Is There an Influence of Electrically Stimulated Osteoblasts on the Induction of Osteoclastogenesis?
Abstract
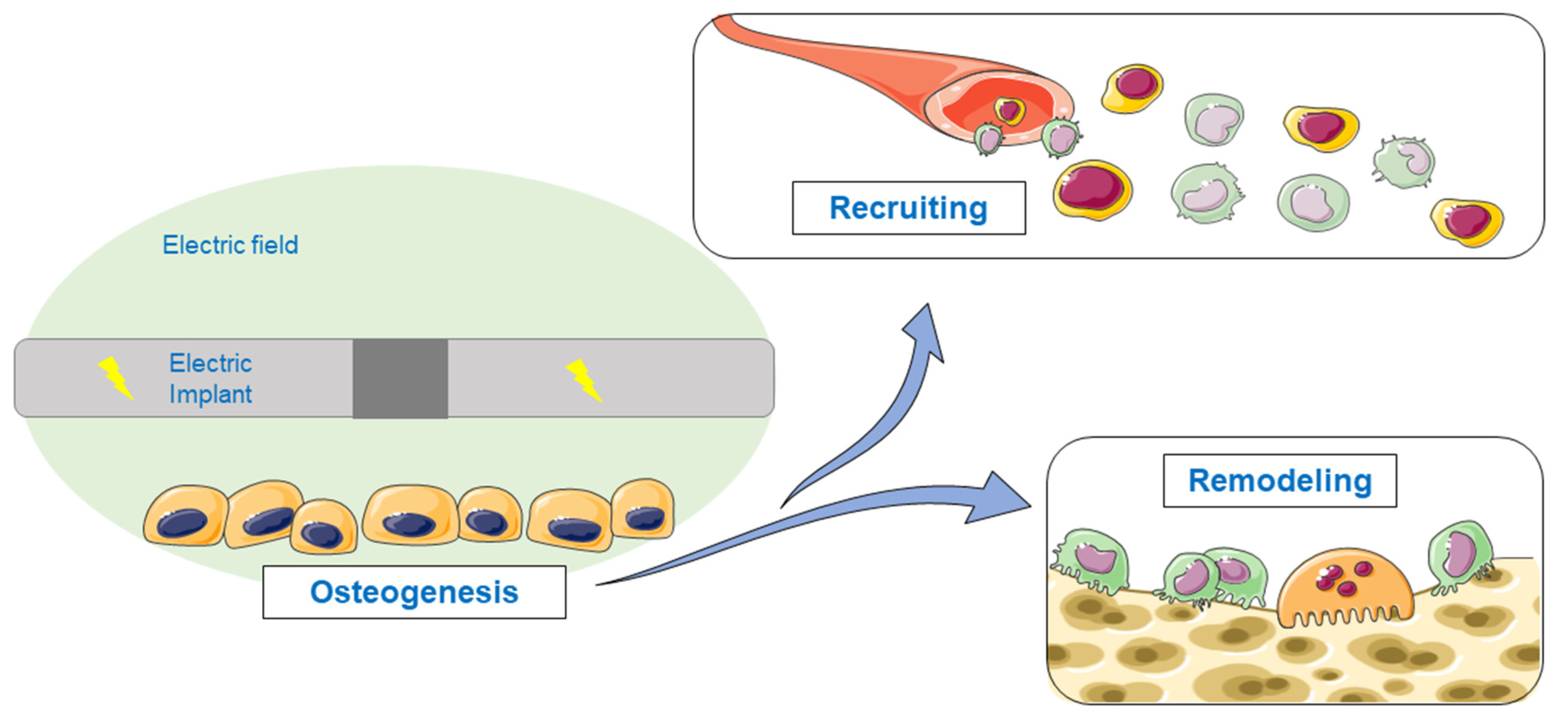
1. Introduction
2. Materials and Methods
2.1. Electrical Stimulation of Human Primary Osteoblasts
2.2. Isolation and Differentiation of Peripheral Blood Mononuclear Cells
2.3. Indirect Stimulation
2.4. Cell Viability
2.5. Actin-DAPI Staining
2.6. Gene Expression Analysis
2.7. Quantification of the Secreted Proteins
2.8. Display of the Data and Statistical Analysis
3. Results
3.1. Initial Concentration of Cytokines in the Supernatants
3.2. Influence of Conditioned Osteoblastic Supernatants on Peripheral Blood Mononuclear Cell (PBMC)
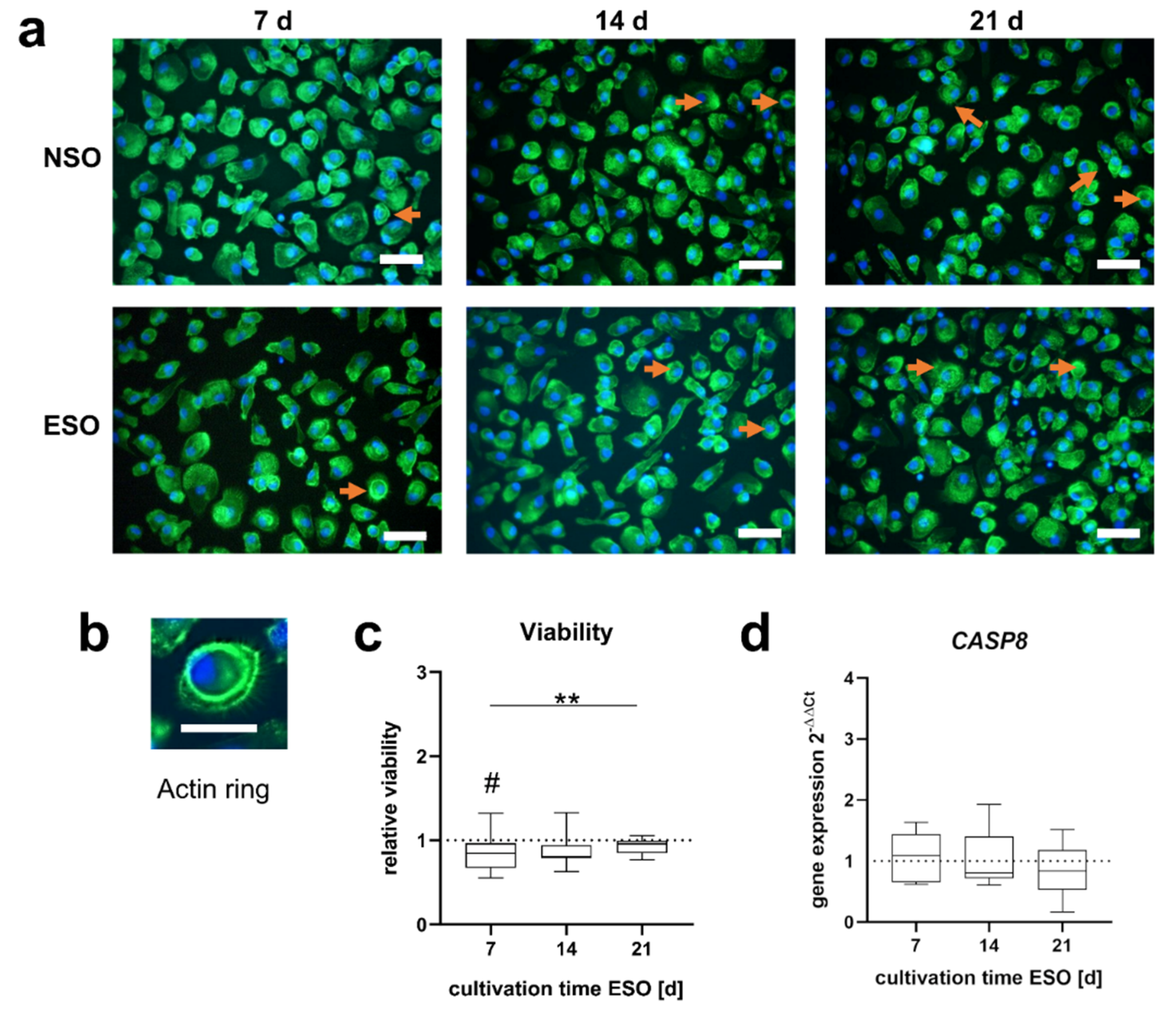
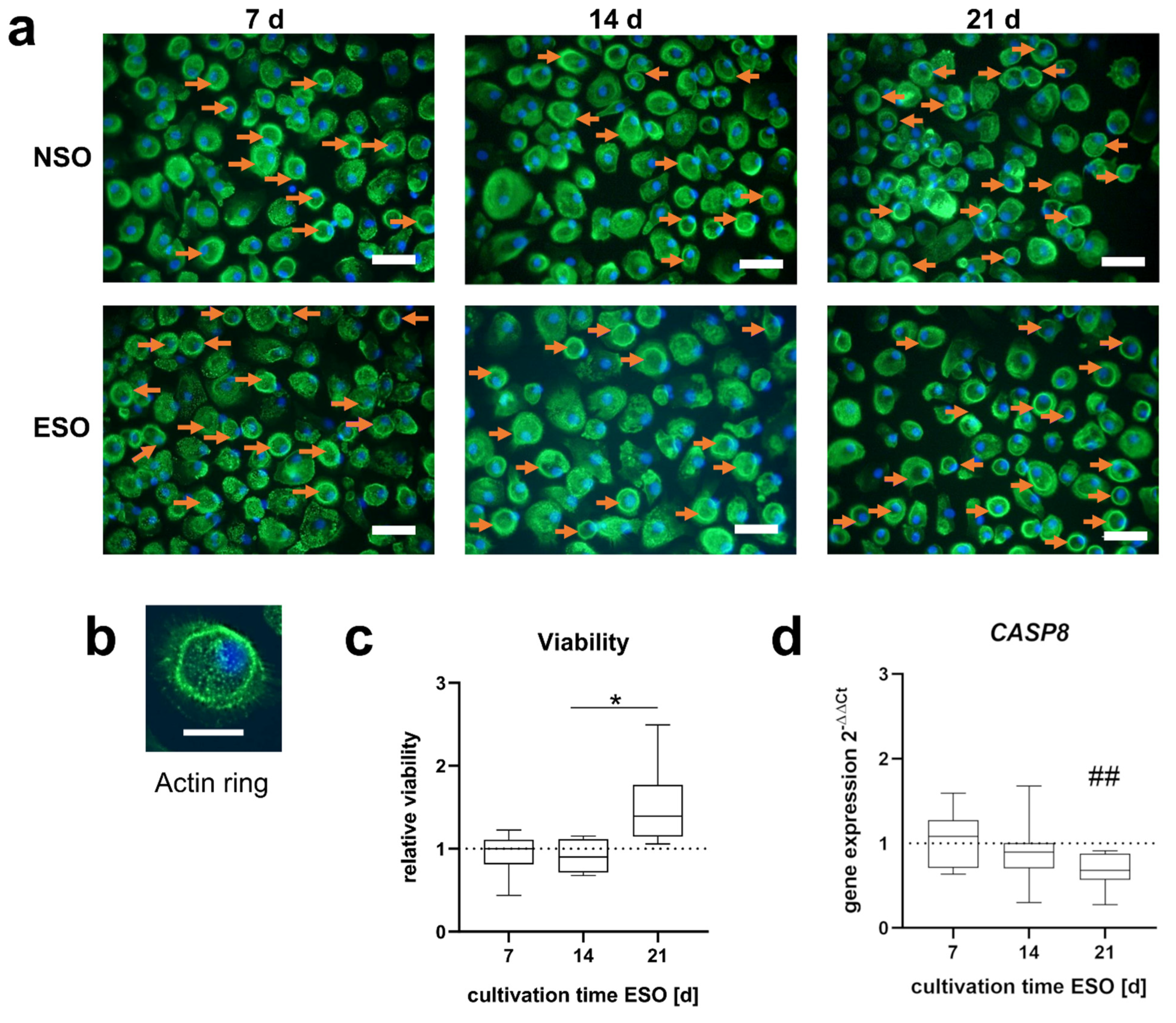
3.2.1. Morphology and Viability
3.2.2. Gene Expression
3.2.3. Released Mediators
3.3. Influence of Conditioned Osteoblastic Supernatants on Pre-Osteoclasts
3.3.1. Morphology and Viability
3.3.2. Gene Expression
3.3.3. Released Mediators
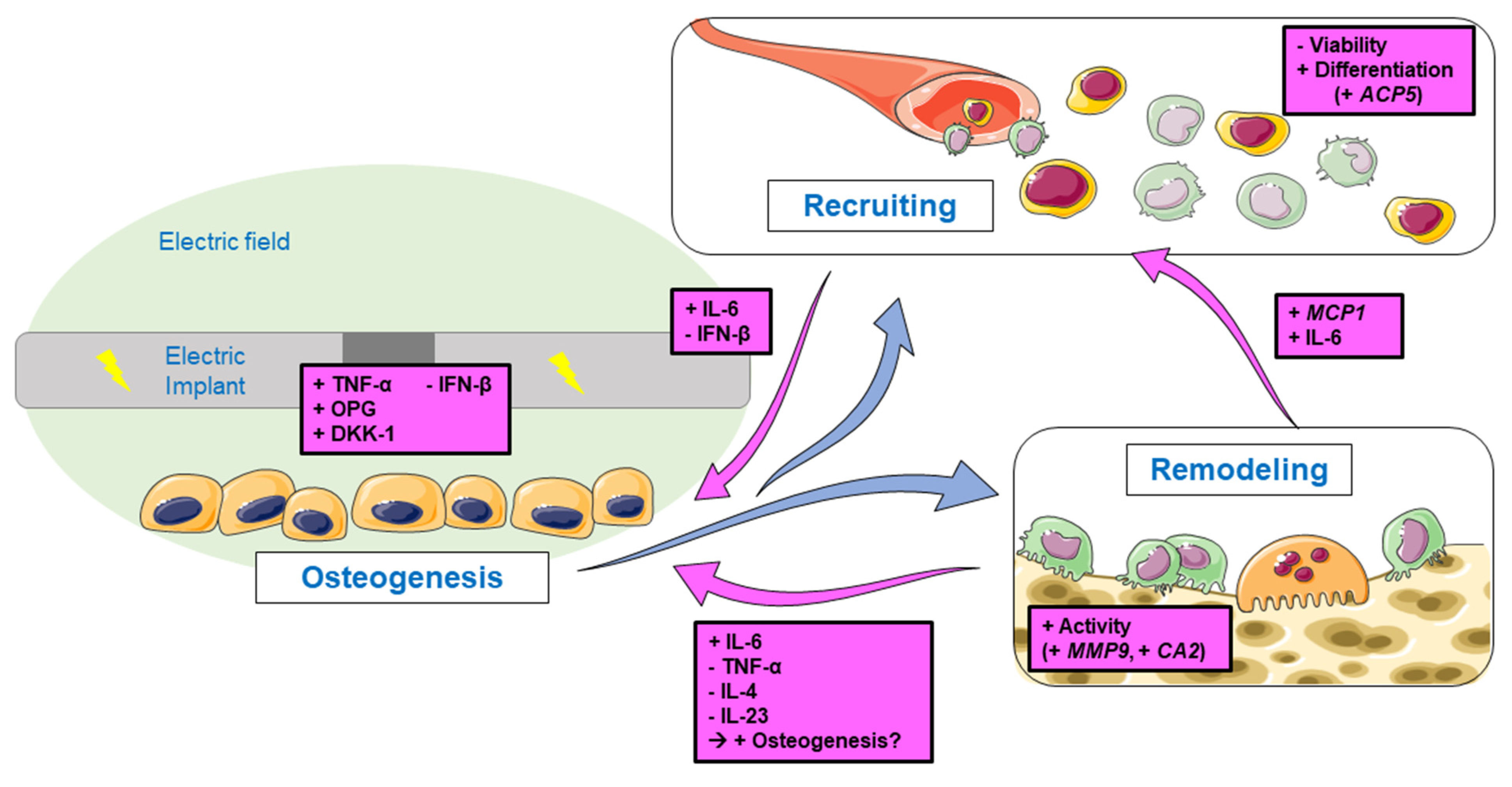
4. Discussion
4.1. Differentiation of PBMCs
4.2. Activation of Pre-Osteoclasts
4.3. Influence on Cell Viability during Osteoclastogenesis
4.4. Feedback Signaling on Osteogenesis and Bone Remodeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part. B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Japan 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- deVet, T.; Jhirad, A.; Pravato, L.; Wohl, G.R. Bone Bioelectricity and Bone-Cell Response to Electrical Stimulation: A Review. Crit. Rev. Biomed. Eng. 2021, 49, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.N.M.; Doulgkeroglou, M.N.; Zeugolis, D.I. Electric Field Stimulation for Tissue Engineering Applications. BMC Biomed. Eng. 2021, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical Stimulation as a Novel Tool for Regulating Cell Behavior in Tissue Engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Griffin, M.; Bayat, A. Electrical Stimulation in Bone Healing: Critical Analysis by Evaluating Levels of Evidence. Eplasty 2011, 11, e34. [Google Scholar]
- Ercan, B.; Webster, T.J. The Effect of Biphasic Electrical Stimulation on Osteoblast Function at Anodized Nanotubular Titanium Surfaces. Biomaterials 2010, 31, 3684–3693. [Google Scholar] [CrossRef]
- Srirussamee, K.; Xue, R.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Changes in the Extracellular Microenvironment and Osteogenic Responses of Mesenchymal Stem/Stromal Cells Induced by in Vitro Direct Electrical Stimulation. J. Tissue Eng. 2021, 12, 2041731420974147. [Google Scholar] [CrossRef]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef]
- Supronowicz, P.R.; Ajayan, P.M.; Ullmann, K.R.; Arulanandam, B.P.; Metzger, D.W.; Bizios, R. Novel Current-Conducting Composite Substrates for Exposing Osteoblasts to Alternating Current Stimulation. J. Biomed. Mater. Res. 2001, 59, 499–506. [Google Scholar] [CrossRef]
- Creecy, C.M.; O’Neill, C.F.; Arulanandam, B.P.; Sylvia, V.L.; Navara, C.S.; Bizios, R. Mesenchymal Stem Cell Osteodifferentiation in Response to Alternating Electric Current. Tissue Eng. Part A 2013, 19, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and Mechanisms of Exogenous Electromagnetic Field on Bone Cells: A Review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Boccaccini, A.R. The Role of Osteoclasts in Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chang, W.H.-S.; Tsai, M.-T.; Shih, C. Pulsed Electromagnetic Fields Accelerate Apoptotic Rate in Osteoclasts. Connect. Tissue Res. 2006, 47, 222–228. [Google Scholar] [CrossRef]
- Chang, K.; Chang, W.H.-S.; Wu, M.-L.; Shih, C. Effects of Different Intensities of Extremely Low Frequency Pulsed Electromagnetic Fields on Formation of Osteoclast-like Cells. Bioelectromagnetics 2003, 24, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; McLeod, K.J.; Titus, L.; Nanes, M.S.; Catherwood, B.D.; Rubin, C.T. Formation of Osteoclast-like Cells Is Suppressed by Low Frequency, Low Intensity Electric Fields. J. Orthop. Res. 1996, 14, 7–15. [Google Scholar] [CrossRef]
- Shankar, V.S.; Simon, B.J.; Bax, C.M.R.; Pazianas, M.; Moonga, B.S.; Adebanjo, O.A.; Zaidi, M. Effects of Electromagnetic Stimulation on the Functional Responsiveness of Isolated Rat Osteoclasts. J. Cell Physiol. 1998, 176, 537–544. [Google Scholar] [CrossRef]
- Ferrier, J.; Ross, S.M.; Kanehisa, J.; Aubin, J.E. Osteoclasts and Osteoblasts Migrate in Opposite Directions in Response to a Constant Electrical Field. J. Cell Physiol. 1986, 129, 283–288. [Google Scholar] [CrossRef]
- He, Z.; Selvamurugan, N.; Warshaw, J.; Partridge, N.C. Pulsed Electromagnetic Fields Inhibit Human Osteoclast Formation and Gene Expression via Osteoblasts. Bone 2018, 106, 194–203. [Google Scholar] [CrossRef]
- Lei, Y.; Su, J.; Xu, H.; Yu, Q.; Zhao, M.; Tian, J. Pulsed Electromagnetic Fields Inhibit Osteoclast Differentiation in RAW264.7 Macrophages via Suppression of the Protein Kinase B/Mammalian Target of Rapamycin Signaling Pathway. Mol. Med. Rep. 2018, 18, 447–454. [Google Scholar] [CrossRef]
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely Low Frequency Electromagnetic Field and Wound Healing: Implication of Cytokines as Biological Mediators. Eur. Cytokine Netw. 2013, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Menger, M.M.; Braun, B.J.; Schweizer, S.; Linnemann, C.; Falldorf, K.; Ronniger, M.; Wang, H.; Histing, T.; Nussler, A.K.; et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering 2021, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Groiss, S.; Lammegger, R.; Brislinger, D. Anti-Oxidative and Immune Regulatory Responses of THP-1 and PBMC to Pulsed EMF Are Field-Strength Dependent. Int. J. Environ. Res. Public Health 2021, 18, 9519. [Google Scholar] [CrossRef] [PubMed]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct Electrical Stimulation Enhances Osteogenesis by Inducing Bmp2 and Spp1 Expressions from Macrophages and Preosteoblasts. Biotechnol. Bioeng. 2019, 116, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Peng, S.-W.; Cheng, J.-Y. In Vitro Electrical-Stimulated Wound-Healing Chip for Studying Electric Field-Assisted Wound-Healing Process. Biomicrofluidics 2012, 6, 34117. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Mayr, H.; Seitz, D.; Ziegler, G. Is Hydroxyapatite Ceramic Included in the Bone Remodelling Proccess? An In Vitro Study of Resorption and Formation Processes. Key Eng. Mater. 2008, 361–363, 1123–1126. [Google Scholar] [CrossRef]
- Zehnder, T.; Boccaccini, A.R.; Detsch, R. Biofabrication of a Co-Culture System in an Osteoid-like Hydrogel Matrix. Biofabrication 2017, 9, 025016. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co-Culture Systems of Osteoblasts and Osteoclasts: Simulating in Vitro Bone Remodeling in Regenerative Approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonca, F.; Caetano, G.F.; Bartolo, P. Engineered 3D Printed Poly(Varepsilon-Caprolactone)/Graphene Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Sahm, F.; Freiin Grote, V.; Zimmermann, J.; Haack, F.; Uhrmacher, A.M.; van Rienen, U.; Bader, R.; Detsch, R.; Jonitz-Heincke, A. Long-Term Stimulation with Alternating Electric Fields Modulates the Differentiation and Mineralization of Human Pre-Osteoblasts. Front. Physiol. 2022, 13, 965181. [Google Scholar] [CrossRef]
- Grunert, P.C.; Jonitz-Heincke, A.; Su, Y.; Souffrant, R.; Hansmann, D.; Ewald, H.; Krüger, A.; Mittelmeier, W.; Bader, R. Establishment of a Novel In Vitro Test Setup for Electric and Magnetic Stimulation of Human Osteoblasts. Cell Biochem. Biophys. 2014, 70, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Hiemer, B.; Krogull, M.; Bender, T.; Ziebart, J.; Krueger, S.; Bader, R.; Jonitz-Heincke, A. Effect of Electric Stimulation on Human Chondrocytes and Mesenchymal Stem Cells under Normoxia and Hypoxia. Mol. Med. Rep. 2018, 18, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Markhoff, J.; Jonitz-Heincke, A.; Sterna, P.; Salamon, A.; Bader, R. Comparison of Different Cell Culture Plates for the Enrichment of Non-Adherent Human Mononuclear Cells. Exp. Ther. Med. 2019, 17, 2004–2012. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Han, Z.; et al. Electrical Stimulation-Based Bone Fracture Treatment, If It Works so Well Why Do Not More Surgeons Use It? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Sieberath, A.; Della Bella, E.; Ferreira, A.M.; Gentile, P.; Eglin, D.; Dalgarno, K. A Comparison of Osteoblast and Osteoclast In Vitro Co-Culture Models and Their Translation for Preclinical Drug Testing Applications. Int. J. Mol. Sci. 2020, 21, 912. [Google Scholar] [CrossRef]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the Functional Response of Bone to Mechanical Strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and Macrophages in Tissue Repair: Implications for Immunoregenerative Biomaterial Design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef]
- Wilson, S.R.; Peters, C.; Saftig, P.; Brömme, D. Cathepsin K Activity-Dependent Regulation of Osteoclast Actin Ring Formation and Bone Resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef]
- Kim, N.; Kadono, Y.; Takami, M.; Lee, J.; Lee, S.-H.; Okada, F.; Kim, J.H.; Kobayashi, T.; Odgren, P.R.; Nakano, H.; et al. Osteoclast Differentiation Independent of the TRANCE–RANK–TRAF6 Axis. J. Exp. Med. 2005, 202, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Ko, N.Y.; Kim, H.S.; Kim, J.W.; Kim, D.K.; Kim, A.-R.; Lee, S.H.; Kim, Y.-G.; Lee, C.K.; Lee, S.H.; et al. Interleukin-33 Stimulates Formation of Functional Osteoclasts from Human CD14+ Monocytes. Cell. Mol. Life Sci. 2010, 67, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor Necrosis Factor α Stimulates Osteoclast Differentiation by a Mechanism Independent of the Odf/Rankl–Rank Interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Murphy, C.; Kirstein, B.; Fox, S.W.; Chambers, T.J. TNFα Potently Activates Osteoclasts, through a Direct Action Independent of and Strongly Synergistic with RANKL. Endocrinology 2002, 143, 1108–1118. [Google Scholar] [CrossRef]
- Heiland, G.R.; Zwerina, K.; Baum, W.; Kireva, T.; Distler, J.H.; Grisanti, M.; Asuncion, F.; Li, X.; Ominsky, M.; Richards, W.; et al. Neutralisation of Dkk-1 Protects from Systemic Bone Loss during Inflammation and Reduces Sclerostin Expression. Ann. Rheum. Dis. 2010, 69, 2152–2159. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Evans, K.E.; Fox, S.W. Interleukin-10 Inhibits Osteoclastogenesis by Reducing NFATc1 Expression and Preventing Its Translocation to the Nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef]
- Cho, H.; Lee, J.; Jang, S.; Lee, J.; Oh, T.I.; Son, Y.; Lee, E. Casr-Mediated Hbmscs Activity Modulation: Additional Coupling Mechanism in Bone Remodeling Compartment. Int. J. Mol. Sci. 2021, 22, 325. [Google Scholar] [CrossRef]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Guo, S.; Zhang, W.; Wang, J.; Lin, Y. Dynamic Expression of Matrix Metalloproteinases 2, 9 and 13 in Ovariectomy-Induced Osteoporosis Rats. Exp. Ther. Med. 2018, 16, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.C.; Barker, J.H.; Berezikov, E.; Pindur, L.; Kynigopoulos, S.; Eischen-Loges, M.; Han, Z.; Bhavsar, M.B.; Henrich, D.; Leppik, L. Electrical Stimulation Shifts Healing/Scarring towards Regeneration in a Rat Limb Amputation Model. Sci. Rep. 2019, 9, 11433. [Google Scholar] [CrossRef] [PubMed]
- Lehenkari, P.; Hentunen, T.A.; Laitala-Leinonen, T.; Tuukkanen, J.; Väänänen, H.K. Carbonic Anhydrase II Plays a Major Role in Osteoclast Differentiation and Bone Resorption by Effecting the Steady State Intracellular PH and Ca2+. Exp. Cell Res. 1998, 242, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Arendt, B.K.; Velazquez-Dones, A.; Tschumper, R.C.; Howell, K.G.; Ansell, S.M.; Witzig, T.E.; Jelinek, D.F. Interleukin 6 Induces Monocyte Chemoattractant Protein-1 Expression in Myeloma Cells. Leukemia 2002, 16, 2142–2147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biswas, P.; Delfanti, F.; Bernasconi, S.; Mengozzi, M.; Cota, M.; Polentarutti, N.; Mantovani, A.; Lazzarin, A.; Sozzani, S.; Poli, G. Interleukin-6 Induces Monocyte Chemotactic Protein-1 in Peripheral Blood Mononuclear Cells and in the U937 Cell Line. Blood 1998, 91, 258–265. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ninomiya, K.; Sonoda, K.-H.; Miyauchi, Y.; Hoshi, H.; Iwasaki, R.; Miyamoto, H.; Yoshida, S.; Sato, Y.; Morioka, H.; et al. MCP-1 Expressed by Osteoclasts Stimulates Osteoclastogenesis in an Autocrine/Paracrine Manner. Biochem. Biophys. Res. Commun. 2009, 383, 373–377. [Google Scholar] [CrossRef]
- Takahashi, N.; Ejiri, S.; Yanagisawa, S.; Ozawa, H. Regulation of Osteoclast Polarization. Odontology 2007, 95, 1–9. [Google Scholar] [CrossRef]
- Azari, A.; Schoenmaker, T.; de Souza Faloni, A.P.; Everts, V.; de Vries, T.J. Jaw and Long Bone Marrow Derived Osteoclasts Differ in Shape and Their Response to Bone and Dentin. Biochem. Biophys. Res. Commun. 2011, 409, 205–210. [Google Scholar] [CrossRef]
- Detsch, R.; Schaefer, S.; Deisinger, U.; Ziegler, G.; Seitz, H.; Leukers, B. In Vitro-Osteoclastic Activity Studies on Surfaces of 3D Printed Calcium Phosphate Scaffolds. J. Biomater. Appl. 2011, 26, 359–380. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, B. The Multi-Differentiation Potential of Peripheral Blood Mononuclear Cells. Stem Cell Res. Ther. 2012, 3, 48. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating Cell Proliferation and Differentiation: Antagonism between Cell Cycle Regulators and Cell Type-Specific Gene Expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 Is the Molecular Switch for Apoptosis, Necroptosis and Pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of Osteoblast Differentiation by Tumor Necrosis Factor-Alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016, 1318256. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/Interleukin-6 Receptor Complex Promotes Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 13. [Google Scholar] [CrossRef]
- Park, Y.-T.; Lee, S.-M.; Kou, X.; Karabucak, B. The Role of Interleukin 6 in Osteogenic and Neurogenic Differentiation Potentials of Dental Pulp Stem Cells. J. Endod. 2019, 45, 1342–1348. [Google Scholar] [CrossRef]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T. IL-6 Is Produced by Osteoblasts and Induces Bone Resorption. J. Immunol. 1990, 145, 3297–3303. [Google Scholar]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Combination of IL-6 and SIL-6R Differentially Regulate Varying Levels of RANKL-Induced Osteoclastogenesis through NF-ΚB, ERK and JNK Signaling Pathways. Sci. Rep. 2017, 7, 41411. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Sakamoto, K.; Minamizato, T.; Katsube, K.; Nakanishi, S. Regulation of Osteoblast Differentiation Mediated by BMP, Notch, and CCN3/NOV. Jpn. Dent. Sci. Rev. 2008, 44, 48–56. [Google Scholar] [CrossRef]
- Remmers, S.J.A.; de Wildt, B.W.M.; Vis, M.A.M.; Spaander, E.S.R.; de Vries, R.B.M.; Ito, K.; Hofmann, S. Osteoblast-Osteoclast Co-Cultures: A Systematic Review and Map of Available Literature. PLoS ONE 2021, 16, e0257724. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-L.; Wu, Q.; Miao, Z.-N.; Xu, M.-H.; Xu, R.-S.; Jiang, D.-L.; Ye, J.-X.; Chen, F.; Zhao, M.-D.; Wang, H.; et al. Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast–Osteoblasts Communication in Bone Remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef] [PubMed]



| Gene | Abbreviation | Sequence |
|---|---|---|
| Caspase 8 | CASP8 | For: TGTTTTCACAGGTTCTCCTCCTTT Rev: GAGAATATAATCCGCTCCACCTT |
| Receptor Activator of NFκB | RANK | For: AGAAAACCACCAAATGAACCCC Rev: GCCAGAGCCTCATTGATTC |
| Tartrate Resistant Acid phosphatase 5 | ACP5 | For: GGGAGATGTGTGAGCCAGTG Rev: GTCCACATGTCCATCCAGGG |
| Carbonic anhydrase 2 | CA2 | For: TGGGGTTCACTTGATGGACAA Rev: CAGCACTCTTGCCCTTTGTT |
| Cathepsin K | CTSK | For: GGAAGCAATTAACAACAAGGTGGA Rev: GGGGCCTACCTTCCCATTC |
| Matrix Metallopeptidase 9 | MMP9 | For: ACGCAGACATCGTCATCCAG Rev: AACCGAGTTGGAACCACGAC |
| Hypoxanthine-Guanine Phosphoribosyl Transferase | HPRT | For: CCCTGGCGTCGTGATTAGTG Rev: TCGAGCAAGACGTTCAGTCC |
| ESO | NSO | |||||
|---|---|---|---|---|---|---|
| Time [d] | 7 | 14 | 21 | 7 | 14 | 21 |
| Pro-osteoclastic | ||||||
| TNF-α [pg/mg] | 6.00 | 6.20 | 5.70 | 2.56 | 3.27 | 2.55 |
| Anti-osteoclastic | ||||||
| IL-10 [pg/mg] | 0.00 | 0.92 | 0.00 | 0.96 | 0.00 | 1.47 |
| IFN-β [pg/mg] | 0.00 | 1.48 | 0.00 | 1.3 | 0.76 | 2.36 |
| Bone remodeling | ||||||
| TGF-β1 [pg/mg] | 0.00 | 0.00 | 0.59 | 0.69 | 0.00 | 0.62 |
| IL-6 [pg/mg] | 176.45 | 213.29 | 387.33 | 231.20 | 318.82 | 278.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahm, F.; Jakovljevic, A.; Bader, R.; Detsch, R.; Jonitz-Heincke, A. Is There an Influence of Electrically Stimulated Osteoblasts on the Induction of Osteoclastogenesis? Appl. Sci. 2022, 12, 11840. https://doi.org/10.3390/app122211840
Sahm F, Jakovljevic A, Bader R, Detsch R, Jonitz-Heincke A. Is There an Influence of Electrically Stimulated Osteoblasts on the Induction of Osteoclastogenesis? Applied Sciences. 2022; 12(22):11840. https://doi.org/10.3390/app122211840
Chicago/Turabian StyleSahm, Franziska, Ana Jakovljevic, Rainer Bader, Rainer Detsch, and Anika Jonitz-Heincke. 2022. "Is There an Influence of Electrically Stimulated Osteoblasts on the Induction of Osteoclastogenesis?" Applied Sciences 12, no. 22: 11840. https://doi.org/10.3390/app122211840
APA StyleSahm, F., Jakovljevic, A., Bader, R., Detsch, R., & Jonitz-Heincke, A. (2022). Is There an Influence of Electrically Stimulated Osteoblasts on the Induction of Osteoclastogenesis? Applied Sciences, 12(22), 11840. https://doi.org/10.3390/app122211840







