Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside
Abstract
:1. Introduction
- “Priority 1 (critical): carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant ESBL-producing Enterobacteriaceae
- Priority 2 (high): vancomycin-resistant Enterococcus faecium, methicillin-resistant, vancomycin-intermediate and resistant Staphylococcus aureus, clarithromycin-resistant Helicobacter pylori, fluoroquinolone-resistant Campylobacter spp., fluoroquinolone-resistant Salmonellae, cephalosporin-resistant, fluoroquinolone-resistant Neisseria gonorrhoeae
- Priority 3 (medium): penicillin-non-susceptible Streptococcus pneumoniae, ampicillin-resistant Haemophilus influenzae and fluoroquinolone-resistant Shigella spp.” [2]
2. Results of Systematic Search for Clinical Trials
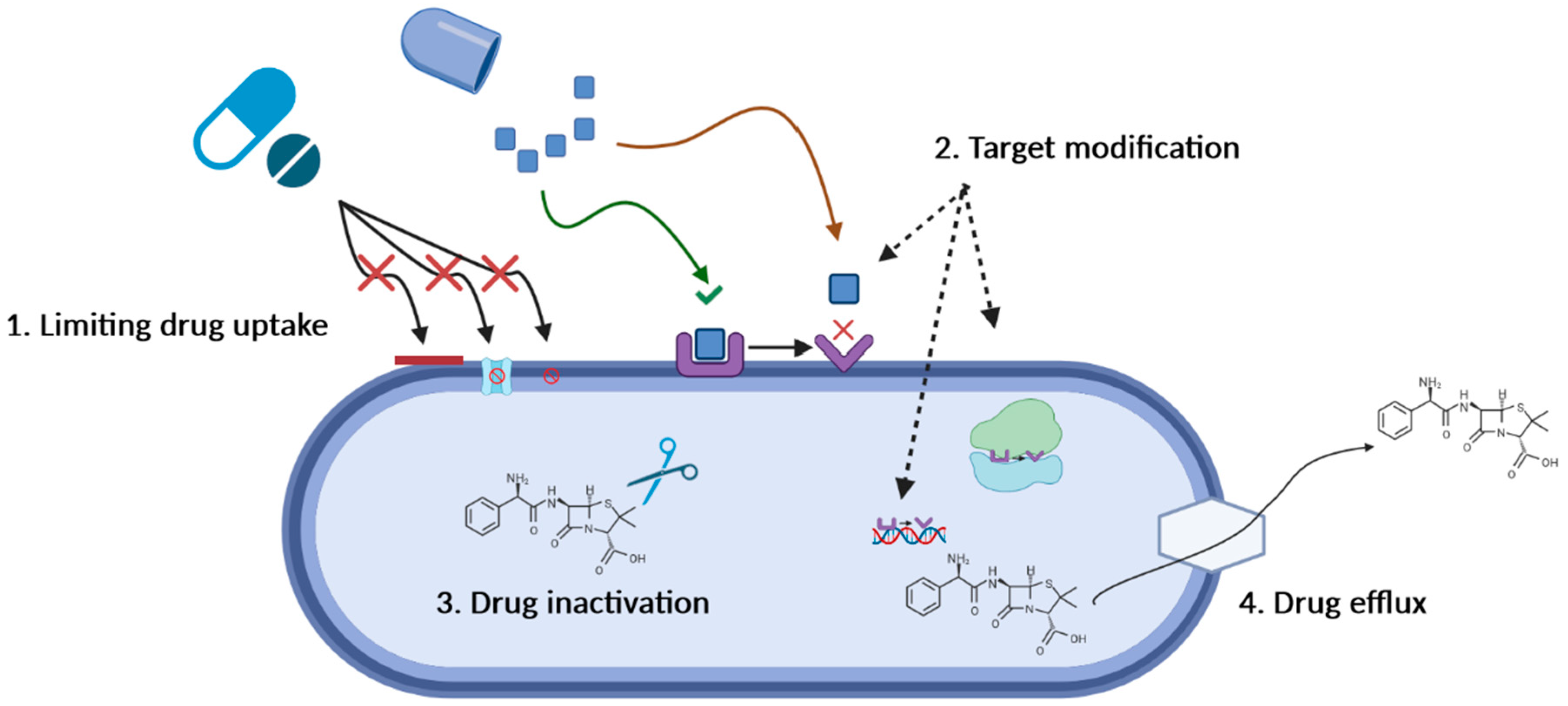
3. Antimicrobial Resistance—Mechanisms and Mitigation Strategies
3.1. Limiting Drug Uptake
3.2. Target Modification
3.3. Drug Inactivation
3.4. Active Drug Efflux
3.5. Strategies for Mitigating the Occurrence of Antimicrobial Resistance
4. Drug Delivery Systems—Applications in Infectious Diseases
4.1. Targeted Antibiotic Release
4.2. Sustained Local Release for Bone Applications
4.3. Stimuli-Responsive DDS for Triggered Antibiotic Release
4.3.1. pH-Sensitive DDS
4.3.2. Pathogen-Sensitive DDS
4.4. Ultrasound- and Light-Sensitive DDS
4.5. Enhanced Antimicrobial Activity
4.6. “Trojan Horse” Strategies in DDS for Infectious Diseases
4.7. Bench-to-Bedside and Technology Readiness in DDS for Infectious Diseases
5. Novel Targets—Antivirulence Therapeutics
5.1. Disrupting Quorum Sensing
5.2. Inhibiting Bacterial Adherence
5.3. Inhibiting Biofilm Formation
5.4. Silencing Virulence Traits
5.5. Inhibiting Betalactamases
5.6. Neutralization of Toxins—Bench-to-Bedside in Antivirulence Therapeutics
5.7. Theragnostics in Infectious Diseases
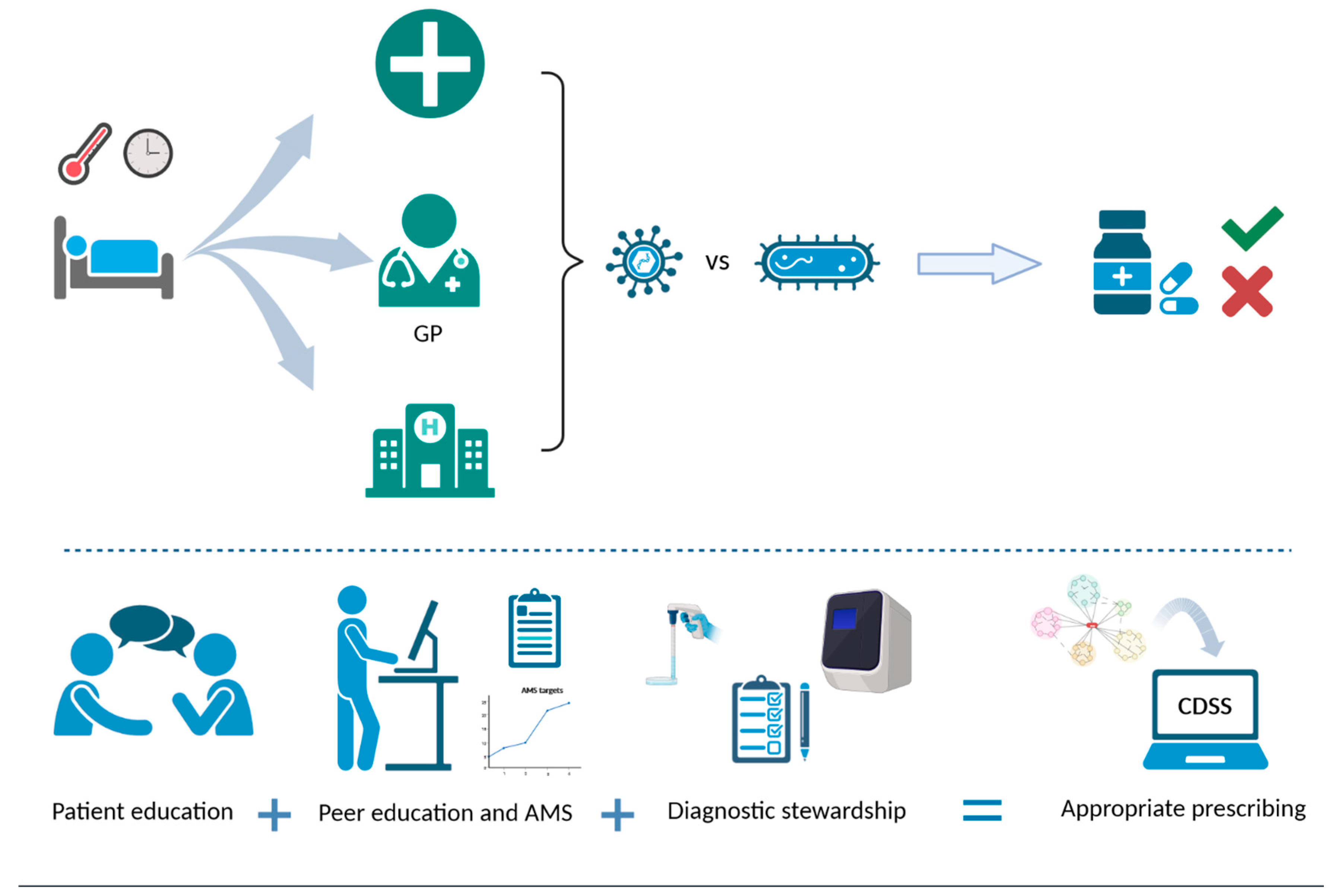
6. Rational Antimicrobial Use Practices
6.1. Antimicrobial Stewardship
6.2. Diagnostic Stewardship
6.3. Education on Rational Antimicrobial Use
6.4. International Standards on Rational Antimicrobial Use
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 26 February 2022).
- Antimicrobial Resistance Collaboratos. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Miron, V.D.; Filimon, C.; Cabel, T.; Mihăescu, R.I.; Bar, G.; Leu, D.; Craiu, M. Urinary tract infections in children: Clinical and antimicrobial resistance data from Bucharest area, Romania. Germs 2021, 11, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Durand-Reville, T.F.; Guler, S.; Comita-Prevoir, J.; Chen, B.; Bifulco, N.; Huynh, H.; Lahiri, S.; Shapiro, A.B.; McLeod, M.S.; Carter, M.D.; et al. ETX2514 is a broad-spectrum beta-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat. Microbiol. 2017, 2, 17104. [Google Scholar] [CrossRef]
- Ali, S.O.; Yu, X.Q.; Robbie, G.J.; Wu, Y.; Shoemaker, K.; Yu, L.; Di Giandormenico, A.; Keller, A.E.; Anude, C.; Hernandez-Illas, M.; et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019, 25, 629.e1–629.e6. [Google Scholar] [CrossRef] [Green Version]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Trejo, A.; Ruiz-Ruiz, J.M.; Gonzalez-Avila, L.U.; Saldaña-Padilla, A.; Hernández-Cortez, C.; Loyola-Cruz, M.A.; Bello-López, J.M.; Castro-Escarpulli, G. Evasion of Antimicrobial Activity in Acinetobacter baumannii by Target Site Modifications: An Effective Resistance Mechanism. Int. J. Mol. Sci. 2022, 23, 6582. [Google Scholar] [CrossRef]
- Bhujbalrao, R.; Gavvala, K.; Singh, R.K.; Singh, J.; Boudier, C.; Chakrabarti, S.; Patwari, G.N.; Mély, Y.; Anand, R. Identification of allosteric hotspots regulating the ribosomal RNA binding by antibiotic resistance-conferring Erm methyltransferases. J. Biol. Chem. 2022, 298, 102208. [Google Scholar] [CrossRef] [PubMed]
- Krucinska, J.; Lombardo, M.N.; Erlandsen, H.; Estrada, A.; Si, D.; Viswanathan, K.; Wright, D.L. Structure-guided functional studies of plasmid-encoded dihydrofolate reductases reveal a common mechanism of trimethoprim resistance in Gram-negative pathogens. Commun. Biol. 2022, 5, 459. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Wright, G.D. Antibiotic resistance by enzyme inactivation: From mechanisms to solutions. ChemBioChem 2010, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-Silva, F.; Gaspar, M.; Aguiar, S. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ranzoni, A.; Phetsang, W.; Blaskovich, M.A.T.; Cooper, M.A. Surface Ligand Density of Antibiotic-Nanoparticle Conjugates Enhances Target Avidity and Membrane Permeabilization of Vancomycin-Resistant Bacteria. Bioconjugate Chem. 2017, 28, 353–361. [Google Scholar] [CrossRef]
- Ambrose, C.G.; Gogola, G.R.; Clyburn, T.A.; Raymond, A.K.; Peng, A.S.; Mikos, A.G. Antibiotic Microspheres: Preliminary Testing for Potential Treatment of Osteomyelitis. Clin. Orthop. Relat. Res. 2003, 415, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Szczeblinska, J.; Fijalkowski, K.; Kohn, J.; El Fray, M. Antibiotic loaded microspheres as antimicrobial delivery systems for medical applications. Mater. Sci. Eng. C 2017, 77, 69–75. [Google Scholar] [CrossRef]
- Shen, S.-C.; Letchmanan, K.; Chow, P.S.; Tan, R.B.H. Antibiotic elution and mechanical property of TiO2 nanotubes functionalized PMMA-based bone cements. J. Mech. Behav. Biomed. Mater. 2018, 91, 91–98. [Google Scholar] [CrossRef]
- Zaborniak, I.; Macior, A.; Chmielarz, P. Stimuli-Responsive Rifampicin-Based Macromolecules. Materials 2020, 13, 3843. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Xing, X.; Zhang, J.; Zhang, M.-R.; Ma, X.; Shi, R.; Zhang, L. Smart Titanium Coating Composed of Antibiotic Conjugated Peptides as an Infection-Responsive Antibacterial Agent. Macromol. Biosci. 2021, 21, e2000194. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.G.; Wang, T.; Harrington, W.N.; Zharov, V.P.; Johnson, S.A.; Jenkins, S.V.; Oyibo, S.E.; Walker, C.M.; Mills, W.B.; Shirtliff, M.E.; et al. Versatility of targeted antibiotic-loaded gold nanoconstructs for the treatment of biofilm-associated bacterial infections. Int. J. Hyperth. 2018, 34, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Mordovina, E.A.; Plastun, V.O.; Abdurashitov, A.S.; Proshin, P.I.; Raikova, S.V.; Bratashov, D.N.; Inozemtseva, O.A.; Goryacheva, I.Y.; Sukhorukov, G.B.; Sindeeva, O.A. “Smart” Polylactic Acid Films with Ceftriaxone Loaded Microchamber Arrays for Personalized Antibiotic Therapy. Pharmaceutics 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Khan, F.; Hoang, G.; Mondal, S.; Kim, H.; Doan, V.H.M.; Kim, Y.-M.; Oh, J. Thiol chitosan-wrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr. Polym. 2019, 225, 115228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26711–26721. [Google Scholar] [CrossRef] [PubMed]
- Khantamat, O.; Li, C.-H.; Yu, F.; Jamison, A.C.; Shih, W.-C.; Cai, C.; Lee, T.R. Gold Nanoshell-Decorated Silicone Surfaces for the Near-Infrared (NIR) Photothermal Destruction of the Pathogenic Bacterium E. faecalis. ACS Appl. Mater. Interfaces 2015, 7, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2018, 188, 83–95. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Capeletti, L.B.; de Oliveira, L.F.; Goncalves Kde, A.; de Oliveira, J.F.; Saito, A.; Kobarg, J.; Dos Santos, J.H.Z.; Cardoso, M.B. Tailored silica-antibiotic nanoparticles: Overcoming bacterial resistance with low cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Raiz, A.; Unni, A.D.; Murhekar, S.; Donose, B.; Floetenmeyer, M.; Cock, I.; Brown, C.L. Combating Antibiotic-Resistant Gram-Negative Bacteria Strains with Tetracycline-Conjugated Carbon Nanoparticles. Adv. Biosyst. 2020, 4, e2000074. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, S.; Gasser, V.; Peukert, C.; Pinkert, L.; Kuhn, L.; Perraud, Q.; Normant, V.; Brönstrup, M.; Schalk, I.J. Uptake Mechanisms and Regulatory Responses to MECAM- and DOTAM-Based Artificial Siderophores and Their Antibiotic Conjugates in Pseudomonas aeruginosa. ACS Infect. Dis. 2022, 8, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Nolan, E.M. Heavy-metal Trojan horse: Enterobactin-directed delivery of platinum(IV) prodrugs to Escherichia coli. J. Am. Chem. Soc. 2022, 144, 12756–12768. [Google Scholar] [CrossRef]
- Porosk, L.; Langel, U. Approaches for evaluation of novel CPP-based cargo delivery systems. Front. Pharmacol. 2022, 13, 1056467. [Google Scholar] [CrossRef]
- Krivic, H.; Himbert, S.; Sun, R.; Feigis, M.; Rheinstadter, M.C. Erythro-PmBs: A Selective Polymyxin B Delivery System Using Antibody-Conjugated Hybrid Erythrocyte Liposomes. ACS Infect. Dis. 2022, 8, 2059–2072. [Google Scholar] [CrossRef]
- Kajihara, K.K.; Pantua, H.; Hernandez-Barry, H.; Hazen, M.; Deshmukh, K.; Chiang, N.; Ohri, R.; Castellanos, E.R.; Martin, L.; Matsumoto, M.L.; et al. Potent Killing of Pseudomonas aeruginosa by an Antibody-Antibiotic Conjugate. mBio 2021, 12, e0020221. [Google Scholar] [CrossRef]
- Luong, A.D.; Buzid, A.; Luong, J.H.T. Important Roles and Potential Uses of Natural and Synthetic Antimicrobial Peptides (AMPs) in Oral Diseases: Cavity, Periodontal Disease, and Thrush. J. Funct. Biomater. 2022, 13, 175. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, R.; Chai, C.; Wang, Y.; Chen, T.; Li, H.; Hu, Y.; Feng, Q.; Li, J. Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications. Front. Bioeng. Biotechnol. 2022, 10, 1030162. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Squire, M.W.; Ludwig, B.J.; Thompson, J.R.; Jagodzinski, J.; Hall, D.; Andes, D. Premixed antibiotic bone cement: An in vitro comparison of antimicrobial efficacy. J. Arthroplasty. 2008, 23, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Executive Summary. Classification of Wound Dressings Combined with Drugs; FDA: Silver Spring, MD, USA, 2016.
- Dehbanipour, R.; Ghalavand, Z. Anti-virulence therapeutic strategies against bacterial infections: Recent advances. Germs 2022, 12, 262–275. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence Strategies for the Treatment of Staphylococcus aureus Infections: A Mini Review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef] [PubMed]
- Fleitas Martinez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent Advances in Anti-virulence Therapeutic Strategies With a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front. Cell Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Palaniappan, B.; Solomon, A.P. Targeting AgrA quorum sensing regulator by bumetanide attenuates virulence in Staphylococcus aureus—A drug repurposing approach. Life Sci. 2021, 273, 119306. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through sRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef] [Green Version]
- Suman, E.; D′souza, S.; Jacob, P.; Sushruth, M.R.; Kotian, M. Anti-biofilm and anti-adherence activity of Glm-U inhibitors. Indian J. Med. Sci. 2011, 65, 387–392. [Google Scholar] [CrossRef]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Sheng, Y.; Zhang, J.; Xu, Y.; Yu, J.; Wang, B.; Zhao, H.; Wang, X.; Guo, Y.; Wu, X.; et al. Small-Molecule Compound SYG-180-2-2 to Effectively Prevent the Biofilm Formation of Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 770657. [Google Scholar] [CrossRef]
- Song, W.; Wang, L.; Jin, M.; Guo, X.; Wang, X.; Guan, J.; Zhao, Y. Punicalagin, an Inhibitor of Sortase A, Is a Promising Therapeutic Drug to Combat Methicillin-Resistant Staphylococcus aureus Infections. Antimicrob. Agents Chemother. 2022, 66, e0022422. [Google Scholar] [CrossRef]
- Shamprasad, B.R.; Lotha, R.; Nagarajan, S.; Sivasubramanian, A. Metal nanoparticles functionalized with nutraceutical Kaempferitrin from edible Crotalaria juncea, exert potent antimicrobial and antibiofilm effects against Methicillin-resistant Staphylococcus aureus. Sci. Rep. 2022, 12, 7061. [Google Scholar] [CrossRef] [PubMed]
- Bajire, S.K.; Jain, S.; Johnson, R.P.; Shastry, R.P. 6-Methylcoumarin attenuates quorum sensing and biofilm formation in Pseudomonas aeruginosa PAO1 and its applications on solid surface coatings with polyurethane. Appl. Microbiol. Biotechnol. 2021, 105, 8647–8661. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Hübner, I.; Sieber, S.A. Tailored Phenyl Esters Inhibit ClpXP and Attenuate Staphylococcus aureus α-Hemolysin Secretion. ChemBioChem 2022, 23, e202200253. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xu, Y.; Shen, L.; Wang, X.; Zhao, H.; Wang, B.; Zhang, J.; Xiao, Y.; Guo, Y.; Sheng, Y.; et al. Small-molecule compound SYG-180-2-2 attenuates Staphylococcus aureus virulence by inhibiting hemolysin and staphyloxanthin production. Front. Cell. Infect. Microbiol. 2022, 12, 1008289. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, A.J.; Avery, L.M.; Nicolau, D.P.; Asempa, T.E. In vivo pharmacokinetics and pharmacodynamics of ceftibuten/ledaborbactam, a novel oral beta-lactam/beta-lactamase inhibitor combination. J. Antimicrob. Chemother. 2022, dkac359. [Google Scholar] [CrossRef] [PubMed]
- Ooi, N.; Lee, V.E.; Chalam-Judge, N.; Newman, R.; Wilkinson, A.J.; Cooper, I.R.; Orr, D.; Lee, S.; Savage, V.J. Restoring carbapenem efficacy: A novel carbapenem companion targeting metallo-beta-lactamases in carbapenem-resistant Enterobacterales. J. Antimicrob. Chemother. 2021, 76, 460–466. [Google Scholar] [CrossRef]
- Han, X.; Ortines, R.; Mukherjee, I.; Kanipakala, T.; Kort, T.; Sherchand, S.P.; Liao, G.; Mednikov, M.; Chenine, A.L.; Aman, M.J.; et al. Hyperimmune Targeting Staphylococcal Toxins Effectively Protect Against USA 300 MRSA Infection in Mouse Bacteremia and Pneumonia Models. Front. Immunol. 2022, 13, 893921. [Google Scholar] [CrossRef]
- François, B.; Jafri, H.S.; Chastre, J.; Sánchez-García, M.; Eggimann, P.; Dequin, P.-F.; Huberlant, V.; Soria, L.V.; Boulain, T.; Bretonnière, C.; et al. Efficacy and safety of suvratoxumab for prevention of Staphylococcus aureus ventilator-associated pneumonia (SAATELLITE): A multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 2 pilot trial. Lancet Infect. Dis. 2021, 21, 1313–1323. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Pollack, M.; Callahan, L.T., 3rd; Taylor, N.S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: Evidence for in vivo toxin production during infection. Infect. Immun. 1976, 14, 942–947. [Google Scholar] [CrossRef]
- Shadman, Z.; Farajnia, S.; Pazhang, M.; Tohidkia, M.; Rahbarnia, L.; Najavand, S.; Toraby, S. Isolation and characterizations of a novel recombinant scFv antibody against exotoxin A of Pseudomonas aeruginosa. BMC Infect. Dis. 2021, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Kong-Ngoen, T.; Chongsa-Nguan, M.; Boonyuen, U.; Pumirat, P.; Sookrung, N.; Chaicumpa, W.; Indrawattana, N. Human Single-Chain Antibodies That Neutralize Elastolytic Activity of Pseudomonas aeruginosa LasB. Pathogens 2021, 10, 765. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Mousavi, R.S.M.; Bahador, A. DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Bahador, A. Aptamer decorated emodin nanoparticles-assisted delivery of dermcidin-derived peptide DCD-1L: Photoactive bio-theragnostic agent for Enterococcus faecalis biofilm destruction. Photodiagnosis Photodyn. Ther. 2022, 39, 103020. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Cissé, C.; Vitale, S.; Ahmadova, A.; Degardin, M.; Pérard, J.; Colas, P.; Miras, R.; Boturyn, D.; Covès, J.; et al. From Peptide Aptamers to Inhibitors of FUR, Bacterial Transcriptional Regulator of Iron Homeostasis and Virulence. ACS Chem. Biol. 2016, 11, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Grau, S.; Pano-Pardo, J.R.; Lopez, A.; Oliver, A.; Cisneros, J.M. Antimicrobial stewardship in Spain: Programs for Optimizing the use of Antibiotics (PROA) in Spanish hospitals. Germs 2018, 8, 109–112. [Google Scholar] [CrossRef]
- Aboderin, A.; Adeyemo, A.T.; Olayinka, A.; Oginni, A.S.; Adeyemo, A.T.; Oni, A.; Olabisi, O.F.; Fayomi, O.D.; Anuforo, A.C.; Egwuenu, A.; et al. Antimicrobial use among hospitalized patients: A multi-center, point prevalence survey across public healthcare facilities, Osun State, Nigeria. Germs 2021, 11, 523–535. [Google Scholar] [CrossRef]
- Stivers, T.; Timmermans, S. Arriving at no: Patient pressure to prescribe antibiotics and physicians’ responses. Soc. Sci. Med. 2021, 290, 114007. [Google Scholar] [CrossRef]
- Miron, V.D.; Craiu, M. “Red throat” or acute pharyngitis—Challenges in real life clinical practice. Germs 2021, 11, 351–353. [Google Scholar] [CrossRef]
- Gyssens, I.C. Role of Education in Antimicrobial Stewardship. Med. Clin. N. Am. 2018, 102, 855–871. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Prescribing and Use. Core Elements of Antibiotic Stewardship. 2022. Available online: https://www.cdc.gov/antibiotic-use/core-elements/index.html (accessed on 19 September 2022).
- Pezzani, M.D.; Carrara, E.; Sibani, M.; Presterl, E.; Gastmeier, P.; Renk, H.; Kanj, S.S.; Velavan, T.P.; Song, L.H.; Leibovici, L.; et al. White Paper: Bridging the gap between human and animal surveillance data, antibiotic policy and stewardship in the hospital sector-practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J. Antimicrob. Chemother. 2020, 75, ii20–ii32. [Google Scholar] [CrossRef] [PubMed]
- Arieti, F.; Göpel, S.; Sibani, M.; Carrara, E.; Pezzani, M.D.; Murri, R.; Mutters, N.T.; Lòpez-Cerero, L.; Voss, A.; Cauda, R.; et al. White Paper: Bridging the gap between surveillance data and antimicrobial stewardship in the outpatient sector—Practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J. Antimicrob. Chemother. 2020, 75, ii42–ii51. [Google Scholar] [CrossRef] [PubMed]
- Sibani, M.; Mazzaferri, F.; Carrara, E.; Pezzani, M.D.; Arieti, F.; Göpel, S.; Paul, M.; Tacconelli, E.; Mutters, N.T.; Voss, A.; et al. White Paper: Bridging the gap between surveillance data and antimicrobial stewardship in long-term care facilities—Practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J. Antimicrob. Chemother. 2020, 75, ii33–ii41. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. The Core Elements of Hospital Antibiotic Stewardship Programs: 2019; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Hwang, S.; Kwon, K.T. Core Elements for Successful Implementation of Antimicrobial Stewardship Programs. Infect. Chemother. 2021, 53, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Bonine, N.G.; Ye, J.M.; Folse, H.J.; Gillard, P. Development of a bedside tool to predict the probability of drug-resistant pathogens among hospitalized adult patients with gram-negative infections. BMC Infect. Dis. 2019, 19, 718. [Google Scholar] [CrossRef] [Green Version]
- Schaut, M.; Schaefer, M.; Trost, U.; Sander, A. Integrated antibiotic clinical decision support system (CDSS) for appropriate choice and dosage: An analysis of retrospective data. Germs 2022, 12, 203–213. [Google Scholar] [CrossRef]
- Vieceli, T.; Rello, J. Optimization of antimicrobial prescription in the hospital. Eur. J. Intern. Med. 2022, S0953-6205(22)00313-2. [Google Scholar] [CrossRef]
- Amin, A.N.; Dellinger, E.P.; Harnett, G.; Kraft, B.D.; LaPlante, K.L.; LoVecchio, F.; McKinnell, J.A.; Tillotson, G.; Valentine, S. It’s about the patients: Practical antibiotic stewardship in outpatient settings in the United States. Front. Med. 2022, 9, 901980. [Google Scholar] [CrossRef]
- Miron, V.D. COVID-19 in the pediatric population and parental perceptions. Germs 2020, 10, 294. [Google Scholar] [CrossRef]
- O’Sullivan, J.W.; Harvey, R.T.; Glasziou, P.P.; McCullough, A. Written information for patients (or parents of child patients) to reduce the use of antibiotics for acute upper respiratory tract infections in primary care. Cochrane Database Syst. Rev. 2016, 11, CD011360. [Google Scholar] [CrossRef]
- De Bont, E.G.; Alink, M.; Falkenberg, F.C.; Dinant, G.J.; Cals, J.W. Patient information leaflets to reduce antibiotic use and reconsultation rates in general practice: A systematic review. BMJ Open 2015, 5, e007612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magill, S.S.; O’Leary, E.; Ray, S.M.; Kainer, M.A.; Evans, C.; Bamberg, W.M.; Johnston, H.; Janelle, S.J.; Oyewumi, T.; Lynfield, R.; et al. Assessment of the Appropriateness of Antimicrobial Use in US Hospitals. JAMA Netw. Open 2021, 4, e212007. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Proposals for EU Guidelines on the Prudent Use of Antimicrobials in Humans; ECDC: Stockholm, Sweden, 2017.
- European Commission. Commission Notice EU Guidelines for the Prudent Use of Antimicrobials in Human Health, OJ EU 2017/C 212/01; European Commission: Brussels, Belgium, 2017.
| Site of Infection or Study Population | Novel Agent | Phase of the Clinical Trial | Expected Completion of the Clinical Trial (Year) | ClinicalTrials.gov Reference |
|---|---|---|---|---|
| Indication: carbapenem-resistant Acinetobacter baumannii | ||||
| Healthy volunteers | ETX2514 and 14C-ETX2514 | Phase 1 (excretion and metabolism) | 2019 (completed) | NCT04018950 |
| Healthy volunteers | ETX2514 vs. placebo vs. moxifloxacin | Phase 1 (cardiac repolarization) | 2019 (completed) | NCT03985410 |
| Volunteers with and without renal impairment | ETX2514 plus sulbactam | Phase 1 (renal safety) | 2018 (completed) | NCT03310463 |
| Healthy volunteers | ETX2514 vs. ETX2514 plus sulbactam vs. ETX2514 plus sulbactam plus imipenem/cilastatin | Phase 1 (safety, tolerability and PK) | 2017 (completed) | NCT02971423 |
| Healthy volunteers | ETX2514 plus sulbactam | 1 (plasma, epithelial lining fluid and alveolar macrophage concentrations) | 2017 (completed) | NCT03303924 |
| HABP, VABP, bacteremia | ETX2514/sulbactam + imipenem/cilastin vs. colistin + imipenem/cilastin | Phase 3 | 2021 (completed) | NCT03894046 |
| Pneumonia or bacteremia/septicemia | Phage treatment | Expanded access study | 2020-not specified | NCT04636554 |
| Diabetic foot ulcers | Local bacteriophage therapy TP-102 | (Local study: Israel) | 2021-recruiting | NCT04803708 |
| Critical illness with multi-drug resistant pathogens | Polymyxin B vs. polymyxin B plus imipenem | Phase 3 (local study: Puerto Rico) | 2019 (completed) | NCT03159078 |
| Resistant Pseudomonas aeruginosa | ||||
| Healthy volunteers | MEDI3902 | Phase 1 dose-escalation study | 2018 (completed) | NCT02255760 |
| Prevention of nosocomial pneumonia caused by P. aeruginosa in mechanically ventilated patients | MEDI3902 vs. placebo | Phase 2 | 2016-2020 (completed) | NCT02696902 |
| Carbapenem-resistant Enterobacteriaceae (CRE) | ||||
| Colonization and clinical infection with CRE | Capsulized FMT | Local study: Israel | April 2023 | NCT04790565 |
| Clinical infection with CRE | Capsulized FMT | Phase 2, 3 | June 2022 | NCT04146337 |
| Colonization with CRE or VRE | FMT with frozen stool from donors | Not mentioned | March 2022 | NCT04583098 |
| Colonization with CRE or VRE | FMT with donor stool samples from stool bank | Phase 2 | December 2021 | NCT03479710 |
| Colonization with CRE | FMT from donors | Phase 1, 2 | February 2023 | NCT04759001 |
| Colonization with CRE | Capsulized FMT | Phase 1, 2 | December 2019 | NCT03167398 |
| Colonization with KPC | Capsulized FMT | Phase 2 | September 2024 | NCT04760665 |
| Colonization with MDR organisms after infection, in renal transplant recipients | FMT using allogeneic human stool in glycerol | Phase 1 | December 2022 | NCT02922816 |
| Prevention and decolonization of MDR bacteria | Probiotics (Lactobacillus casei, Lactobacillus plantarum, Streptococcus faecalis, Bifidobacterium brevis) | Not mentioned | December 2019 | NCT03967301 |
| Colonization with MDR Gram-negative bacilli | Probiotic: 4 Lactobacillus strains, 3 bifidobacteria strains, and Streptococcus thermophilus 24,731 vs. capsulized FMT from healthy donor | Not mentioned | July 2023 | NCT04431934 |
| Serious infections due CRE (complicated UTI, acute pyelonephritis, HABP, VABP, bacteremia, abdominal infection) | Meropenem + vaborbactam vs. best available therapy | Phase 3 | July 2017 | NCT02168946 |
| Colonization with MDR bacteria | Colistin sulphate + neomycin sulphate days 1–5 then capsulized FMT days 7 and 8 | Phase 2 | March 2018 | NCT02472600 |
| CRE or KPC infections | Imipenem/cilastatin/relebactam | Phase 4 | August 2022 | NCT04785924 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Săndulescu, O.; Viziteu, I.; Streinu-Cercel, A.; Miron, V.D.; Preoțescu, L.L.; Chirca, N.; Albu, S.E.; Craiu, M.; Streinu-Cercel, A. Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside. Appl. Sci. 2022, 12, 11615. https://doi.org/10.3390/app122211615
Săndulescu O, Viziteu I, Streinu-Cercel A, Miron VD, Preoțescu LL, Chirca N, Albu SE, Craiu M, Streinu-Cercel A. Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside. Applied Sciences. 2022; 12(22):11615. https://doi.org/10.3390/app122211615
Chicago/Turabian StyleSăndulescu, Oana, Ioana Viziteu, Anca Streinu-Cercel, Victor Daniel Miron, Liliana Lucia Preoțescu, Narcis Chirca, Simona Elena Albu, Mihai Craiu, and Adrian Streinu-Cercel. 2022. "Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside" Applied Sciences 12, no. 22: 11615. https://doi.org/10.3390/app122211615
APA StyleSăndulescu, O., Viziteu, I., Streinu-Cercel, A., Miron, V. D., Preoțescu, L. L., Chirca, N., Albu, S. E., Craiu, M., & Streinu-Cercel, A. (2022). Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside. Applied Sciences, 12(22), 11615. https://doi.org/10.3390/app122211615








