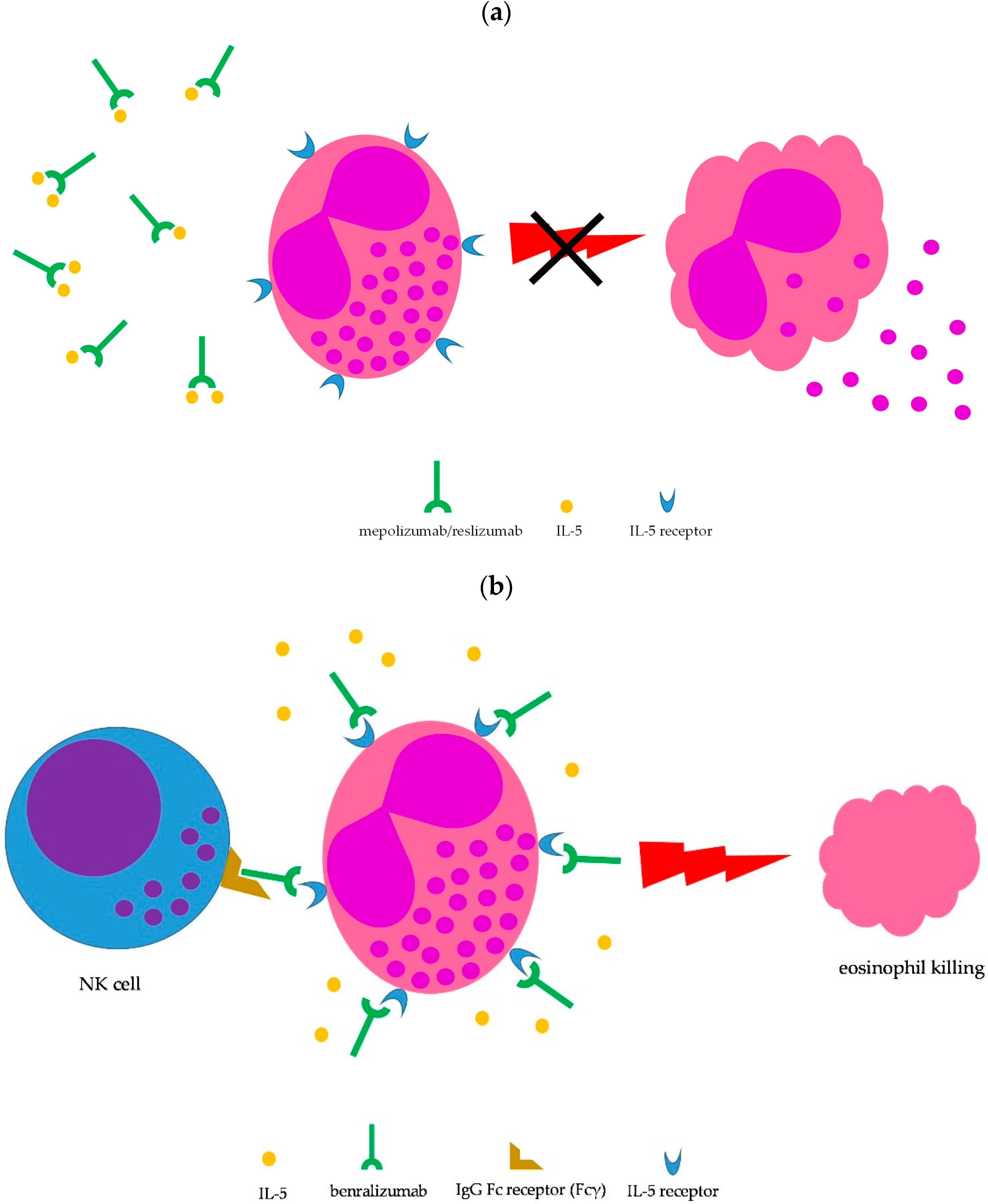
Lung involvement is not included among the factors defining severe EGPA (Table 1); however, the lung is the most commonly involved organ in EGPA (≥90% of cases) [1,2,8,11]. Moderate-to-severe asthma is the main clinical manifestation and chest X-ray may show transient pulmonary infiltrates, which are due to the accumulation of eosinophils. Upper airway involvement is also common, in the form of chronic rhinosinusitis with nasal polyps [1,2,8]. Asthma is managed according to international guidelines [16]; however, the severity of symptoms may require prolonged courses with oral steroids, which are difficult to taper. Therefore, IL-5-targeted therapy may be implemented to spare glucocorticoid side effects. To date, therapeutic monoclonal antibodies capable to interfere with the IL-5 pathway and, consequently, on eosinophil growth, recruitment, activation, and survival, include mepolizumab, reslizumab, and benralizumab [17,18]. Mepolizumab and reslizumab act by sequestering soluble IL-5, making it not available to eosinophils, whereas benralizumab binds to the IL-5 receptor, thus blocking access to IL-5, but is also able to induce eosinophil killing by natural killer cells through antibody-dependent cell toxicity (ADCC) (Figure 3) [17,18]. All three monoclonal antibodies have been approved for treatment of moderate-to-severe eosinophilic asthma as add-on therapy in patients unsatisfactorily controlled by standard therapy [19], thus being theoretically administrable in EGPA-associated eosinophilic asthma as well.
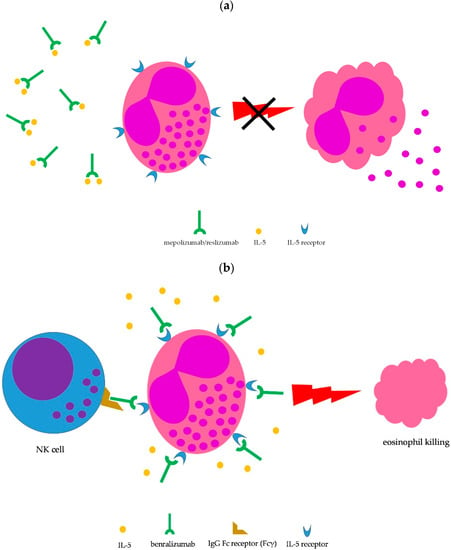
Figure 3.
Mechanism of action of IL-5-targeted monoclonal antibodies. (a) Blocking IL-5 by mepolizumab and reslizumab prevents eosinophil maturation, proliferation, recruitment, survival, priming, and activation (the latter shown here); (b) benralizumab binds to the IL-5 receptor on eosinophils, thus inhibiting cytokine-mediated cell stimulation; in addition, the Fc fragment of benralizumab is able to mediate eosinophil killing by NK cells through antibody-dependent cell cytotoxicity.
Moreover, apart from the upper and lower airways, eosinophils are also believed to play a crucial role in determining tissue damage in other typical manifestations of EGPA, such as peripheral neuritis and vasculitis [7]. Therefore, IL-5-targeted therapies may also result in control of non-asthma features of EGPA.
3.2.1. Mepolizumab
To date, only mepolizumab has obtained indication for relapsing/remitting or refractory EGPA [20]. In fact, following reports of encouraging results primarily in small series [21,22,23,24], a multicenter, double-blind, parallel-group, phase 3 randomized trial was eventually carried out to evaluate the efficacy and safety of mepolizumab in patients with refractory or relapsing EGPA on stable oral steroid treatment [25]. Relapses considered by the investigators included active vasculitis (Birmingham Vasculitis Activity Score [BVAS] >0 [26]), active asthma symptoms or signs, or active nasal or sinus disease, prompting an increase in the glucocorticoid dose, an initiation of or increase in immunosuppressive therapy, or hospitalization. Relapses could be of more than one type. The study confirmed the effectiveness of mepolizumab in maintaining remission throughout the study duration and in delaying major relapses when compared with placebo, with no significant between-group differences in adverse events [25]. Specifically, the study met the two primary endpoints, i.e., (1) the accrued weeks of remission (28% vs. 3%, mepolizumab vs. placebo, respectively, with the treated group showing 24+ weeks of remission) plus prednisone daily dose ≤4 mg over 52 weeks (44% successfully lowering the dose to ≤4 mg and 18% discontinuing prednisone vs. 7% and 3%, mepolizumab vs. placebo, respectively) and (2) the proportion of patients in remission at weeks 36 and 48 (32% vs. 3%, mepolizumab vs. placebo, respectively). Rates of relapses were as follows (mepolizumab vs. placebo, respectively): vasculitis 43% vs. 65%; asthma 37% vs. 60%; sinusitis 35% vs. 51%. Overall, 20% of the patients had a vasculitic relapse only, while 54% had a combined vasculitic and asthmatic/sinonasal relapse. Finally, a subgroup analysis revealed that patients with an absolute eosinophil count greater than 150 cells/mm3 at baseline were more likely to experience a greater efficacy when compared with patients with a lower value of eosinophils, who did not actually obtain a significant benefit from treatment [25]. This observation clearly paves the way for a more effective selection of EGPA patients likely responding to IL-5 therapeutic inhibition in future studies. Therefore, based on these results, the Food and Drug Administration (FDA) licensed mepolizumab in the USA in 2017 as an add-on therapy with a steroid-sparing effect in adult patients with relapsing or refractory EGPA, at a dose of 300 mg subcutaneously (s.c.) every 4 weeks.
Subsequent reports from real-world clinical practice confirmed the safety and efficacy of mepolizumab in small series of patients with relapsing or refractory EGPA. Specifically, Ueno et al. [27] treated 16 patients, all of them with comorbid asthma, for one year with the licensed 300 mg dose. At the 12-month assessment, mepolizumab treatment resulted in a decreased disease activity, a remission rate of 75%, a glucorticoid-sparing effect, and a reduced number of patients using concomitant immunosuppressants. The retention rate was excellent (100% of patients). Respiratory symptoms improved immediately after starting mepolizumab, with only 2 patients still complaining of chest discomfort at the 12-month evaluation. Only three patients had an infection. The same authors also retrospectively compared the effectiveness of mepolizumab (300 mg/monthly) with i.v. cyclophosphamide pulse therapy for remission induction in EGPA patients [28]. Treatment was ≥6 months and both groups of patients were also administered high-dose steroids at the initiation of therapy. Even considering the critical issues of the study, mainly related to the small groups evaluated (7 patients in the mepolizumab group and 13 patients in the cyclophosphamide group), the results were promising: the retention rate for mepolizumab was again complete (100%), while only 61.5% of patients completed the pulse therapy (6 pulses total); adverse events were nearly twice as frequent in the cyclophosphamide group (53.8% vs. 28.6%, cyclophosphamide vs. mepolizumab, respectively). With regard to efficacy, there were no significant differences in BVAS and eosinophil count reductions between the two groups, however, mepolizumab-treated patients benefitted from a greater steroid dose reduction compared with cyclophosphamide-treated patients. Regarding lung involvement, chest manifestations decreased from 71.4% to 0% and from 58.3% to 8.3% at 6 months, mepolizumab vs. i.v. cyclophosphamide, respectively. Thus, mepolizumab for remission induction therapy in severe EGPA appeared to allow control of disease activity as effectively as cyclophosphamide, but with a greater reduction in concomitant steroid doses and fewer adverse events [28]. Finally, a small series (11 EGPA patients, 3 with uncontrolled asthma), characterized by steroid dependence and unsatisfactory disease control, also achieved improvement of disease activity (no evidence of asthma and vasculitic manifestations at the last study follow-up) following treatment with mepolizumab [29]. Again, the improvement in disease activity allowed notable glucocorticoid tapering.
Interestingly, mepolizumab efficacy was also investigated at a lower dose (100 mg every 4 weeks) in real-life EGPA patients. Vultaggio et al. [30] evaluated whether mepolizumab 100 mg/month could allow control of asthma symptoms, tapering of glucocorticoids and/or immunosuppressants, maintenance of remission, and prevention of relapses in 18 EGPA patients over a 12-month study period. At the final assessment, more than two thirds of the patients were shown to have been free from asthma exacerbations during treatment, the daily glucocorticoid dose could be at least halved in 77.7% of the subjects, and immunosuppressants could be discontinued in 4 patients. Nearly all patients obtained clinical remission (94.3%) and no relapses were recorded during the study time frame [30].
Further data on the effectiveness of the 100 mg dose were subsequently published. A retrospective European collaborative study compared different biologics, including rituximab, omalizumab, and mepolizumab, for treatment of relapsing or refractory EGPA [31]. While rituximab was primarily chosen to address the treatment of prominent vasculitic features, mepolizumab and omalizumab were administered for the control of steroid-dependent asthma. Mepolizumab was administered to 51 patients and was shown to be highly effective with a good safety profile in patients with steroid-dependent asthma, with a clear advantage over omalizumab in this category of patients (rates of remission and partial responses: 78% and 10% vs. 15% and 33%, mepolizumab vs. omalizumab, respectively). In addition, mepolizumab was administered at the reduced dose of 100 mg monthly in 57% of patients, while the remaining participants received the approved 300 mg dose. Overall, remission rates at 12 months were 76% and 82% among patients receiving mepolizumab at a dose of 100 mg and 300 mg, respectively, with no significant difference [31]. Caminati et al. [32] showed that in 16 EGPA patients in remission phase but persisting severe steroid-dependent asthma, not only did mepolizumab 100 mg every 4 weeks prevent vasculitis relapse, but it also achieved asthma control during the 6 months of the study duration. Specifically, both at the 3- and 6-month time points, a significant improvement in Asthma Control Test (ACT) scores, forced expiratory volume in one second (FEV1) %, and exacerbations was observed. Moreover, a significant decrease in the average corticosteroid daily intake was recorded at any time point, with 56.2% of patients even completely discontinuing the drug. At the 6-month follow-up, 2 of 11 patients under immunosuppressants suspended the treatment, while 4 of them could taper the dose with no relapse. No adverse events were recorded. In summary, mepolizumab 100 mg at 28-day intervals both prevented EGPA relapse and maintained asthma control over the 6-month study period. Thus, this regimen may have a role in the maintenance phase as a steroid/immunosuppressant-sparing agent in EGPA patients with persisting severe steroid-dependent eosinophilic asthma in spite of remission of systemic vasculitis [32].
Finally, Bettiol et al. [33] reported the results observed in the largest series of EGPA patients (no. 158) treated so far with mepolizumab 100 mg every 4 weeks and compared the outcome with a smaller series of EGPA patients (no. 33) treated with the conventional dose (300 mg every 4 weeks). Complete response rates and reductions in BVAS scores, prednisone doses, and eosinophil counts were comparable between the two regimens. Asthma exacerbations were recorded in 36% of patients on the 100 mg dose and in 52% of patients who received the 300 mg dose. Based on these results, both mepolizumab regimens (100 mg and 300 mg every 4 weeks) appeared to be effective for the treatment of EGPA [33]. However, due to the retrospective nature of the study and the disparity in the sample size between the two doses, definitive conclusions cannot be drawn until a rigorous comparison is carried out in the setting of a controlled trial. Nevertheless, evidence on the effectiveness of the 100 mg dose keeps accumulating [34,35,36].
3.2.2. Reslizumab
Fewer evidence is available for reslizumab as a controller drug in EGPA. A study involving nine patients with refractory, steroid-dependent EGPA patients with severe eosinophilic asthma were treated with reslizumab infusions at a dose of 3 mg/kg every 28 days [37], according to the regimen approved as add-on therapy for treatment of severe asthma with an eosinophilic phenotype [38]. Patients also had other EGPA features, namely, paranasal sinus disease (100% of patients), neurological involvement (44% of patients), and cardiac involvement (33% of patients). At the end of the 48 weeks of treatment, 7 out of 9 patients (~78%) had their steroid doses reduced to or below 7.5 mg/day (consistent with remission [39]), while two patients were able to completely stop the drug. No increases in exacerbation frequency or hospitalization were seen and no treatment-limiting adverse effects were recorded. Since no significant improvement in overall BVAS scores was seen, the authors concluded that reslizumab may be less effective in controlling extrapulmonary manifestations of EGPA, namely, neuropathy, than in quenching activity of airway disease [37]. Thus, reslizumab may be a therapeutic option in EGPA patients mainly troubled by severe eosinophilic asthma. Another open-label, pilot study involving 10 patients with EGPA [40] demonstrated a significant reduction in the daily oral glucocorticoid dose after 7 monthly reslizumab treatments (equivalent to a 24-week treatment phase). Although only 6 of the 10 subjects completed all the study visits, 8 patients could be nonetheless analyzed; 5 out of these 8 patients were considered true responders to reslizimumab because of successful tapering of oral steroids, with no exacerbations during the treatment phase. Overall, three exacerbations were recorded during the treatment, and the most common was the worsening of asthma symptoms. One patient suffered from a severe adverse event, deemed to be causally linked with the study drug, requiring removal from the study [40]. Because of the small numbers of patients and the design of these studies, randomized, controlled clinical trials are needed to validate the efficacy and safety of reslizumab for EGPA.
3.2.3. Benralizumab
Efficacy of benralizumab in EGPA is mainly supported by case reports [41]. In addition, a small open-label pilot study including 10 EGPA patients suggested that benralizumab was effective in reducing both the daily dose of oral steroids and the exacerbation rate during treatment [42]. Importantly, 8 of 10 subjects reached a minimum oral steroid dose of <5 mg daily, with 5 (50%) able to stop oral glucocorticoids after 40 weeks of treatment [42]. Intriguingly, benralizumab has been reported to be effective even in EGPA patients failing previous treatment with mepolizumab or reslizumab. In 2 case studies (16 EGPA patients overall), including 6 patients failing previous treatment with mepolizumab and 1 patient failing both mepolizumab and reslizumab, benralizumab (30 mg s.c. every 4 weeks for the first 3 injections, then 30 mg s.c. every 8 weeks) significantly reduced oral glucocorticoid doses and improved both BVAS and ACT scores at the completion of the studies [43,44]. Complete depletion of peripheral eosinophils was seen in most of the patients, consistent with the drug’s mechanism of action (Figure 3b). Currently, a phase three trial comparing benralizumab with mepolizumab in relapsing or refractory EGPA is ongoing (MANDARA trial, NCT04157348) [45].
3.3. Other Treatment Strategies
Experience with other investigational or hypothesis-driven monoclonal antibody-based treatment strategies (e.g., omalizumab, belimumab, obinutuzumab, etc.) is limited or absent and is beyond the scope of this article.
4. Case Reports
Two patients with EGPA referred to our clinical immunology outpatient clinic were considered for implementation of IL-5-targeted therapies due to the difficulty in tapering treatment with high-dose oral steroids. The characteristics of the patients are summarized in Table 2.

Table 2.
Characteristics of the patients administered IL-5-targeted therapies.
4.1. Case 1
A 52-year-old female patient with involvement of upper and lower airways and left leg paresthesia as a result of peripheral neuropathy due to EGPA diagnosed 10 years earlier at another institution required consultation because of unsatisfactory asthma control. She had been administered high-dose steroids at onset of disease and several immunosuppressants (azathioprine, methotrexate, and cyclophosphamide) thereafter in an attempt to taper the daily glucocorticoid dose. Although the patient attained remission of most of the several EGPA manifestations she was suffering from (Table 2), she still complained of daily asthma exacerbations despite being on both oral prednisone 25 mg/day and a combination of high-dose inhaled steroids and long-acting bronchodilators. Mepolizumab was therefore prescribed according to the severe asthma schedule, i.e., 100 mg s.c. every 28 days, since the 300 mg dose every 4 weeks for EGPA treatment had not yet been approved at the time of recruitment. One year after commencement of anti-IL-5 treatment, the patient was no longer on oral steroids, eosinophils were in the normal range (450/µL), anti-MPO were slightly above the upper limit of detection (6.8 UI/mL, normal range: 0.0–5.0), inflammatory markers were in the normal range, no further asthma exacerbations had occurred, and taper of inhaled therapy was started. Low-dose mepolizumab (100 mg every 28 days) was confirmed as the only maintenance therapy and is still ongoing.
4.2. Case 2
A 31-year-old female patient received diagnosis of EGPA because of sinonasal disease, severe asthma, peripheral eosinophilia, peripheral neuropathy, purpura, p-ANCA, and anti-MPO positivity. Because of the remarkable peripheral eosinophilia (Table 2), other hypereosinophilic syndromes [46] were considered in the differential before making a definitive diagnosis of EGPA. Specifically, hematologic, parasitic, and allergic diseases were ruled out by means of specific investigations. The gastrointestinal (GI) complaints (Table 2) were addressed by means of upper and lower endoscopy of the GI tract, which only showed a spastic colon; random biopsies carried out along the upper and lower GI tract did not show eosinophil infiltration consistent with eosinophilic gastroenteritis, colitis, or esophagitis [47]. The patient was initially started on prednisone 1 mg/kg per day and obtained acceptable asthma control as well as remission of purpura, gastrointestinal complaints, and constitutional symptoms just after one month of therapy. An improvement in symptoms related to peripheral neuropathy was also observed. A 50% reduction in peripheral eosinophilia (~4000/μL) was recorded after 4 weeks of therapy, along with normalization of inflammatory markers. At the 3-month follow-up, with the patient on oral prednisone 20 mg/day, clinical manifestations were in remission and eosinophilia was mild (~600/µL). However, tapering of oral steroids could not be further accomplished without incipient rebound of the circulating eosinophil count (~1200/µL) and new asthma exacerbations. Because of the exceedingly high count of peripheral blood eosinophils at diagnosis, benralizumab (30 mg s.c. every 4 weeks for the first 3 injections, then 30 mg s.c. every 8 weeks, according to the schedule approved for eosinophilic asthma [48]) was empirically chosen for its ability to efficaciously deplete these cells. One month later, asthma was in remission again and oral prednisone could be successfully tapered off in the subsequent weeks. Three months into benralizumab treatment, the patient was still in complete remission in all aspects of EGPA, her peripheral eosinophils were undetectable (0 cells/µL) and oral and inhaled steroids had been completely suspended. Benralizumab is still ongoing as the only maintenance therapy, 30 mg s.c. every 8 weeks.
5. Discussion
Although often limited by small sample sizes, short duration of the therapeutic interventions, the lack of double-blind placebo controls or other comparators, as well as the uncertainty about long-term adverse effects, particularly in case of complete eosinophil depletion, the evidence accumulated thus far appears to support the use of IL-5-targeted therapies in the treatment of EGPA-associated eosinophilic asthma, as well as for other significant disease manifestations. Currently, only mepolizumab may be used for in-label treatment of relapsing/remitting or refractory EGPA [20], while waiting for completion of ongoing studies or start of new investigations addressing the role of benralizumab and reslizumab in EGPA management. Moreover, given the likely effectiveness of low-dose mepolizumab in the management of refractory/relapsing EGPA [30,31,32,33,34,35,36], studies are also warranted to assess whether 100 mg every 4 weeks may be used as a stable maintenance therapy in patients obtaining initial drug-induced remission. Comparison should be undertaken with the approved 300 mg dose every 28 days, considering as the main outcomes for lung involvement the rates of asthma exacerbations and the daily dose of maintenance oral steroids in the two patient groups. Reslizumab shares the same mechanism of action as mepolizumab, targeting IL-5 directly [18]. Since mepolizumab can only be prescribed in a fixed dose [20], reslizumab may provide an additional advantage due to the i.v. route of administration allowing for weight-based dose adjustments [38], which may be particularly useful in obese patients. Nevertheless, this potential advantage for dose adjustment needs confirmation through design of ad hoc studies, upon preliminary verification of differential responses to mepolizumab treatment between lean and obese EGPA patients. Finally, the ability of benralizumab to completely deplete circulating eosinophils may theoretically suggest use of this drug in patients with exceedingly high peripheral eosinophils, as in case 2 reported above, or in patients failing mepolizumab (or reslizumab, in case of future indication for EGPA). Again, the hypothetical threshold of the circulating eosinophil count above which the fixed mepolizumab dose may be expected not to be satisfactorily effective needs to be determined through specific studies comparing drug responses in EGPA patients with different levels of eosinophilia, and the same holds true with regard to the utility of benralizumab as a rescue therapy following mepolizumab (or reslizumab) failure. A further issue is represented by how long IL-5-targeted therapies should be continued in patients achieving remission. Relapse rates may be high indeed if EGPA patients in remission on IL-5-targeted therapies are switched to conventional immunosuppressants [24]. Therefore, future trials should also investigate whether a minimally effective dose and/or lengthening dosing intervals may be an acceptable trade-off between therapy suspension and relapse rates, as this strategy has been observed to be rewarding in other chronic conditions requiring long-term therapeutic monoclonal antibody administration [49,50]. Cost-effectiveness may also benefit from this strategy. Meanwhile, further insights on the role of IL-5 targeted therapies in relapsing or refractory EGPA will hopefully be obtained at the completion of the MANDARA trial (NCT04157348), comparing mepolizumab vs. benralizumab [45].
6. Conclusions
Currently, IL-5-targeted therapies represent the best option for relapsing or refractory EGPA patients and, possibly, for remission induction. Apart from silencing most of the disease manifestations, these drugs allow significant sparing of steroid doses, which may pose a significant burden on patients in terms of unwanted side effects in the long term. Refinement of IL-5-targeted therapies with future studies is eagerly awaited to further improve the management of specific subsets of EGPA patients, while hopefully waiting for definitive, etiology-driven therapies.
Author Contributions
Conceptualization, C.R.; methodology, C.R., D.C., A.S. and L.R.; literature search, D.C. and A.S.; data curation, C.R., D.C., A.S. and L.R.; writing—original draft preparation, C.R.; writing—review and editing, C.R., D.C., A.S. and L.R.; supervision, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the two patients reported in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almaani, S.; Fussner, L.A.; Brodsky, S.; Meara, A.S.; Jayne, D. ANCA-Associated Vasculitis: An Update. J. Clin. Med. 2021, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Radice, A.; Bianchi, L.; Sinico, R.A. Anti-neutrophil cytoplasmic autoantibodies: Methodological aspects and clinical significance in systemic vasculitis. Autoimmun. Rev. 2013, 12, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Cornec, D.; Cornec-Le Gall, E.; Fervenza, F.C.; Specks, U. ANCA-associated vasculitis—Clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 2016, 12, 570–579. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Prete, M.; Indiveri, F.; Perosa, F. Vasculitides: Proposal for an integrated nomenclature. Autoimmun. Rev. 2016, 15, 167–173. [Google Scholar] [CrossRef]
- Koike, H.; Nishi, R.; Yagi, S.; Furukawa, S.; Fukami, Y.; Iijima, M.; Katsuno, M. A Review of Anti-IL-5 Therapies for Eosinophilic Granulomatosis with Polyangiitis. Adv. Ther. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French vasculitis study group cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef]
- Chaigne, B.; Guillevin, L. Vasculitis for the internist: Focus on ANCA-associated vasculitis. Intern. Emerg. Med. 2017, 12, 577–585. [Google Scholar] [CrossRef]
- Zarka, F.; Veillette, C.; Makhzoum, J.P. A Review of Primary Vasculitis Mimickers Based on the Chapel Hill Consensus Classification. Int. J. Rheumatol. 2020, 2020, 8392542. [Google Scholar] [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res. 2021, 73, 1088–1105. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Lhote, F.; Gayraud, M.; Cohen, P.; Jarrousse, B.; Lortholary, O.; Thibult, N.; Casassus, P. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine 1996, 75, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Management of Difficult-to-Treat & Severe Asthma—Global Initiative for Asthma—GINA (ginasthma.org). Available online: https://ginasthma.org/severeasthma/ (accessed on 23 October 2022).
- Desai, M.; Oppenheimer, J. Biologics in allergic and immunologic diseases: Promises and challenges in the era of personalized medicine. Ann. Allergy Asthma Immunol. 2018, 120, 350–353. [Google Scholar] [CrossRef]
- van de Veen, W.; Akdis, M. The use of biologics for immune modulation in allergic disease. J. Clin. Investig. 2019, 129, 1452–1462. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines—Recommendations for severe asthma. Allergy 2021, 76, 14–44. [Google Scholar] [CrossRef]
- Nucala, INN-Mepolizumab (Europa.eu). Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 23 October 2022).
- Kahn, J.-E.; Grandpeix-Guyodo, C.; Marroun, I.; Catherinot, E.; Mellot, F.; Roufosse, F.; Blétry, O. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010, 125, 267–270. [Google Scholar] [CrossRef]
- Kim, S.; Marigowda, G.; Oren, E.; Israel, E.; Wechsler, M.E. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010, 125, 1336–1343. [Google Scholar] [CrossRef]
- Moosig, F.; Gross, W.L.; Herrmann, K.; Bremer, J.P.; Hellmich, B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann. Intern. Med. 2011, 155, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Gross, W.L.; Moosig, F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg-Strauss syndrome. Clin. Exp. Rheumatol. 2012, 30 (Suppl. S70), S62–S65. [Google Scholar] [PubMed]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Hoffman, G.S.; Merkel, P.A.; Min, Y.I.; Uhlfelder, M.L.; Hellmann, D.B.; Specks, U.; Allen, N.B.; Davis, J.C.; Spiera, R.F.; et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001, 44, 912–920. [Google Scholar] [CrossRef]
- Ueno, M.; Miyagawa, I.; Nakano, K.; Iwata, S.; Hanami, K.; Fukuyo, S.; Kubo, S.; Miyazaki, Y.; Kawabe, A.; Yoshinari, H.; et al. Effectiveness and safety of mepolizumab in combination with corticosteroids in patients with eosinophilic granulomatosis with polyangiitis. Arthritis Res. Ther. 2021, 23, 86. [Google Scholar] [CrossRef]
- Ueno, M.; Miyagawa, I.; Aritomi, T.; Kimura, K.; Iwata, S.; Hanami, K.; Fukuyo, S.; Kubo, S.; Miyazaki, Y.; Nakayamada, S.; et al. Safety and effectiveness of mepolizumab therapy in remission induction therapy for eosinophilic granulomatosis with polyangiitis: A retrospective study. Arthritis Res. Ther. 2022, 24, 159. [Google Scholar] [CrossRef]
- Ríos-Garcés, R.; Prieto-González, S.; Hernández-Rodríguez, J.; Arismendi, E.; Alobid, I.; Penatti, A.E.; Cid, M.C.; Espígol-Frigolé, G. Response to mepolizumab according to disease manifestations in patients with eosinophilic granulomatosis with polyangiitis. Eur. J. Intern. Med. 2022, 95, 61–66. [Google Scholar] [CrossRef]
- Vultaggio, A.; Nencini, F.; Bormioli, S.; Vivarelli, E.; Dies, L.; Rossi, O.; Parronchi, P.; Maggi, E.; Matucci, A. Low-Dose Mepolizumab Effectiveness in Patients Suffering from Eosinophilic Granulomatosis with Polyangiitis. Allergy Asthma Immunol. Res. 2020, 12, 885–893. [Google Scholar] [CrossRef]
- Canzian, A.; Venhoff, N.; Urban, M.L.; Sartorelli, S.; Ruppert, A.M.; Groh, M.; Girszyn, N.; Taillé, C.; Maurier, F.; Cottin, V.; et al. Use of Biologics to Treat Relapsing and/or Refractory Eosinophilic Granulomatosis with Polyangiitis: Data from a European Collaborative Study. Arthritis Rheumatol. 2021, 73, 498–503. [Google Scholar] [CrossRef]
- Caminati, M.; Crisafulli, E.; Lunardi, C.; Micheletto, C.; Festi, G.; Maule, M.; Giollo, A.; Orsolini, G.; Senna, G. Mepolizumab 100 mg in severe asthmatic patients with EGPA in remission phase. J. Allergy Clin. Immunol. Pract. 2021, 9, 1386–1388. [Google Scholar] [CrossRef]
- Bettiol, A.; Urban, M.L.; Dagna, L.; Cottin, V.; Franceschini, F.; Del Giacco, S.; Schiavon, F.; Neumann, T.; Lopalco, G.; Novikov, P.; et al. Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis: A European Multicenter Observational Study. Arthritis Rheumatol. 2022, 74, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Detoraki, A.; Tremante, E.; Poto, R.; Morelli, E.; Quaremba, G.; Granata, F.; Romano, A.; Mormile, I.; Rossi, F.W.; de Paulis, A.; et al. Real-life evidence of low-dose mepolizumab efficacy in EGPA: A case series. Respir. Res. 2021, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Özdel Öztürk, B.; Yavuz, Z.; Aydın, Ö.; Mungan, D.; Sin, B.A.; Demirel, Y.S.; Bavbek, S. Effectiveness of low-dose mepolizumab in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA): A real-life experience. Int. Arch. Allergy Immunol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Can Bostan, O.; Duran, E.; Tuncay, G.; Cihanbeylerden, M.; Karadag, O.; Damadoglu, E.; Karakaya, G.; Kalyoncu, A.F. Sinonasal and respiratory outcomes of eosinophilic granulomatosis with polyangiitis patients receiving 100 mg mepolizumab in real-life clinical practice: 1-year follow up study. J. Asthma 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Kent, B.D.; d’Ancona, G.; Fernandes, M.; Green, L.; Roxas, C.; Thomson, L.; Nanzer, A.M.; Kavanagh, J.; Agarwal, S.; Jackson, D.J. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020, 6, 00311–2019. [Google Scholar] [CrossRef]
- Available online: https://www.cinqairhcp.com/globalassets/cinqair-hcp-redesign/prescribing-information.pdf (accessed on 23 October 2022).
- Hellmich, B.; Flossmann, O.; Gross, W.L.; Bacon, P.; Cohen-Tervaert, J.W.; Guillevin, L.; Jayne, D.; Mahr, A.; Merkel, P.A.; Raspe, H.; et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: Focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann. Rheum. Dis. 2007, 66, 605–617. [Google Scholar] [CrossRef]
- Manka, L.A.; Guntur, V.P.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Strand, M.J.; Wechsler, M.E. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann. Allergy Asthma Immunol. 2021, 126, 696–701.e1. [Google Scholar] [CrossRef]
- Koga, Y.; Aoki-Saito, H.; Kamide, Y.; Sato, M.; Tsurumaki, H.; Yatomi, M.; Ishizuka, T.; Hisada, T. Perspectives on the Efficacy of Benralizumab for Treatment of Eosinophilic Granulomatosis with Polyangiitis. Front. Pharmacol. 2022, 13, 865318. [Google Scholar] [CrossRef]
- Guntur, V.P.; Manka, L.A.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Gill, M.; Kolakowski, C.; Strand, M.J.; Wechsler, M.E. Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis. J. Allergy Clin. Immunol. Pract. 2021, 9, 1186–1193.e1. [Google Scholar] [CrossRef]
- Padoan, R.; Chieco Bianchi, F.; Marchi, M.R.; Cazzador, D.; Felicetti, M.; Emanuelli, E.; Vianello, A.; Nicolai, P.; Doria, A.; Schiavon, F. Benralizumab as a glucocorticoid-sparing treatment option for severe asthma in eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3225–3227.e2. [Google Scholar] [CrossRef]
- Nanzer, A.M.; Dhariwal, J.; Kavanagh, J.; Hearn, A.; Fernandes, M.; Thomson, L.; Roxas, C.; Green, L.; D’Ancona, G.; Agarwal, S.; et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020, 6, 00451–2020. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04157348 (accessed on 23 October 2022).
- Valent, P.; Klion, A.D.; Roufosse, F.; Simon, D.; Metzgeroth, G.; Leiferman, K.M.; Schwaab, J.; Butterfield, J.H.; Sperr, W.R.; Sotlar, K.; et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.M.; Lenti, M.V.; Merli, S.; Licari, A.; Votto, M.; Marseglia, G.L.; Di Sabatino, A. Primary eosinophilic gastrointestinal disorders and allergy: Clinical and therapeutic implications. Clin. Transl. Allergy 2022, 12, e12146. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf (accessed on 23 October 2022).
- Romano, C.; Sellitto, A.; De Fanis, U.; Esposito, G.; Arbo, P.; Giunta, R.; Lucivero, G. Maintenance of remission with low-dose omalizumab in long-lasting, refractory chronic urticarial. Ann. Allergy Asthma Immunol. 2010, 104, 95–97. [Google Scholar] [CrossRef]
- Romano, C.; Sellitto, A.; De Fanis, U.; Balestrieri, A.; Savoia, A.; Abbadessa, S.; Astarita, C.; Lucivero, G. Omalizumab for difficult-to-treat dermatological conditions: Clinical and immunological features from a retrospective real-life experience. Clin. Drug Investig. 2015, 35, 159–168. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).