Application of Graphene Oxide as a Biomaterial for the Development of Large-Area Cell Culture Vessels
Abstract
:1. Introduction
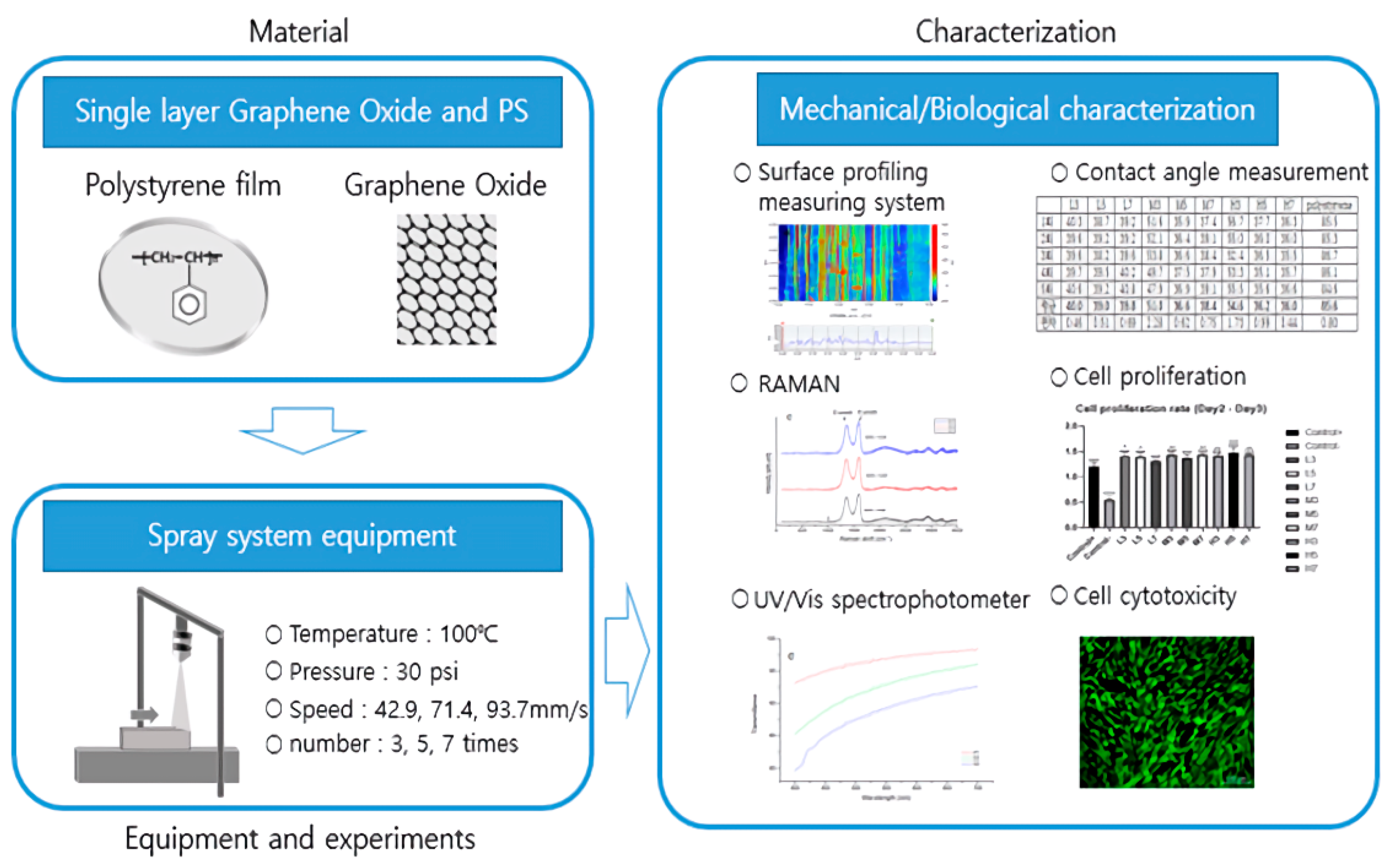
2. Materials and Methods
2.1. Materials
2.1.1. Graphene Oxide
2.1.2. Cell Culture Test
2.2. Equipment
2.3. Experimental Method
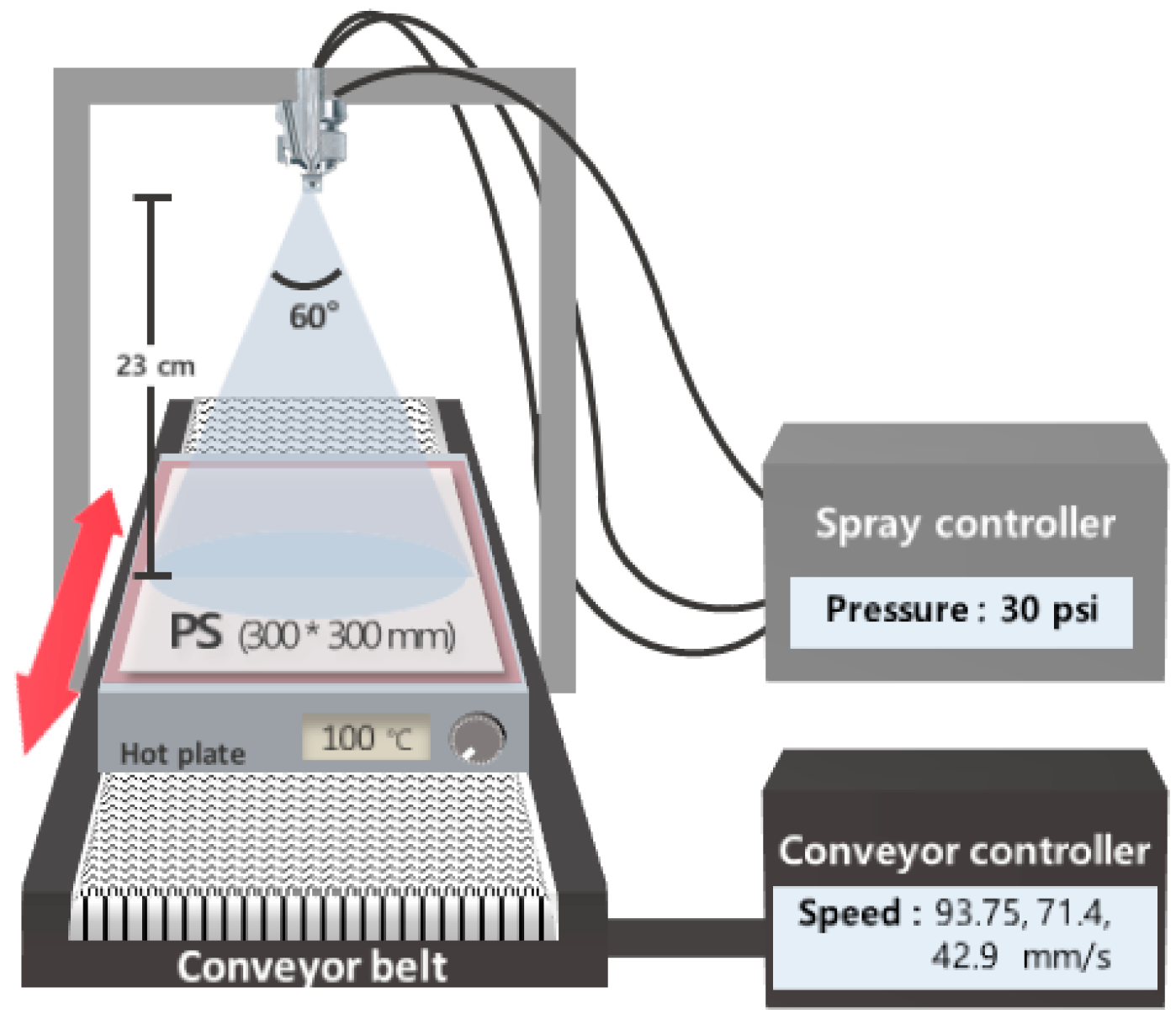
2.3.1. Spray Coating
2.3.2. Surface Profiling Measuring System
2.3.3. Raman Spectroscopy
2.3.4. Ultraviolet/Visible Light Spectrophotometer
2.3.5. Contact Angle Measurements
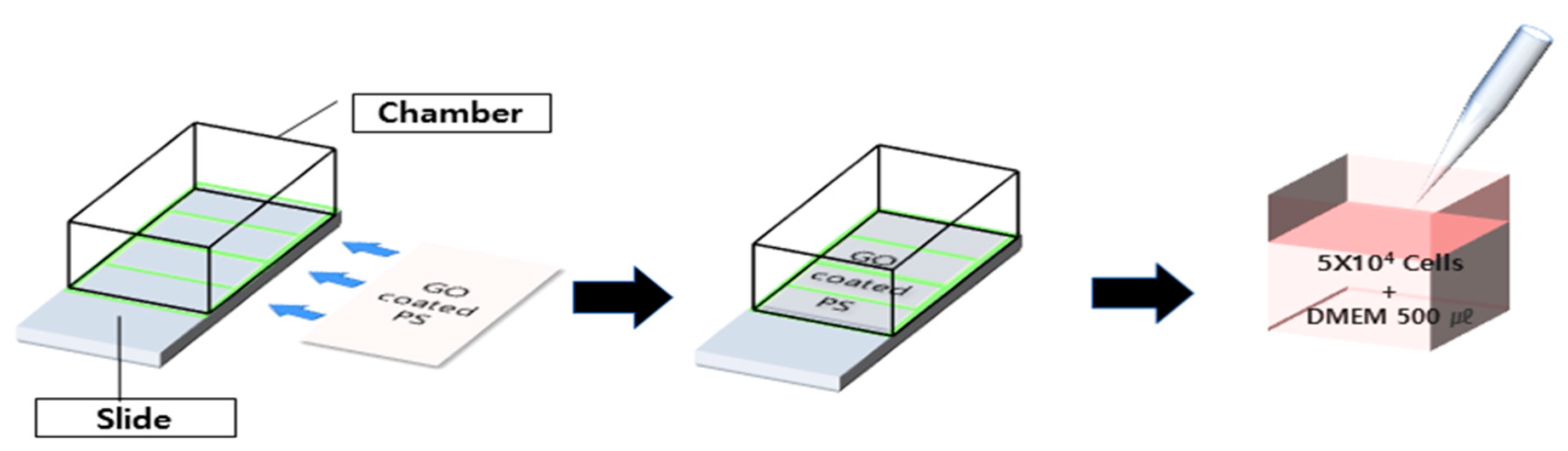
2.3.6. Human Fibroblast Cells Culture Test
2.3.7. Human Bone Marrow-Derived Mesenchymal Stem Cells (hBM-MSCs) Culture Test
Cell Viability and Proliferation Analysis
Flow Cytometry
Enzyme-Linked Immunosorbent Assay (ELISA)
3. Results
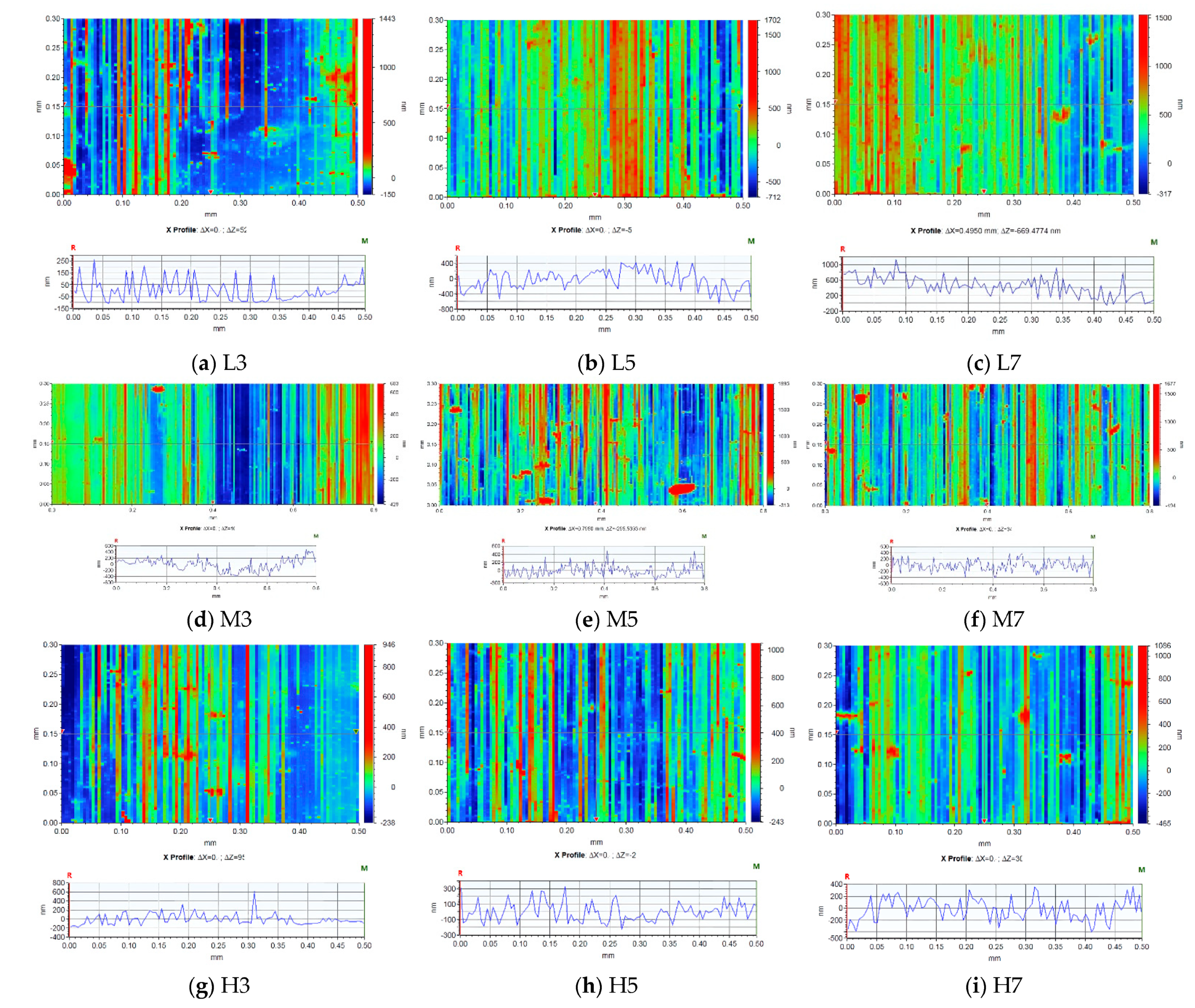
3.1. Step Measurements
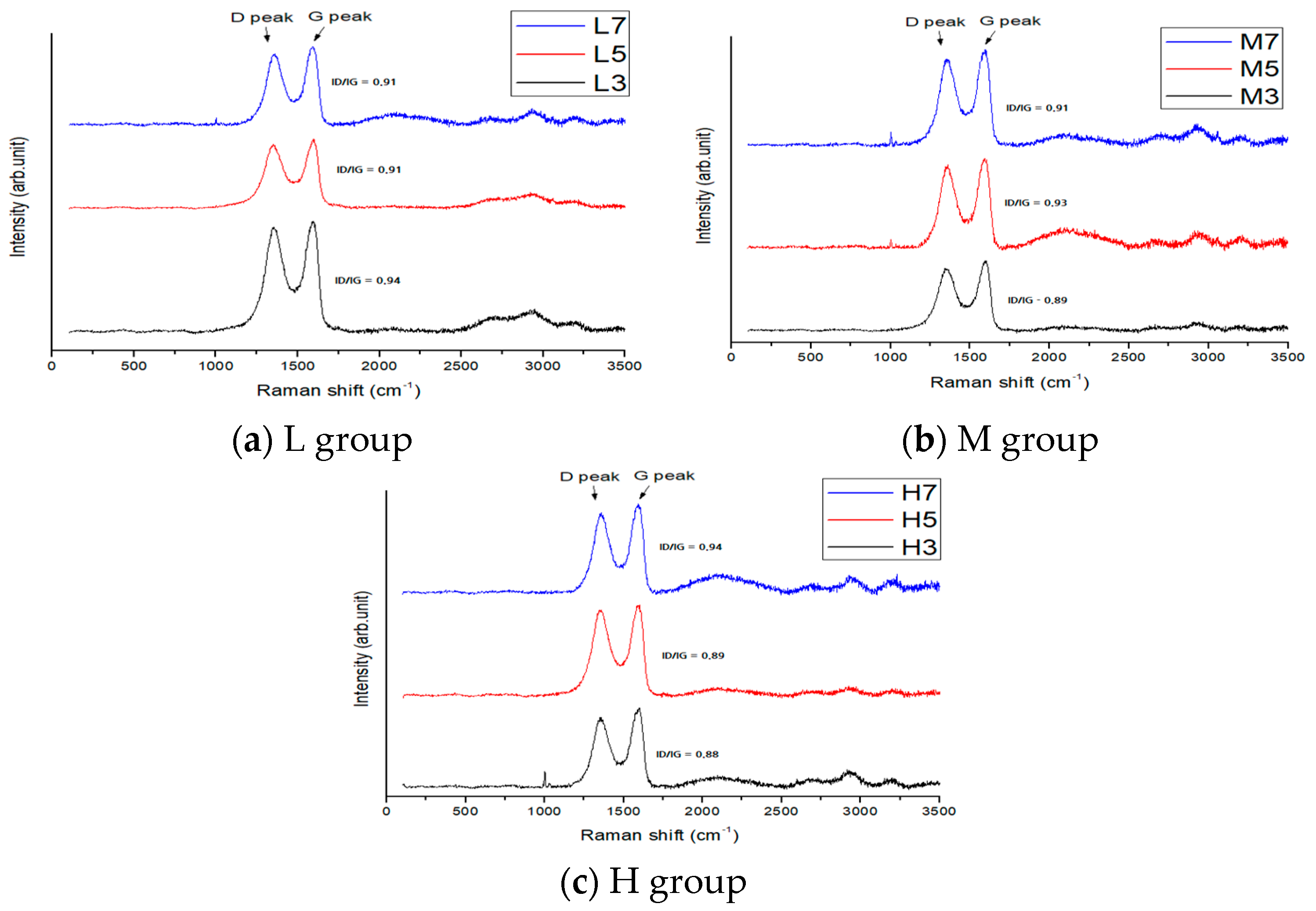
3.2. Raman Spectroscopy
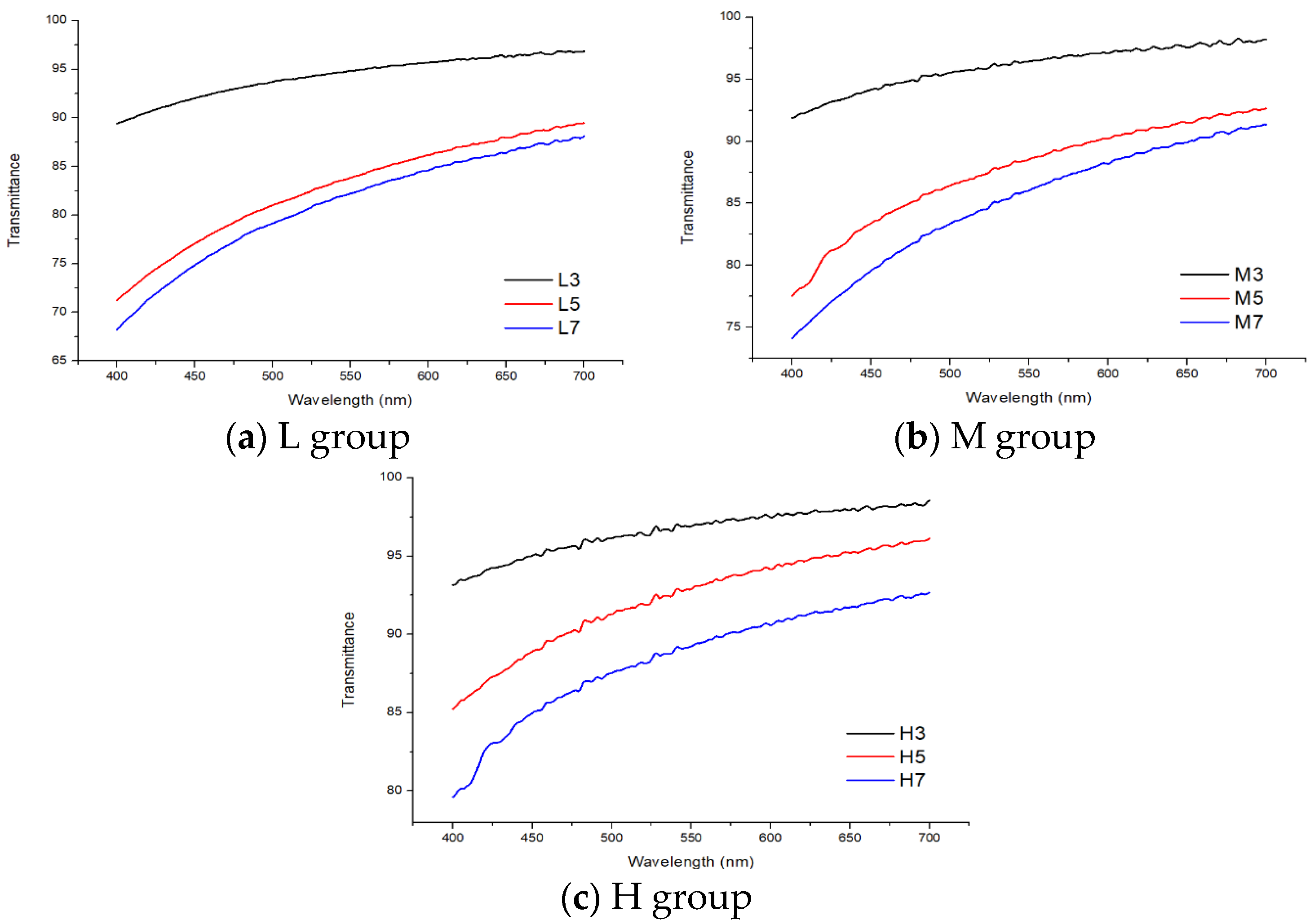
3.3. Permeability
3.4. Contact Angle
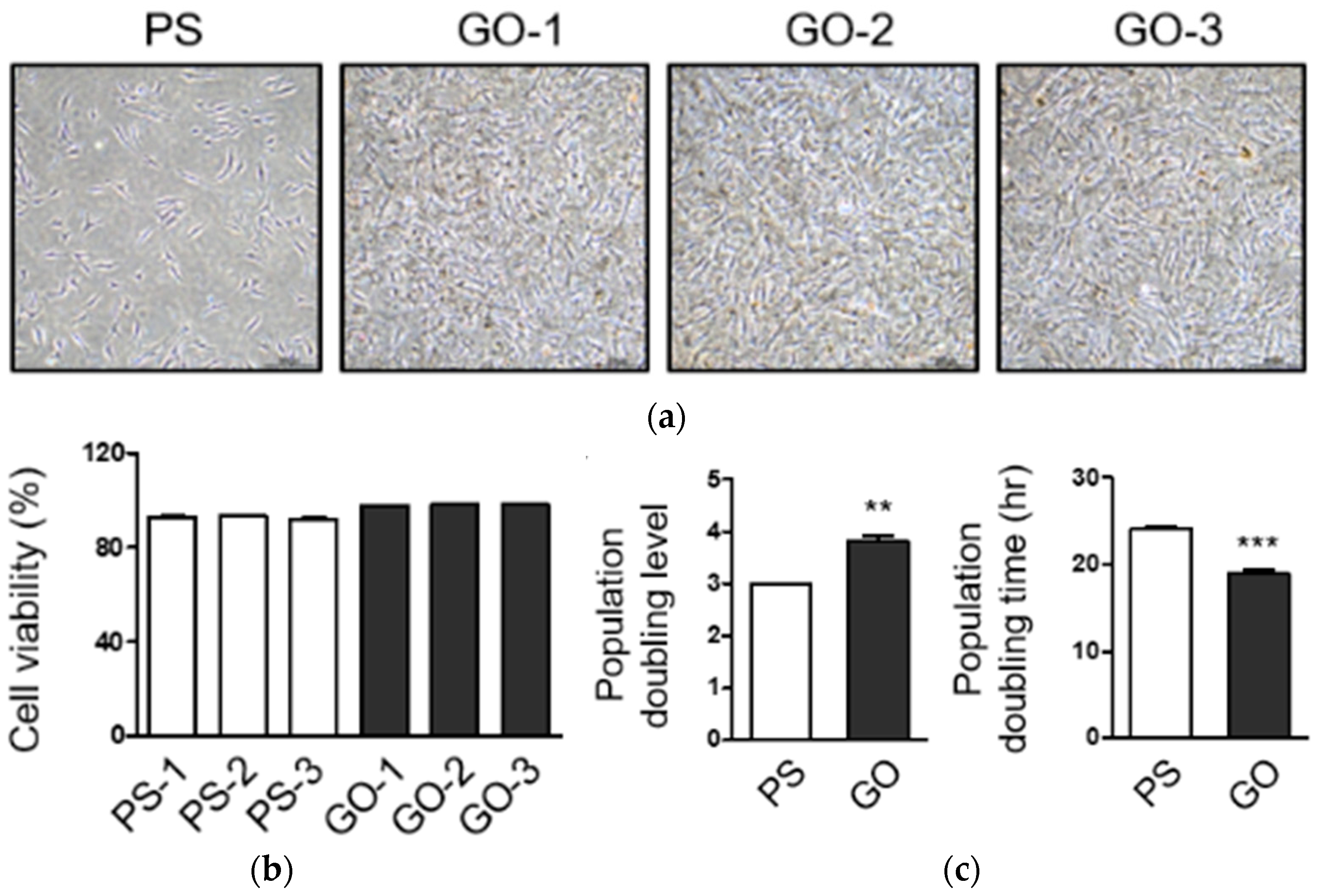
3.5. Human Fibro Blast Cell’s Viability and Proliferation
3.6. hBM-MSCs Cell Culture Test
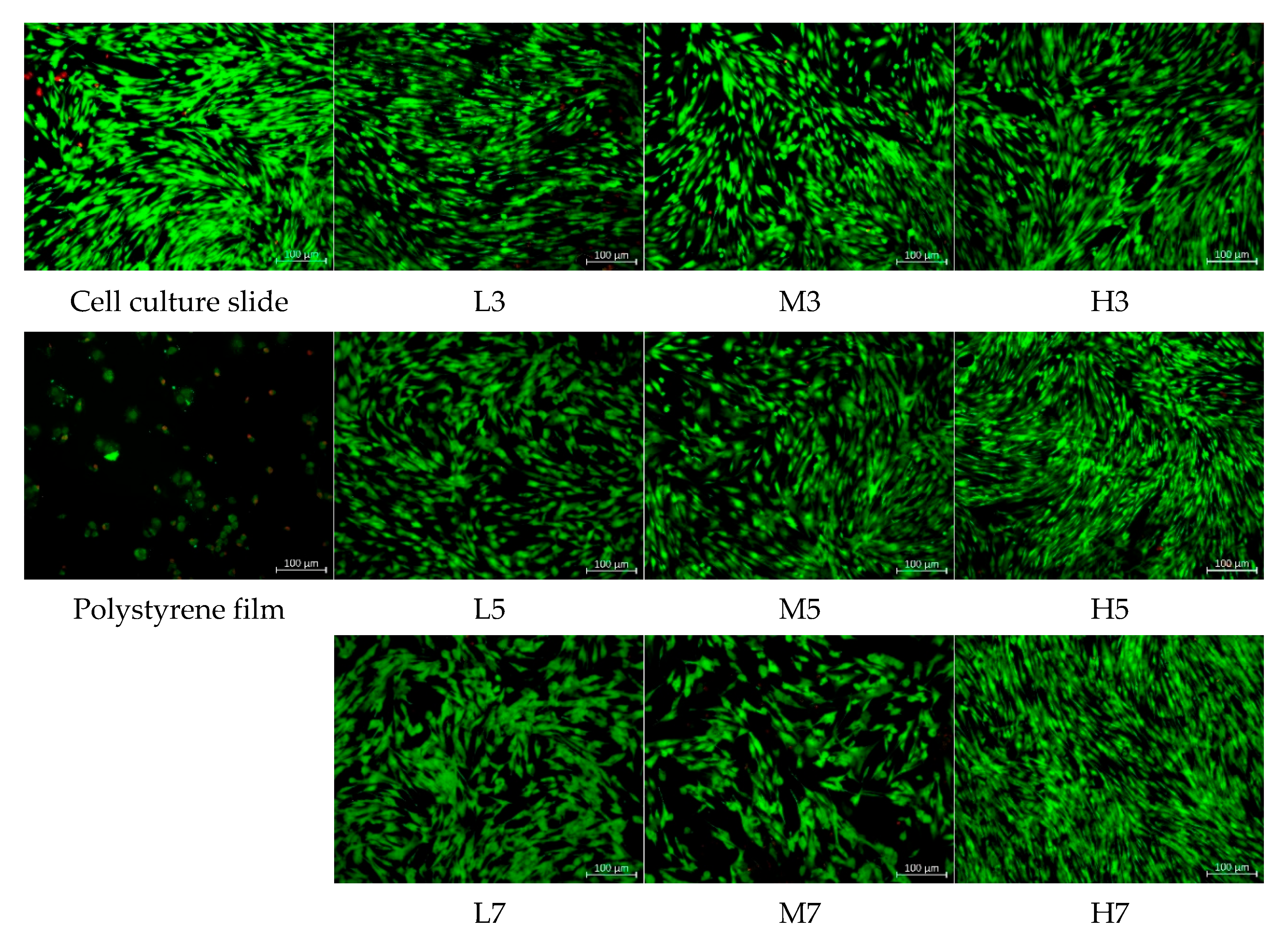
3.6.1. Cell Morphology Analysis
3.6.2. Cell Proliferation Analysis
3.6.3. Flow Cytometric Analysis of MSC Markers
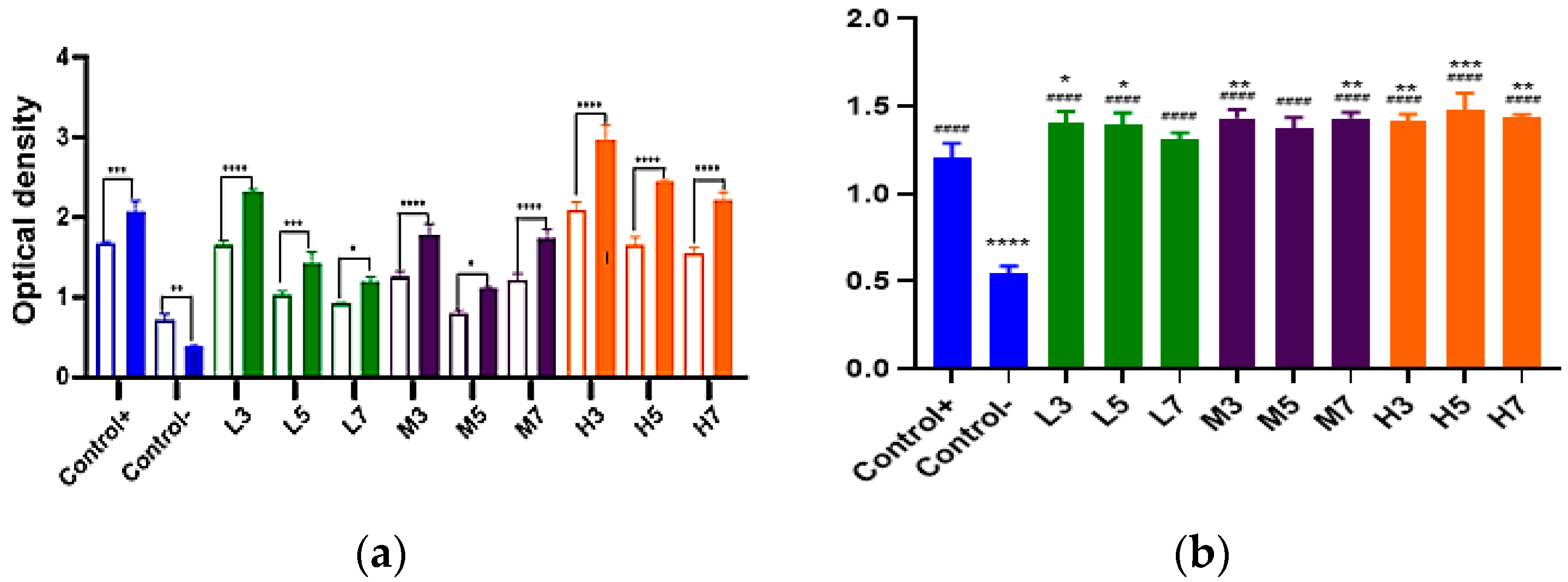
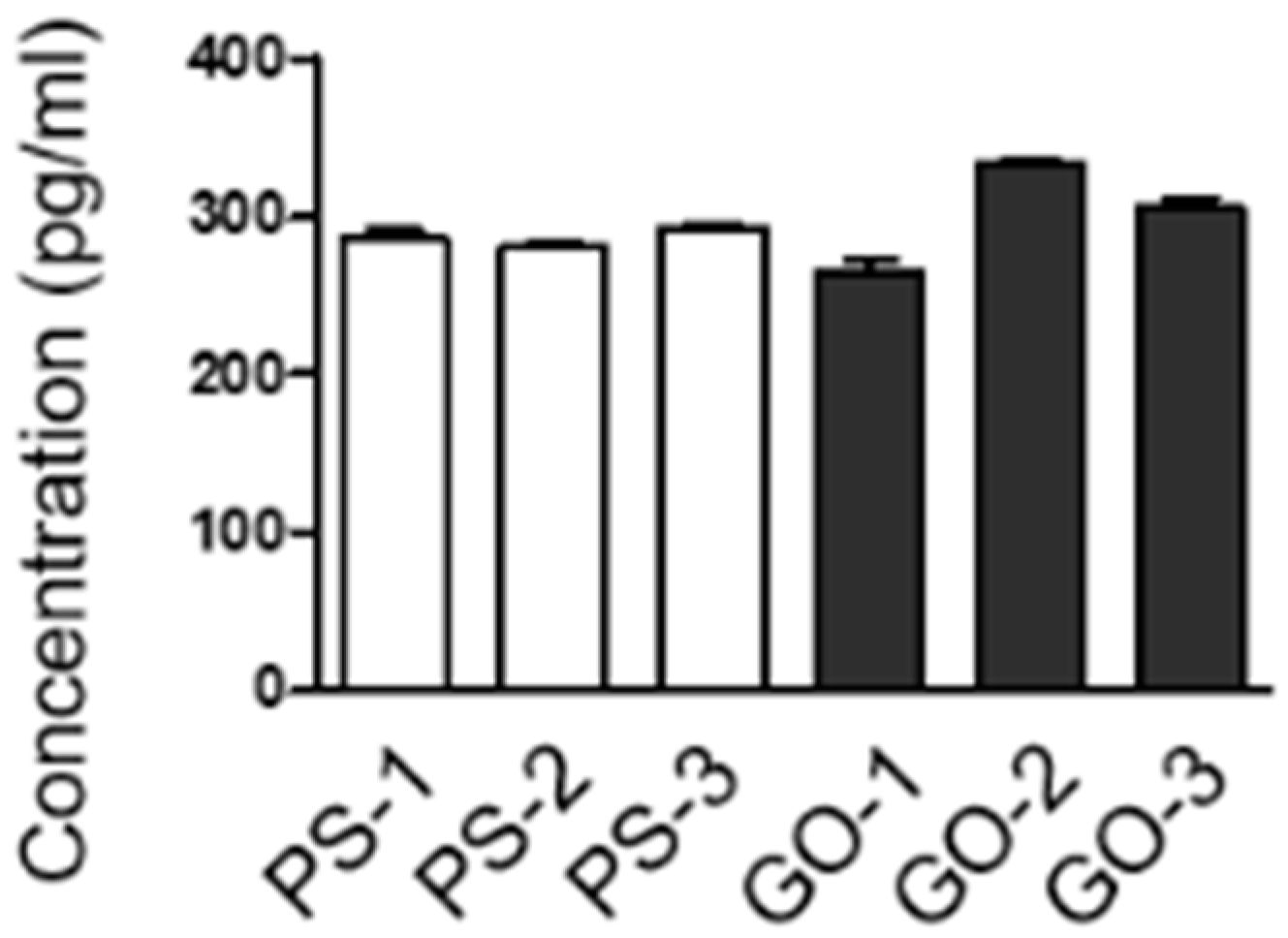
3.6.4. The Secretion of a Cytokine
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Ovid’Ko, I.A. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Han, J.T.; Jeong, S.Y.; Jeong, H.J.; Lee, G.W. Preparation of chemically exfoliated graphene nanosheets and its applications. KIC News 2012, 15, 23–37. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.S.; Li, J.; Zhao, X.S. Improving the thermal and mechanical properties of silicone polymer by incorporating functionalized graphene oxide. J. Mater. Sci. 2013, 48, 5287–5294. [Google Scholar] [CrossRef]
- Han, Z.Y.; Huang, L.J.; Qu, H.J. A review of performance improvement strategies for graphene oxide-based and graphene-based membranes in water treatment. J. Mater. Sci. 2021, 56, 9545–9574. [Google Scholar] [CrossRef]
- Hu, J.; Jia, X.; Li, C.; Ma, Z.; Zhang, G.; Sheng, W.; Zhang, X.; Wei, Z. Effect of interfacial interaction between graphene oxide derivatives and poly (vinyl chloride) upon the mechanical properties of their nanocomposites. J. Mater. Sci. 2014, 49, 2943–2951. [Google Scholar] [CrossRef]
- Politano, G.G.; Versace, C. Electrical and Optical Characterization of Graphene Oxide and Reduced Graphene Oxide Thin Films. Crystals 2022, 12, 1312. [Google Scholar] [CrossRef]
- Park, S. History of graphene oxide and future direction. KIC News 2013, 16, 1–5. [Google Scholar]
- Girão, A.F.; Serrano, M.C.; Completo, A.; Marques, P.A. Do biomedical engineers dream of graphene sheets? Biomater. Sci. 2019, 7, 1228–1239. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M.J.N.T. Can graphene take part in the fight against COVID-19? Nano Today 2020, 33, 100883. [Google Scholar] [CrossRef]
- Raghav, P.K.; Mohanty, S. Are graphene and graphene-derived products capable of preventing COVID-19 infection? Med. Hypotheses 2020, 144, 110031. [Google Scholar]
- Kim, S.J.; Choi, Y.Y.; Lee, W.J.; Kim, K.B. Fabrication Parameter-Dependent Physico-Chemical Properties of hiolated Gelatin/PEGDA Interpenetrating Network Hydrogels. Tissue Eng. Regen. Med. 2021, 19, 309–319. [Google Scholar] [CrossRef]
- Christopherson, G.T.; de Vasconcellos, J.F.; John, C.; Griffin, D.W.; Jones, P.E.; Nesti, L.J. Three-Dimensional Modeling of the Structural Microenvironment in Post-Traumatic War Wounds. Tissue Eng. Regen. Med. 2021, 18, 963–973. [Google Scholar] [CrossRef]
- Park, D.B.; Park, J.B.; Lee, J.H.; Shim, C.J.; Kim, M.S.; Lee, T.Y.; Lim, J.O. Fabrication and Characterization of Graphene Oxide-Coated Plate for Efficient Culture of Stem Cells. Tissue Eng. Regen. Med. 2021, 18, 775–785. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, J.H.; Huh, J.S.; Park, D.B.; Lim, J.O. Investigation on the polystyrene surface coating method of graphene oxide. J. Korean Inst. Surf. Eng. 2021, 54, 77–83. [Google Scholar]
- Lee, J.H.; Park, J.B.; Park, D.B.; Huh, J.S.; Lim, J.O. Coating properties of single and multi-layer graphene oxide on a polystyrene surface. Korean J. Mater. Res. 2021, 31, 420–426. [Google Scholar] [CrossRef]
- Groth, T.; Altankov, G. Studies on cell-biomaterial interaction: Role of tyrosine phosphorylation during fibroblast spreading on surfaces varying in wettability. Biomaterials 1996, 17, 1227–1234. [Google Scholar] [CrossRef]
- Jo, D.A.; Song, K.S. Cell count sensor using graphene FET. J. Inst. Electron. Inf. Eng. 2021, 58, 71–76. [Google Scholar]
- Horbett, T.A. The role of adsorbed proteins in animal cell adhesion. Colloids Surf. B Biointerfaces 1994, 2, 225–240. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Cell adhesion to plasma-treated polymer surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Skarlatos, S.I.; Rao, R.; Kruth, H.S. Accelerated development of human monocyte-macrophages cultured on Plastek-C tissue culture dishes. J. Tissue Cult. Methods 1992, 14, 113–117. [Google Scholar] [CrossRef]
- Hong, J.W.; Shin, J.H. Effects of surface geometrical properties on cellular adhesion behavior. In Proceedings of the Korean Society of Mechanical Engineers, Lectures and Abstracts, Fall Conference, Pyeong Chang, Korea, 4–11 November 2009; pp. 2963–2966. [Google Scholar]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth–facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]



| Speed | 42.90 mm/s | 71.40 mm/s | 93.75 mm/s | |
|---|---|---|---|---|
| Time | ||||
| 3 | L3 | M3 | H3 | |
| 5 | L5 | M5 | H5 | |
| 7 | L7 | M7 | H7 | |
| L3 | L5 | L7 | M3 | M5 | M7 | H3 | H5 | H7 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ra a (nm) | 73 | 210 | 458 | 152 | 118 | 141 | 93 | 98 | 148 | |
| Rq b (nm) | 98 | 262 | 521 | 193 | 166 | 183 | 124 | 125 | 181 | |
| L3 | L5 | L7 | M3 | M5 | M7 | H3 | H5 | H7 | PS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | 40.0 | 39.0 | 39.8 | 51.0 | 36.6 | 38.4 | 54.6 | 36.2 | 36.0 | 85.6 | |
| deviation | 0.46 | 0.51 | 0.69 | 2.28 | 0.62 | 0.75 | 1.73 | 0.99 | 0.44 | 0.80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-b.; Park, D.-b.; Lee, J.-h.; Yang, S.-j.; Lee, J.-e.; Park, J.-K.; Huh, J.-S.; Lim, J.-O. Application of Graphene Oxide as a Biomaterial for the Development of Large-Area Cell Culture Vessels. Appl. Sci. 2022, 12, 11599. https://doi.org/10.3390/app122211599
Park J-b, Park D-b, Lee J-h, Yang S-j, Lee J-e, Park J-K, Huh J-S, Lim J-O. Application of Graphene Oxide as a Biomaterial for the Development of Large-Area Cell Culture Vessels. Applied Sciences. 2022; 12(22):11599. https://doi.org/10.3390/app122211599
Chicago/Turabian StylePark, Jae-bum, Dan-bi Park, Ji-hoon Lee, Su-jeong Yang, Ji-eun Lee, Jin-Kyung Park, Jeung-Soo Huh, and Jeong-Ok Lim. 2022. "Application of Graphene Oxide as a Biomaterial for the Development of Large-Area Cell Culture Vessels" Applied Sciences 12, no. 22: 11599. https://doi.org/10.3390/app122211599
APA StylePark, J.-b., Park, D.-b., Lee, J.-h., Yang, S.-j., Lee, J.-e., Park, J.-K., Huh, J.-S., & Lim, J.-O. (2022). Application of Graphene Oxide as a Biomaterial for the Development of Large-Area Cell Culture Vessels. Applied Sciences, 12(22), 11599. https://doi.org/10.3390/app122211599






