Experimental Study and Numerical Simulation of W/O Emulsion in Developing Heavy Oil Reservoirs
Abstract
:1. Introduction
2. Experiments
2.1. Emulsification Experiment
2.1.1. Materials and Devices
2.1.2. Experiment Preparation
- (1)
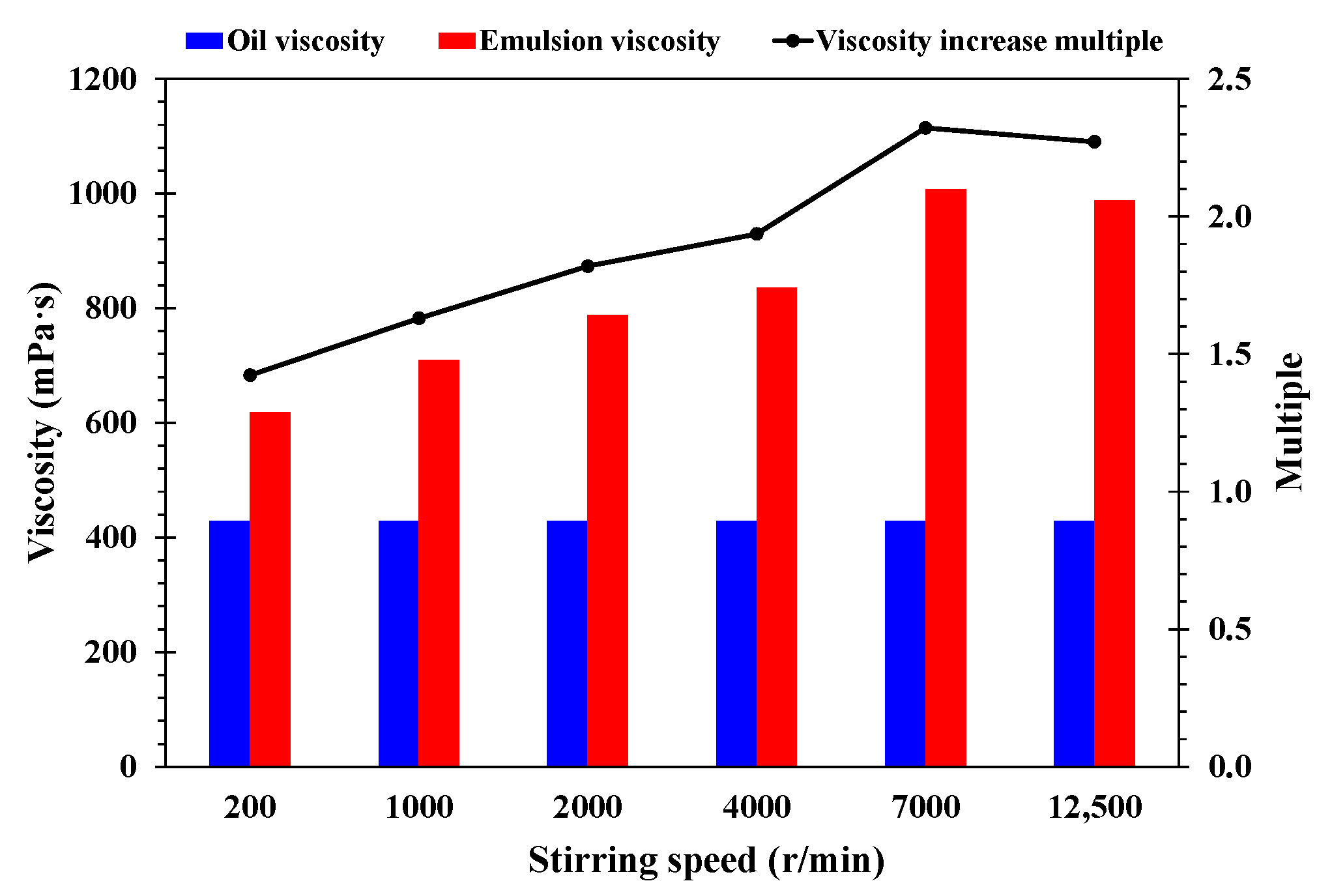
- Stirring speed. In order to study the effect of stirring speed on the stability of emulsion, other experimental conditions remained unchanged. The emulsion was prepared at stirring speeds of 200 r/min, 1000 r/min, 2000 r/min, 4000 r/min, 7000 r/min, and 12,500 r/min. The prepared emulsions were then allowed to stand for 40 days under the experimental conditions.
- (2)
- Temperature. In order to study the effect of temperature on the stability of the emulsion, the emulsions were prepared at 40 °C, 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C. The prepared emulsions were then allowed to stand for 40 days under the experimental conditions.
- (3)
- Water cut of emulsion. In order to study the effect of water cut on the emulsion, the water cuts of the emulsions were set to 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90%. The prepared emulsions were then allowed to stand for 40 days under the experimental conditions.
- (4)
- Mineralization degree. Under the same experimental conditions, the formation water was made into water with different mineralization degrees using deionized water, i.e., 2000 mg/L, 4000 mg/L, 6000 mg/L, 8000 mg/L, and 10,000 mg/L, and then the emulsion with 40% water cut was prepared to study the effect of mineralization degree on the emulsion. The prepared emulsions were then allowed to stand for 40 days under the experimental conditions.
- (5)
- pH value. Under the condition of constant stirring speed and time, temperature, and water cut of the emulsion, the pH value of the formation water was changed using diluted hydrochloric acid solution and sodium bicarbonate solution. Then, it was used to prepare emulsions to study the effect of pH value on the stability of the emulsion. The prepared emulsions were then allowed to stand for 40 days under the experimental conditions.
2.1.3. Investigation of Emulsion Properties
- (1)
- Emulsion stability
- (2)
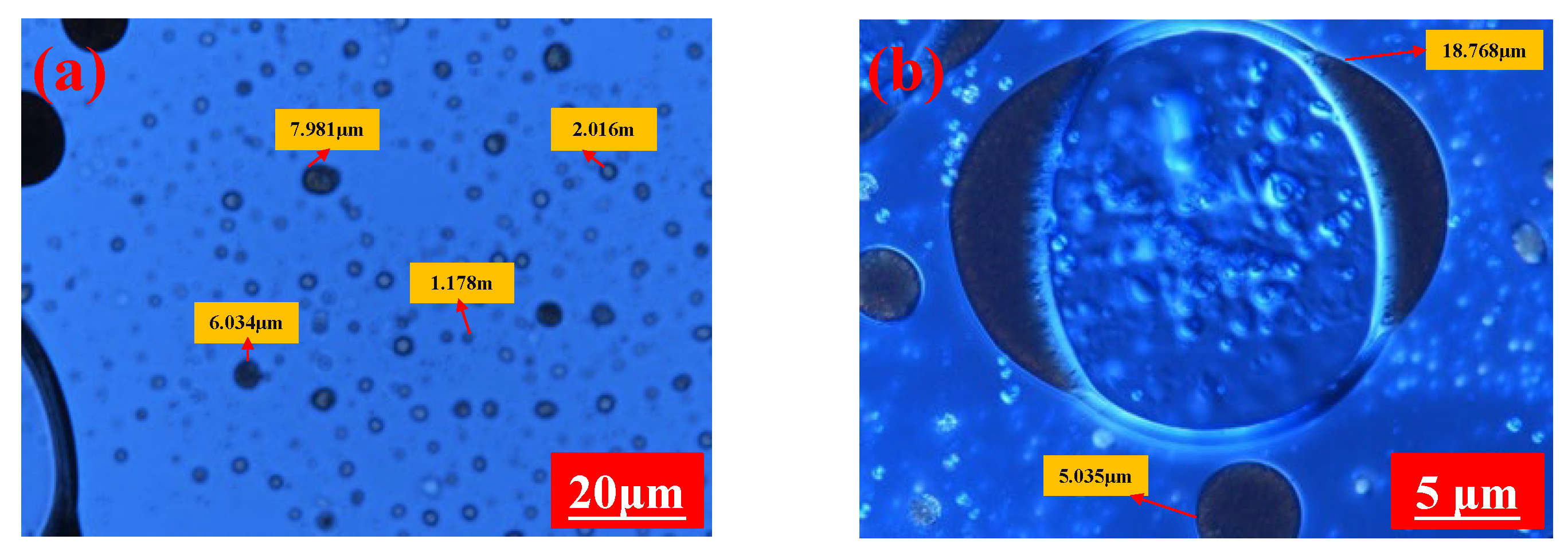
- Emulsion droplets size distribution
2.2. Emulsion Flooding Experiment
2.2.1. Materials
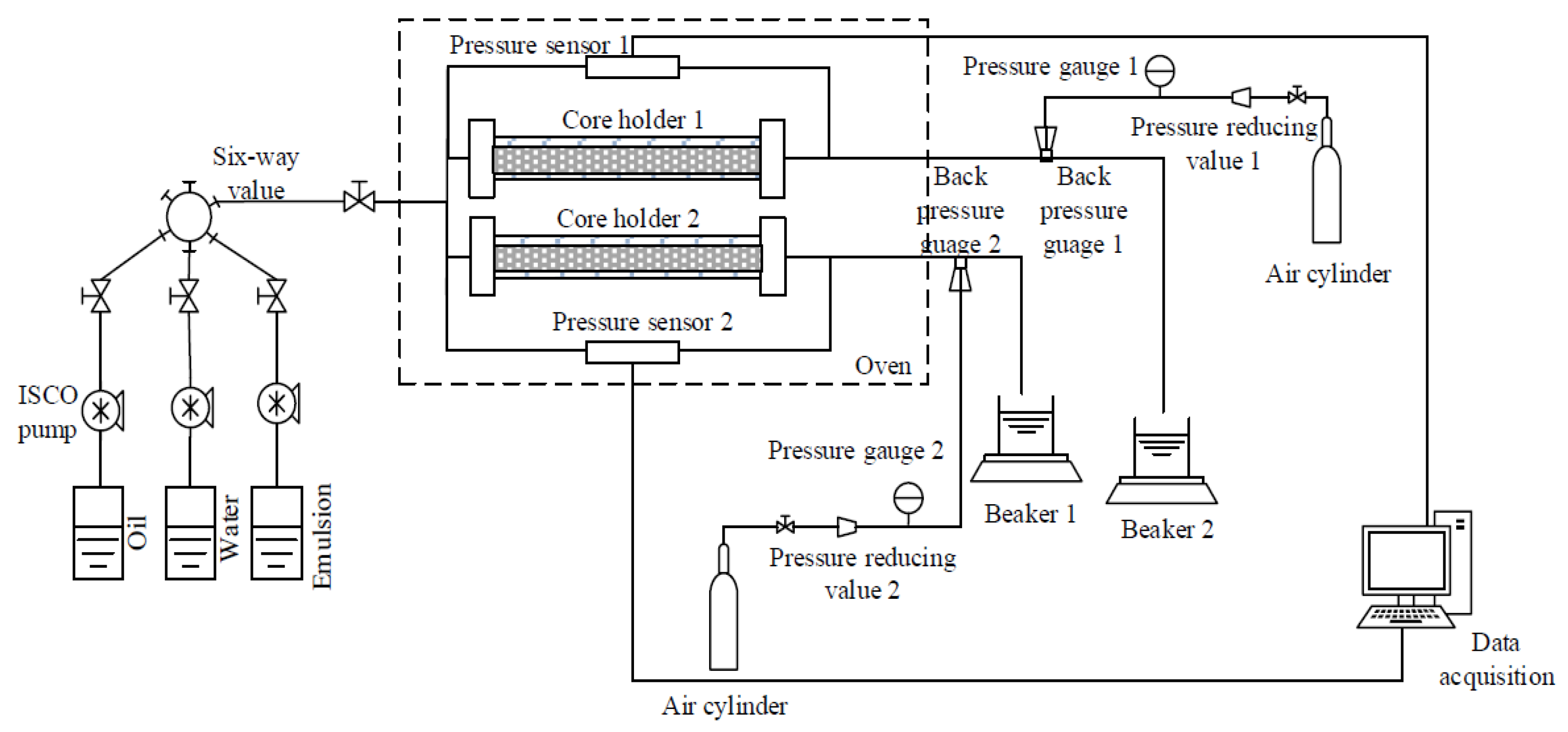
2.2.2. Experimental Apparatus
- (1)
- Injection system. The system consisted of three piston containers and ISCO pumps containing heavy oil samples, formation water, and emulsion. The injection rate was stable.
- (2)
- Pipeline and reservoir simulation system. The system was composed of two core holders and an oven. The cores of the studied oil field were selected to simulate the actual reservoir. The oven could simulate the actual reservoir temperature, and the specifications of the core holder were 25 mm × 300 mm.
- (3)
- Product collection and calculation system. The system used beakers to collect the produced liquid and used the weighing method to measure.
- (4)
- Temperature and pressure control system. The system consisted of a gas cylinder, an oven, a back pressure valve, a pressure reducing valve, two pressure sensors, and two pressure gauges.
2.2.3. Experimental Procedures
- (1)
- Experimental preparation. The core was dried, and the core quality was weighed. It was checked whether the pipeline leaked step by step; the core was extracted for 4–5 h under the condition of −0.1 MPa pressure, and then the core was removed after absorbing water. The surface water was quickly wiped off with paper, the mass was weighed again, and the core pore volume could be obtained from the increase in mass of the core after absorbing water, while the porosity could be calculated.
- (2)
- Permeability to water. The permeability was measured using water at three injection rates, and the average permeability was calculated. The permeability and porosity measured in eight cores are shown in Table 3, and the results were divided into four groups according to the permeability difference.
- (3)
- Saturated oil. The first step involved saturated water. Then, saturated oil was applied at an appropriate injection rate of 0.05 mL/min, and the outlet end cylinder measured the volume of discharged water until there was no more water. The total volume of discharged water was the volume of saturated oil, and the original oil saturation and irreducible water saturation could be calculated. The core aged for 12 h after being saturated with oil.
- (4)
- Emulsion flooding. An injection speed of 0.5 mL/min needed for the experiment was selected to inject the emulsion of 1 PV.
- (5)
- Water flooding. At the end of the emulsion injection stage, subsequent wate flooding was carried out at the same injection speed.
2.2.4. Investigation of Emulsion Flooding
3. Results and Discussion
3.1. Analysis of Emulsion Properties
3.1.1. Emulsion Stability
- (1)
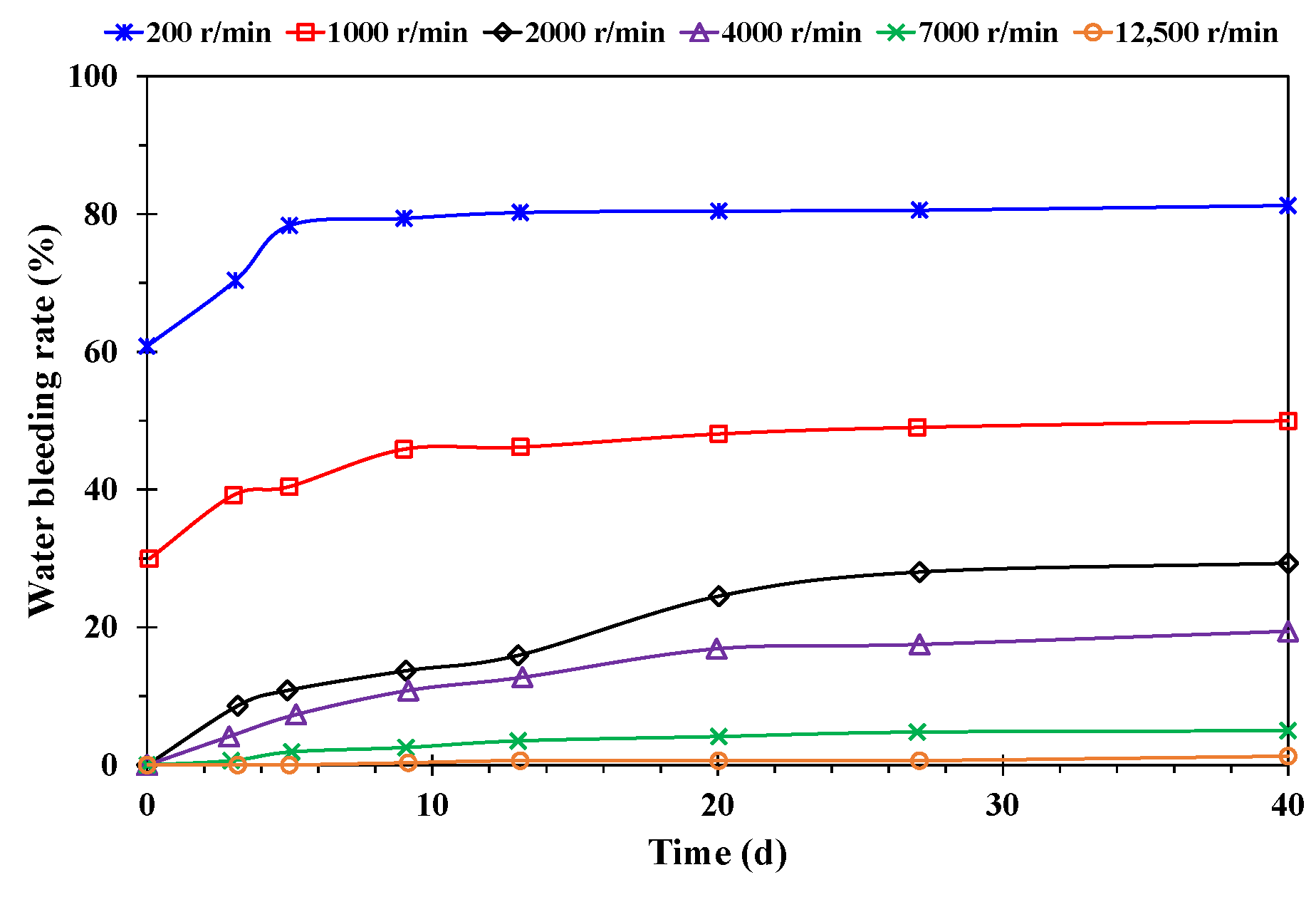
- Effect of stirring speed
- (2)
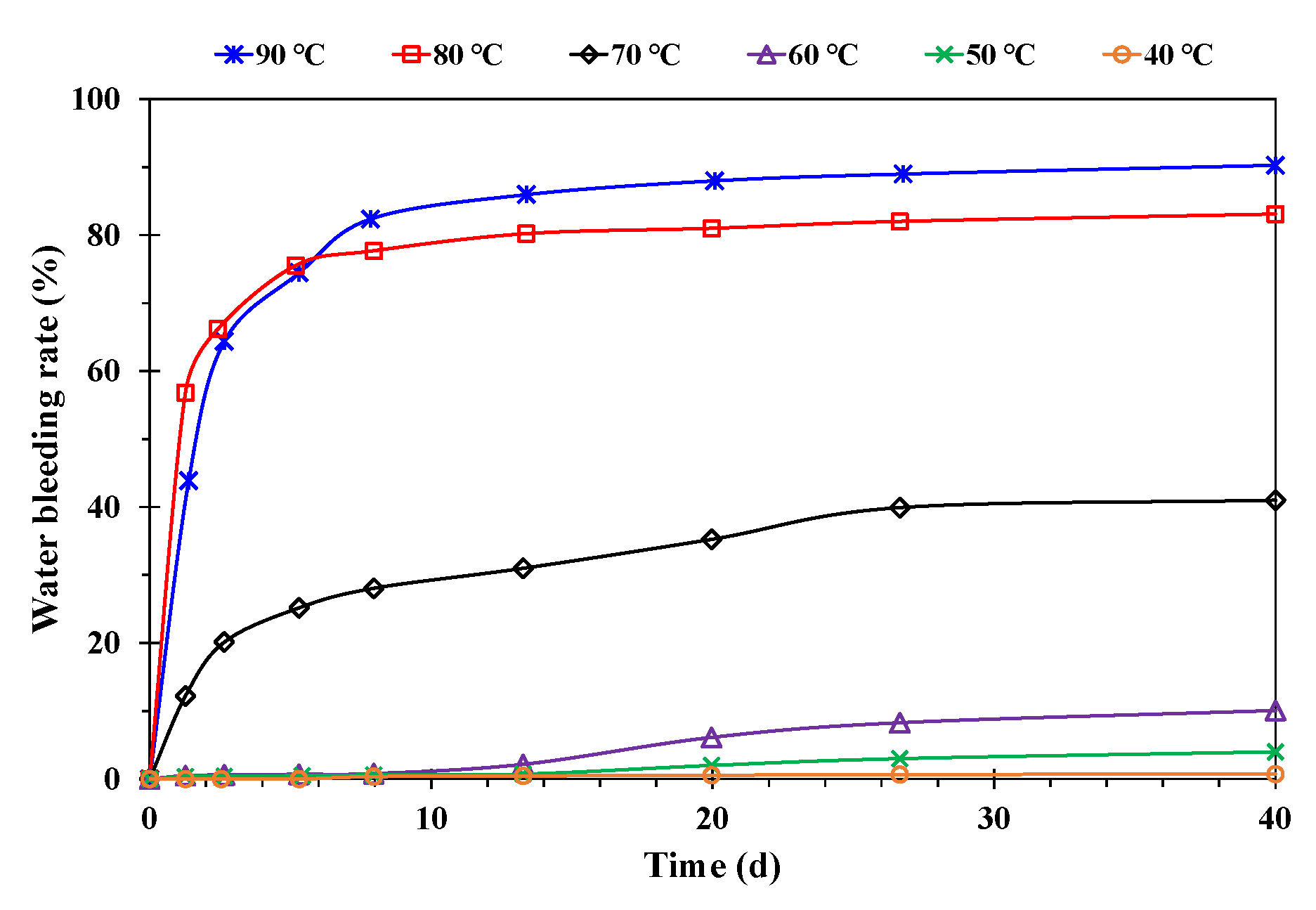
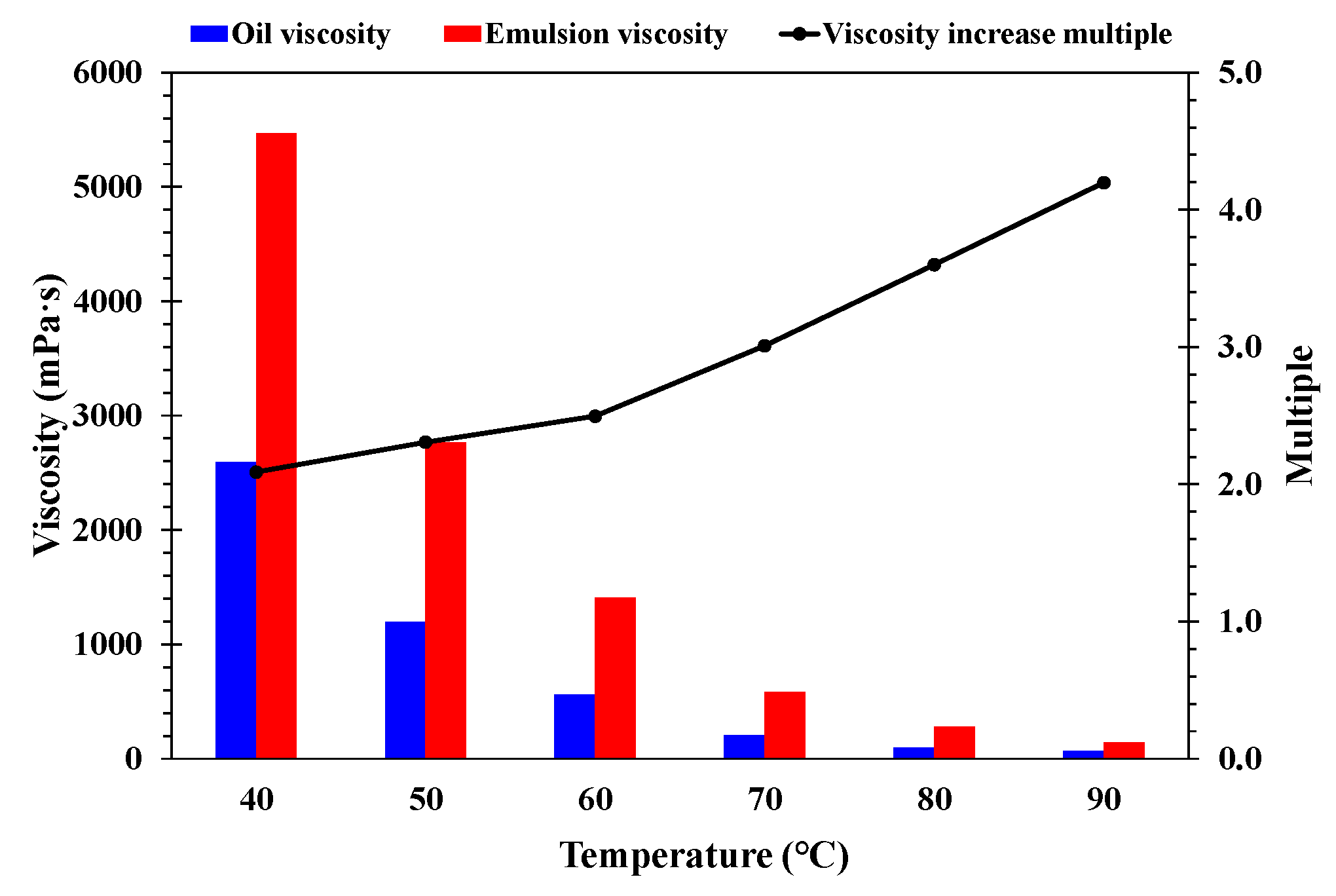
- Effect of temperature
- (3)
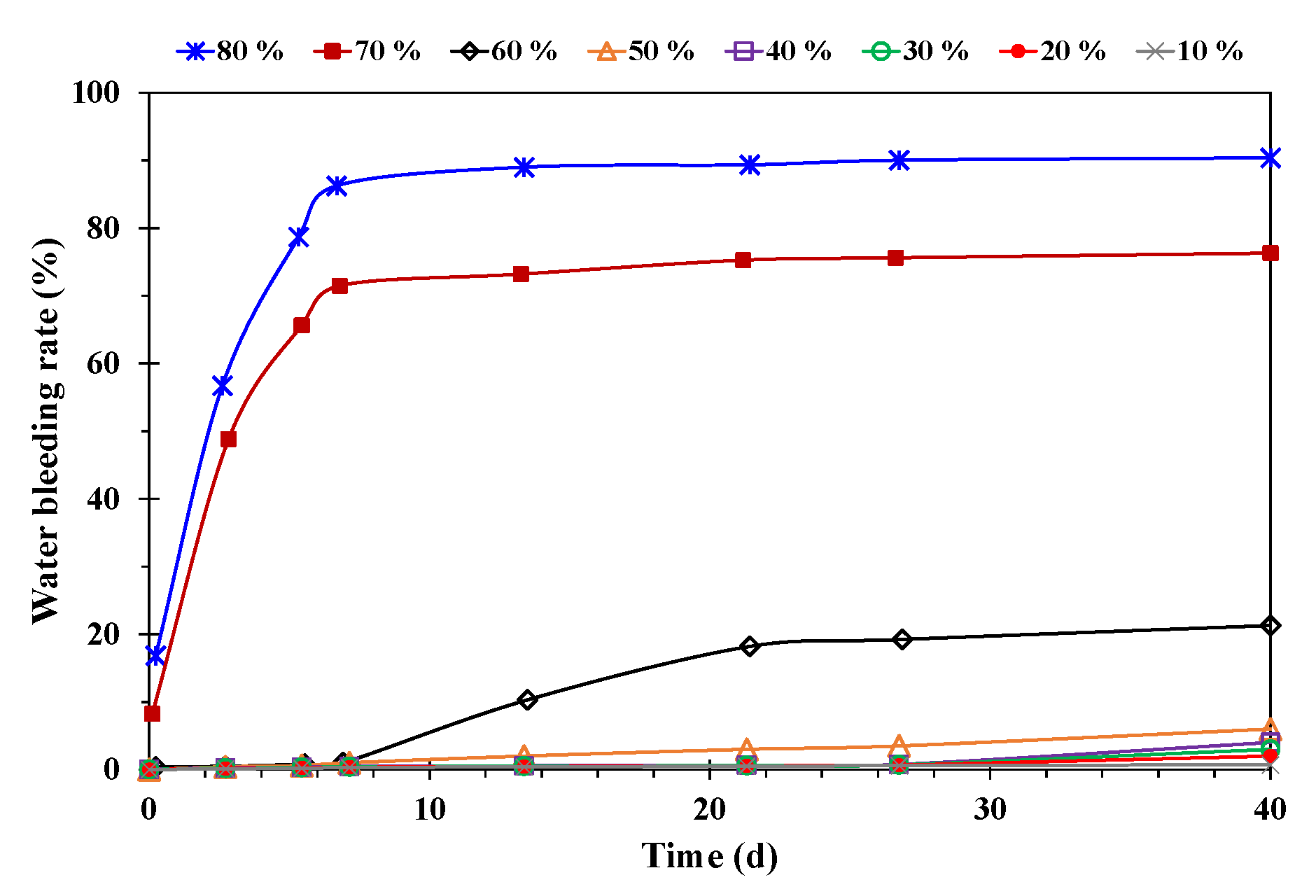
- Effect of water cut of emulsion
- (4)
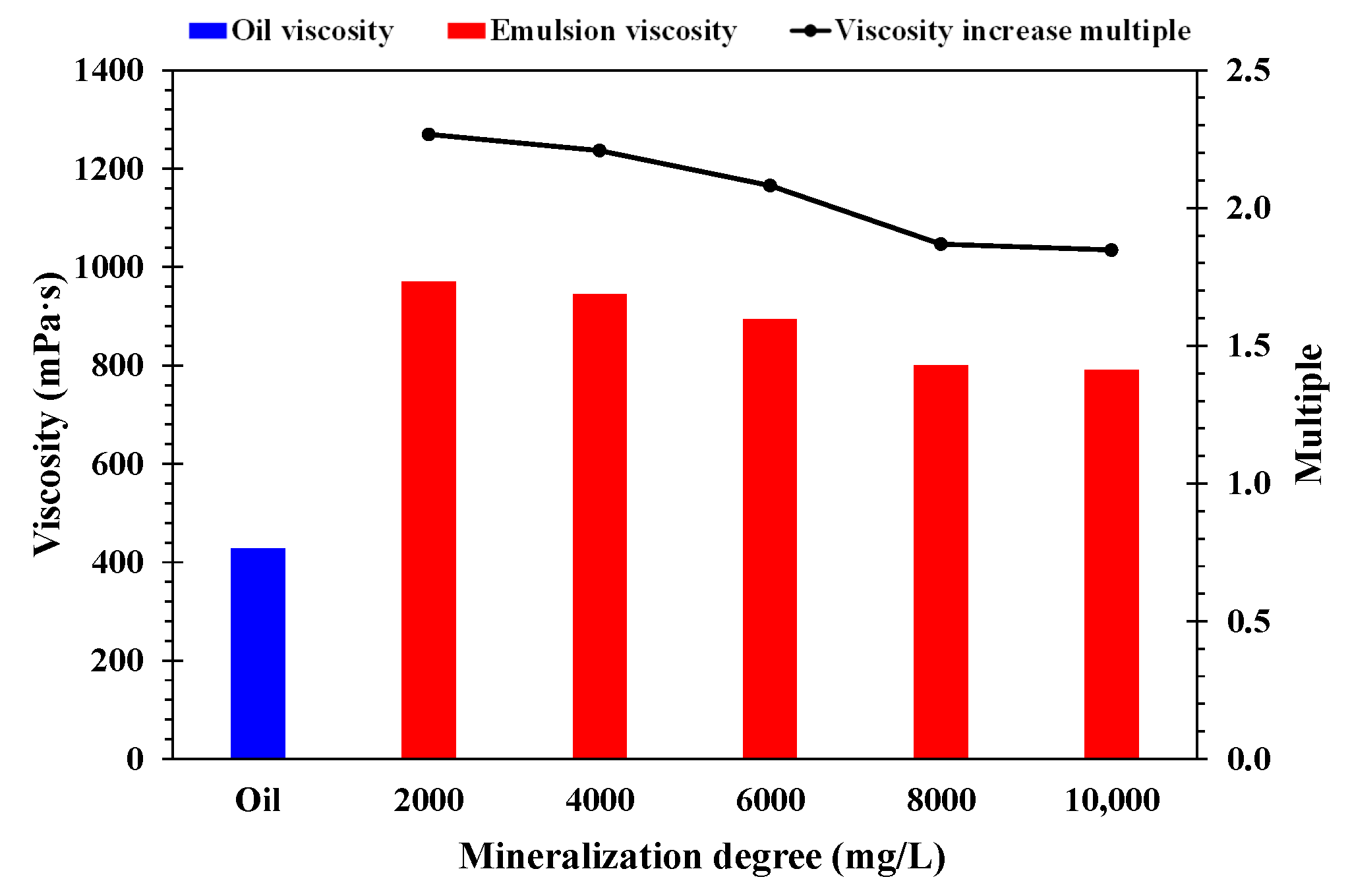
- Effect of mineralization degree
- (5)
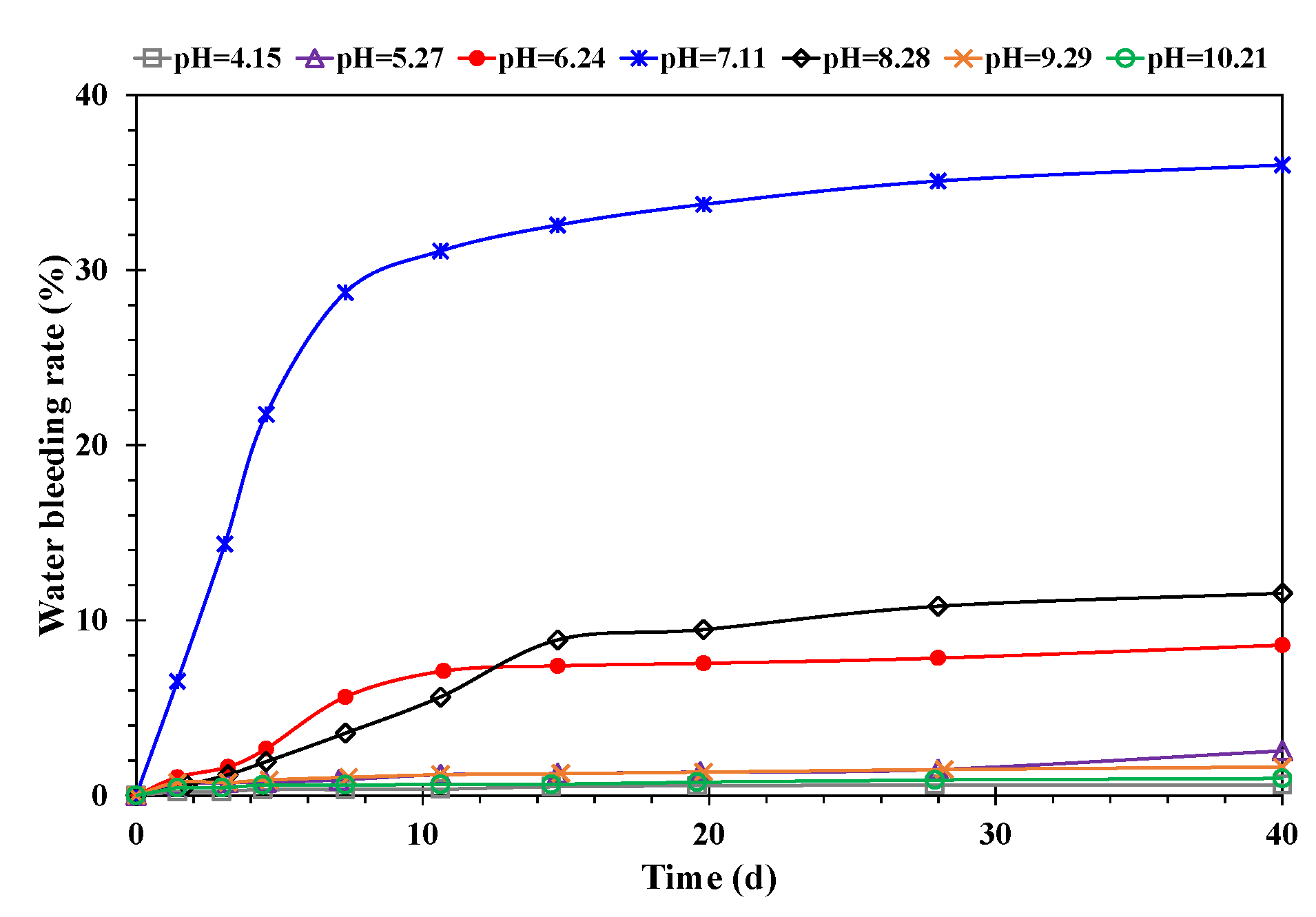
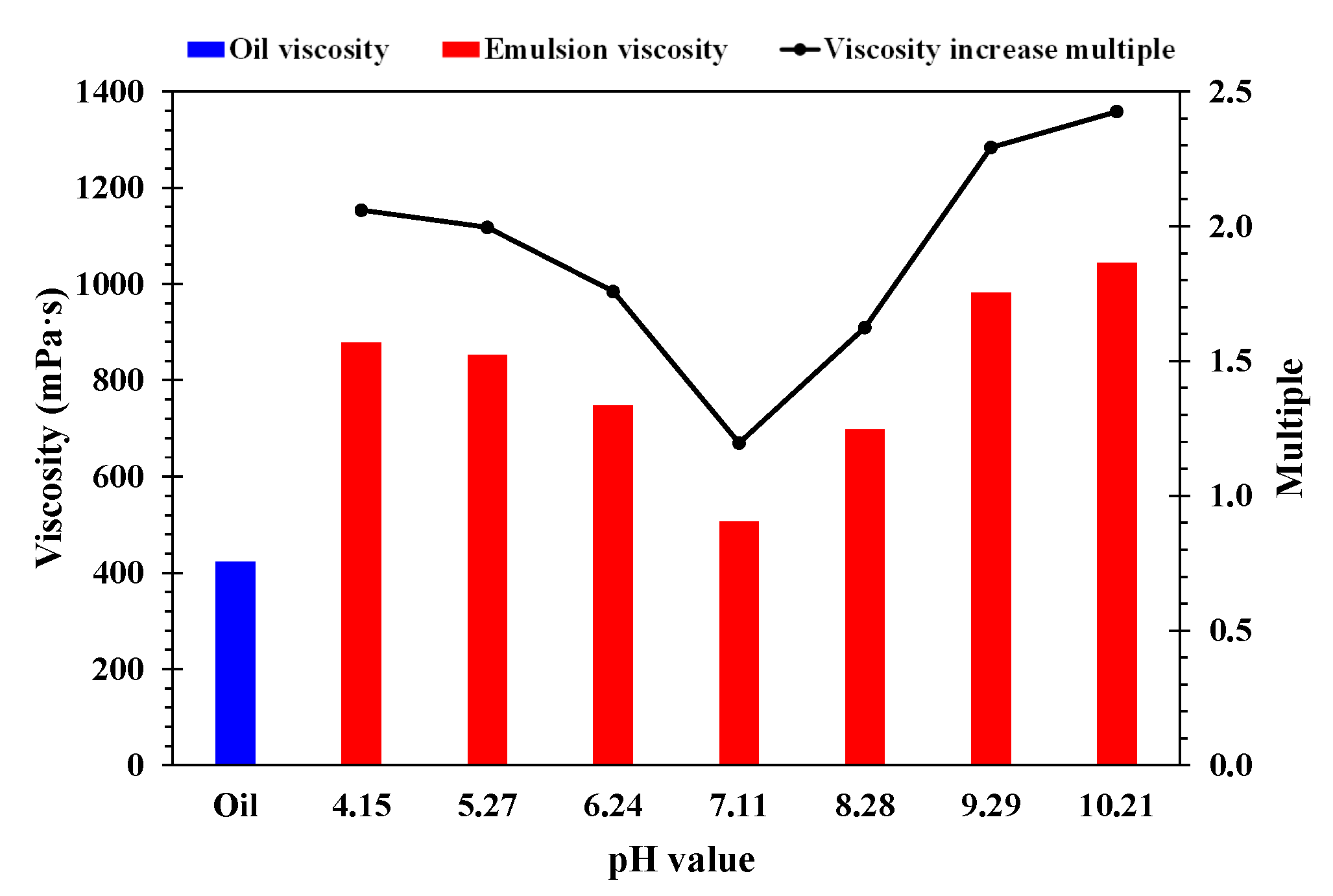
- Effect of pH value
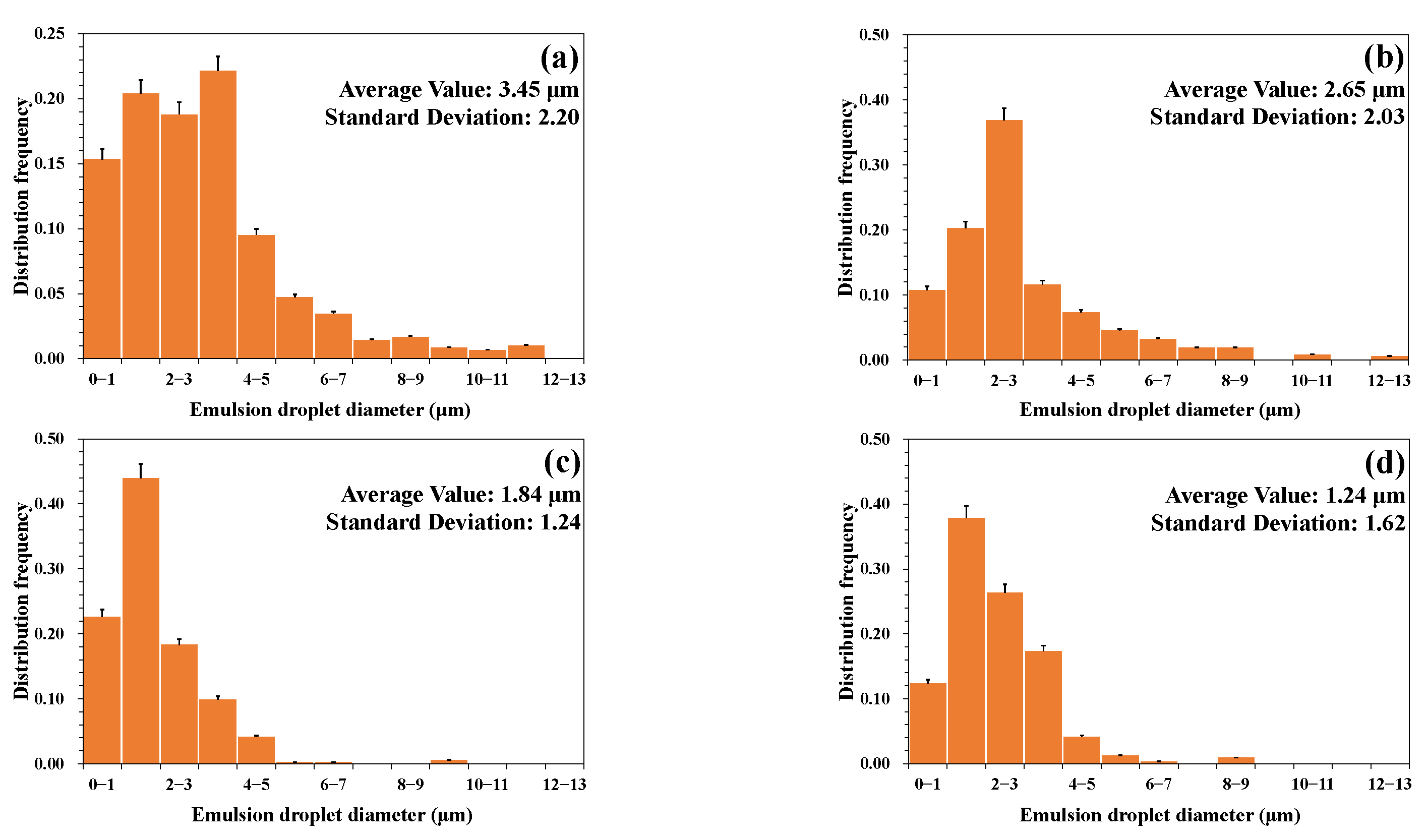
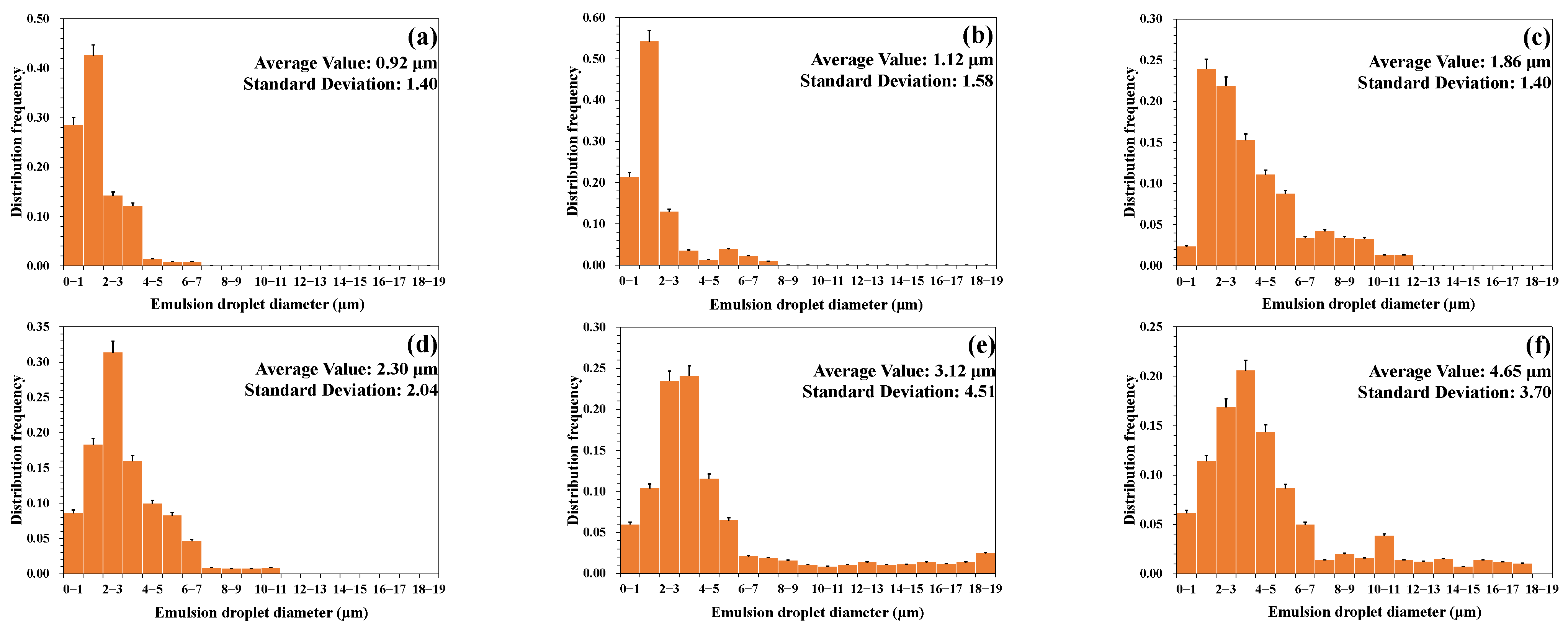
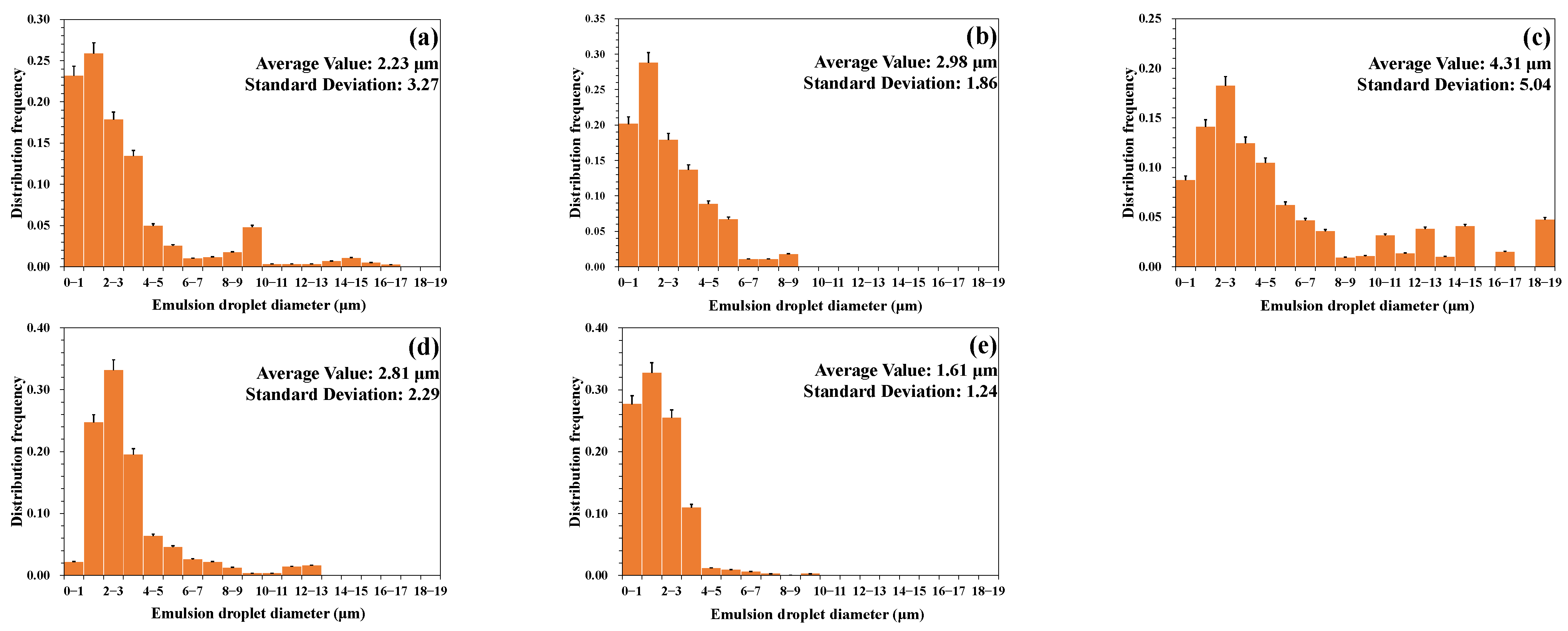
3.1.2. Emulsion Particle Size Distribution
- (1)
- Effect of stirring speed
- (2)
- Effect of temperature
- (3)
- Effect of water cut of emulsion
- (4)
- Effect of mineralization degree
- (5)
- Effect of pH value
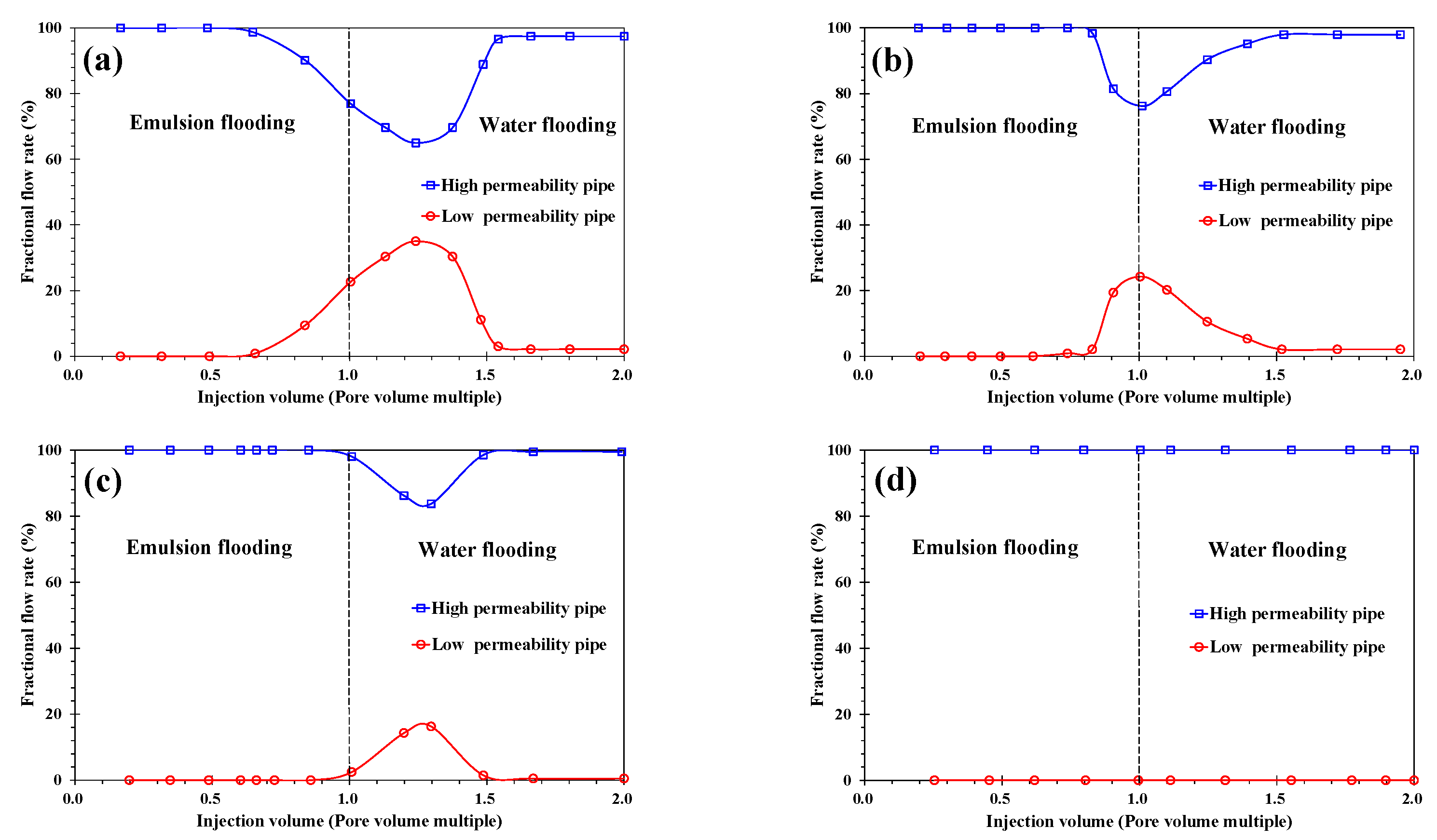
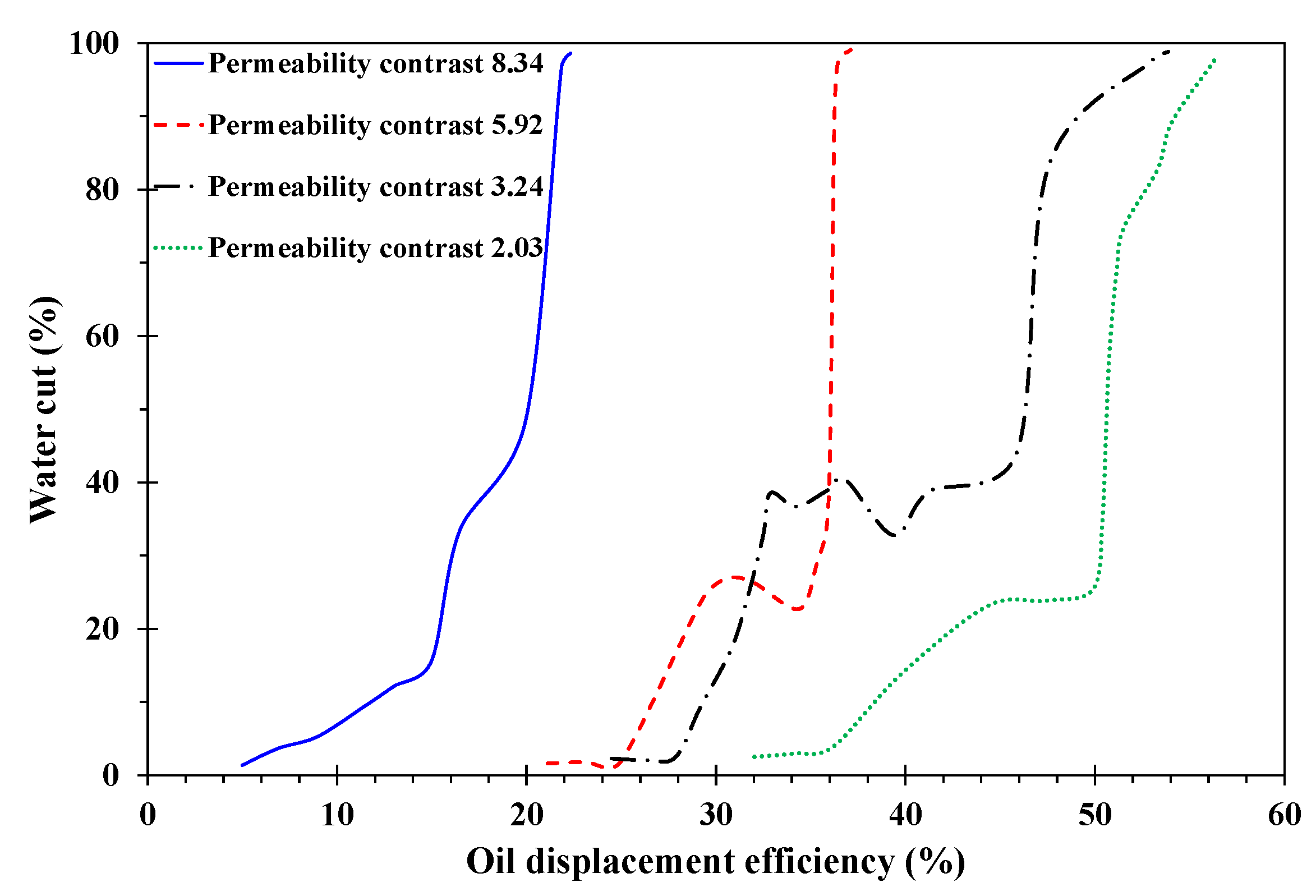
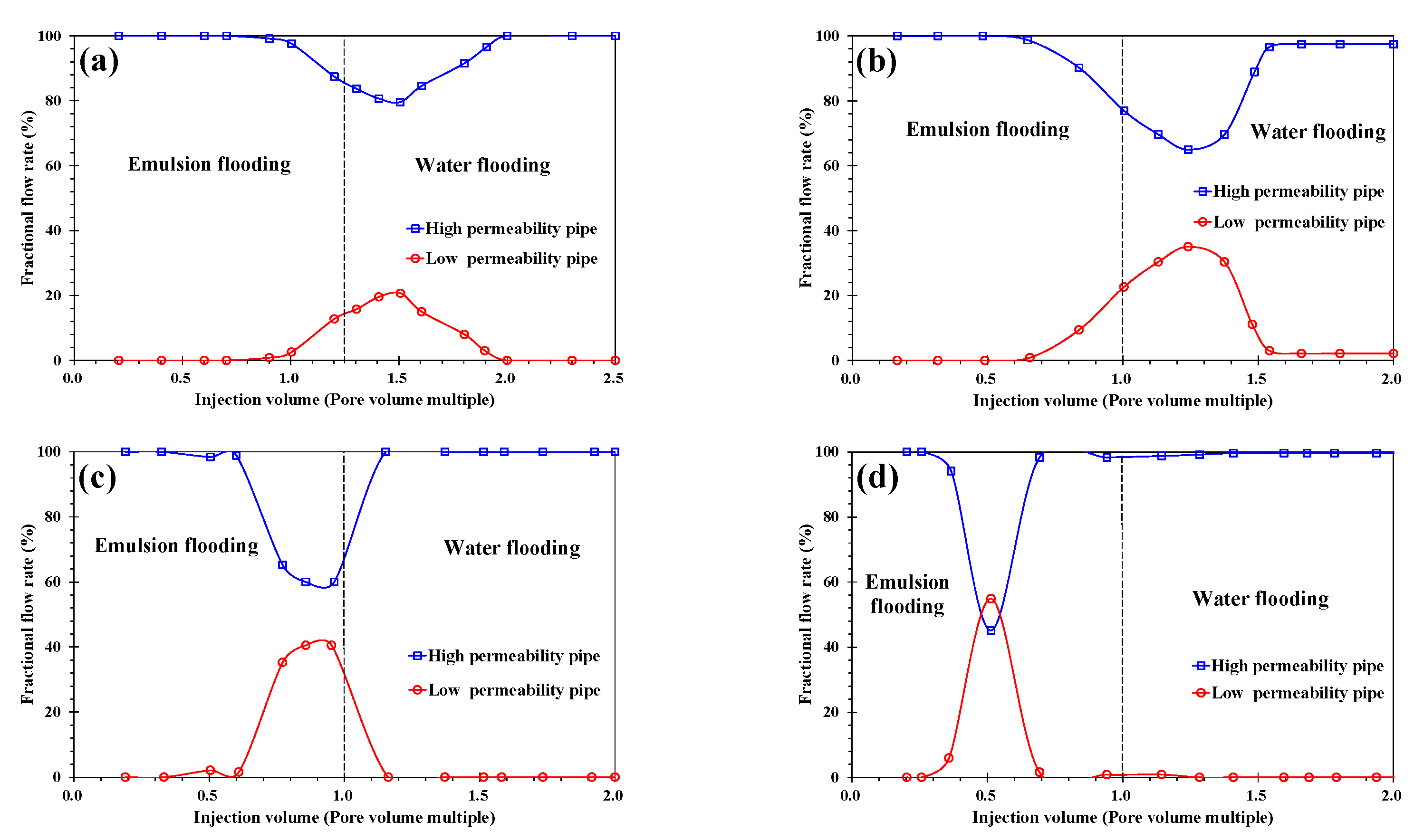
3.2. Analysis of Emulsion Flooding
3.2.1. The Influence of Permeability Contrast
3.2.2. Effect of Water Cut of Emulsion
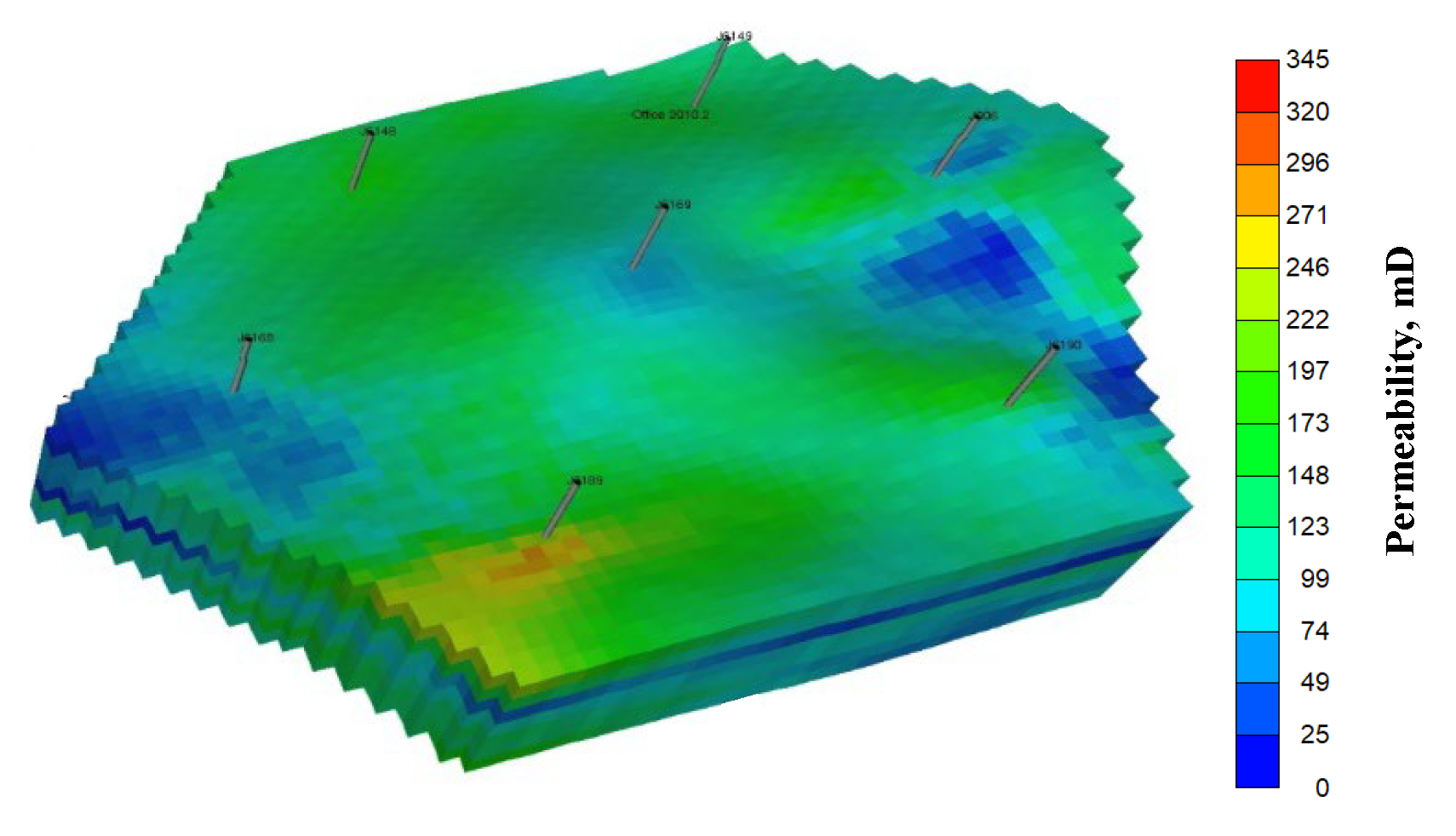
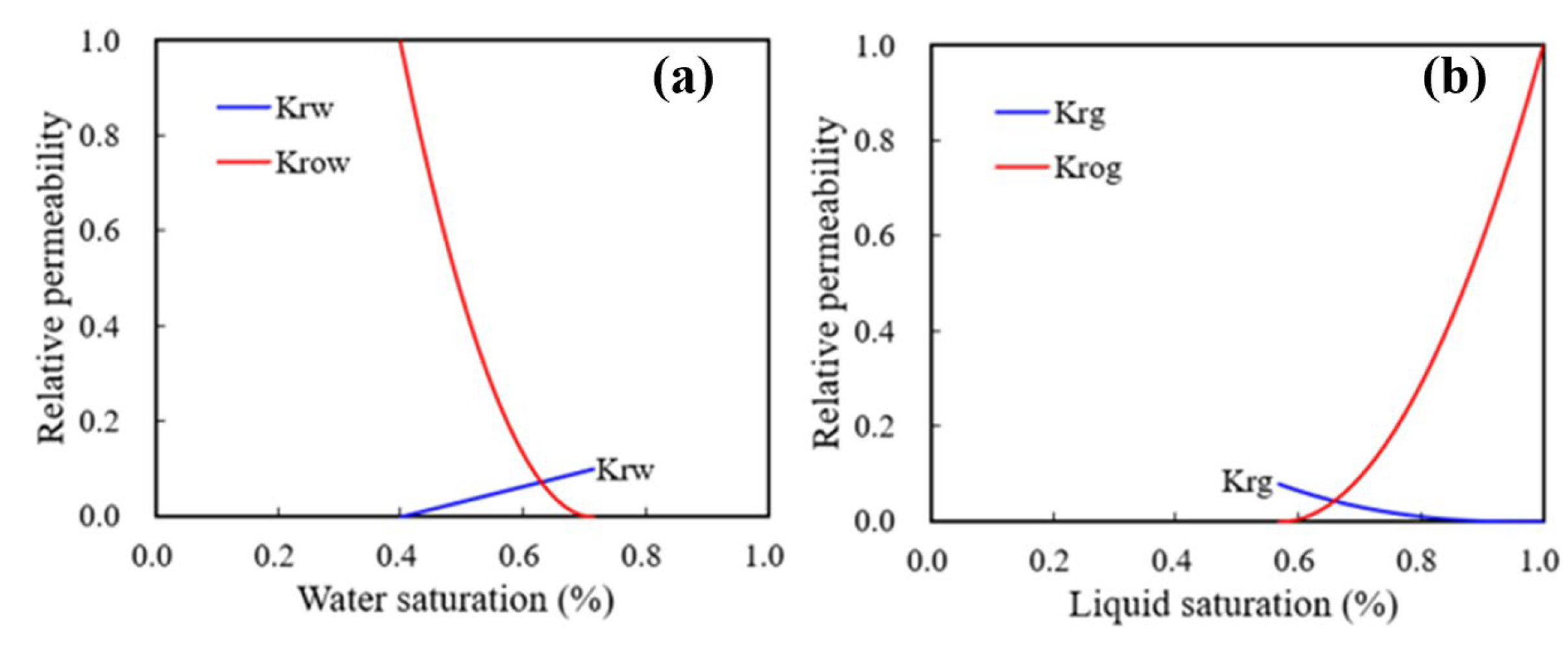
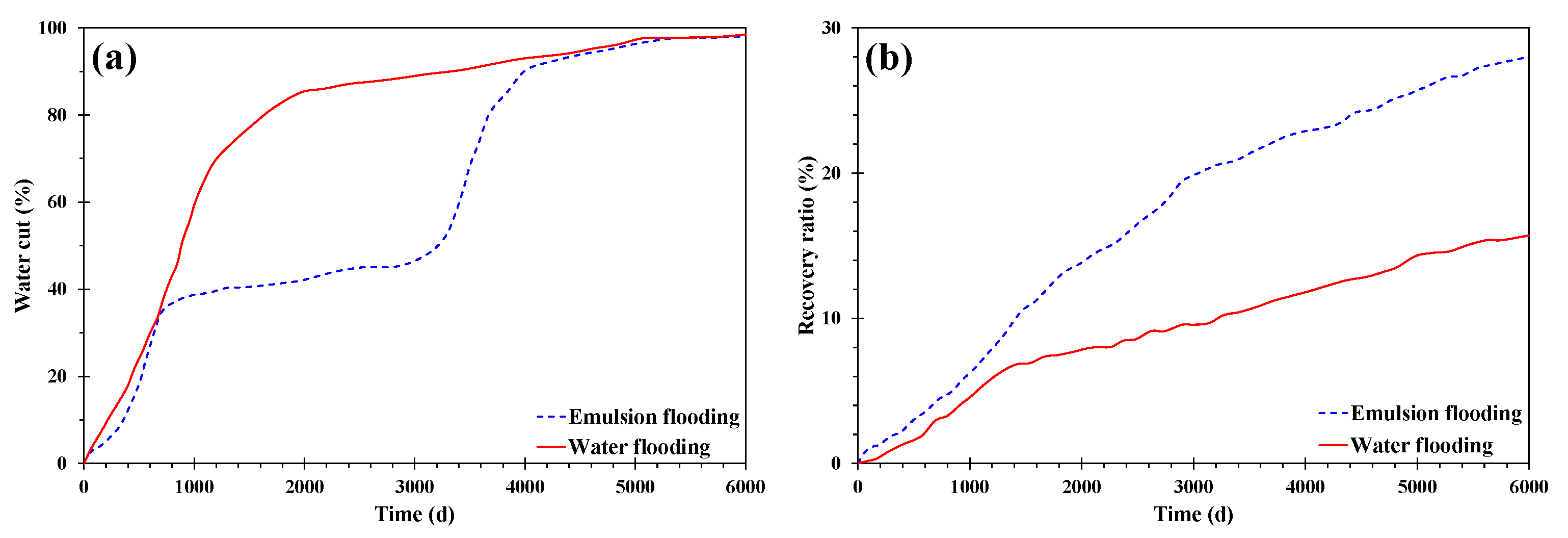
4. Numerical Simulation
5. Conclusions
- (1)
- A higher temperature facilitated emulsification, but a stable emulsion could be formed at temperatures below 60 °C. When the water cut was higher than 60%, emulsification was incomplete, and a lower water cut led to a more stable emulsion. A higher mineralization degree hindered emulsification, and emulsions formed beyond 10,000 mg/L were difficult to stabilize. When the pH was about 7, and the stability of the emulsion was poor in contrast to an acid or alkali environment.
- (2)
- A higher temperature led to a smaller particle size of the emulsion and a more uniform distribution. A lower water cut led to a smaller particle size of the emulsion and a more uniform distribution. A higher mineralization degree led to a larger particle size of the emulsion and a wider distribution range. A more neutral pH value led to a larger particle size of the emulsion and a wider distribution range.
- (3)
- In the emulsion flooding of a heterogeneous heavy oil reservoir, after the permeability contrast reached a certain value, the W/O emulsion lost its ability to block the high-permeability channel.
- (4)
- When the water cut was more than 60%, the emulsion had high viscosity and obvious sweep ability, but its stability worsened; when the water cut was less than 40%, the emulsion was stable, but the viscosity was low and the plugging capacity was insufficient. Therefore, in order to prolong the stable production time of emulsions, it was necessary to maintain the water cut at 40% and 60%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.N.; Liu, Q. On the Position of Heavy Oil in Energy in the 21st Century. Oil Gas Field Surf. Eng. 2000, 19, 4–6. [Google Scholar]
- Shu, Q.L.; Zheng, W.G.; Zhang, Z.P.; Yang, X.M.; Wu, W.; Wu, G.H.; Zhao, H.Y.; Zheng, X. Chemical Viscosity Reduction Compound Flooding Technology for Low-Efficiency Thermal Recovery/Water Flooding Heavy Oil Reservoirs. Pet. Geol. Recovery Effic. 2021, 28, 12–21. [Google Scholar]
- Wang, J.; Dong, M. Simulation of O/W Emulsion Flow in Alkaline/Surfactant Flood for Heavy Oil Recovery. J. Can. Pet. Technol. 2010, 49, 46–52. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Gnanasundaram, N.; Arunagiri, A.; Murugesan, T. A comprehensive review on the recent advances on the petroleum asphaltene aggregation. J. Pet. Sci. Eng. 2019, 176, 249–268. [Google Scholar] [CrossRef]
- Afra, S.; Nasr, H.A.; Socci, D.; Cui, Z. Green phenolic amphiphile as a viscosity modifier and asphaltenes dispersant for heavy and extra-heavy oil. Fuel 2018, 220, 481–489. [Google Scholar] [CrossRef]
- Daaou, M.; Larbi, A.; Martinez, B.; Rogalski, M. A comparative study of the chemical structure of asphaltenes from algerian petroleum collected at different stages of extraction and processing. J. Pet. Sci. Eng. 2016, 138, 50–56. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Pu, W.F.; He, W.; Lu, J.Y.; Chan, J.J.; Li, S.Y. Stability Analysis of Emulsions with Different Moisture Content Magnetic Resonance. Oilfield Chem. 2022, 39, 529–535. [Google Scholar]
- Huang, B.; Nan, X.H.; Fu, C.; Zhu, Y.M.; Zhang, L.; Guo, W.; Wang, S.Q. Study on the macroscopic coalescence mechanism of oil droplets and the microscopic interaction between oil film molecules. Spec. Oil Gas Reserv. 2022, 156, 1–10. [Google Scholar]
- Bai, J.M. Study on the Effect of Heavy Oil Composition and Emulsifier on Oil–Water Interface Properties. China Univ. Pet. 2009, 6, 124–148. [Google Scholar]
- Zhao, Y.L.; Kang, W.L.; Yin, X.; Geng, J.; Tang, X.; Yang, L.; Yuan, H. Relationship between Stability of Changqing Water-in-Oil Emulsion and Asphaltene Content. Acta Pet. Sin. 2018, 34, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.J.; Li, M.Y.; Peng, B.; Wu, Z.L.; Lin, M.Q.; Guo, J.X. Relationship between interfacial shear viscosity of resins and asphaltenes and emulsion stability in water-in-oil emulsions. Acta Pet. Sin. 2007, 3, 107–110. [Google Scholar]
- Guo, J.X.; Wu, Z.L.; Li, M.Y.; Yin, Y. Effect of interfacial shear viscosity on stability of crude oil emulsion. Fine Chem. 2003, 11, 660–662. [Google Scholar]
- Ismail, I.; Kazemzadeh, Y.; Sharifi, M.; Riazi, M.; Malayeri, M.R.; Cortés, F. Formation and stability of W/O emulsions in presence of asphaltene at reservoir thermodynamic conditions. J. Mol. Liq. 2020, 299, 112125. [Google Scholar] [CrossRef]
- Kumar, K.; Nikolov, A.D.; Wasan, D.T. Mechanisms of stabilization of water-in-crude oil emulsions. Ind. Eng. Chem. Res. 2001, 40, 3009–3014. [Google Scholar] [CrossRef]
- Nour, A.H.; Suliman, A.; Hadow, M.M. Stabilization mechanisms of water-in-crude oil emulsions. Appl. Sci. 2008, 8, 1571–1575. [Google Scholar] [CrossRef] [Green Version]
- Czarnecki, J. Stabilization of water in crude oil emulsions. Fuel 2009, 23, 1253–1257. [Google Scholar] [CrossRef]
- Pal, R. Rheology of simple and multiple emulsions. Curr. Opin. Colloid Interface Sci. 2011, 16, 41–60. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H.; Hayder, B. Pipeline transportation of viscous crudes as concentrated oil-in-water emulsions. J. Pet. Sci. Eng. 2012, 90, 139–144. [Google Scholar] [CrossRef]
- Binner, E.R.; Robinson, J.P.; Silvester, S.A.; Kingman, S.; Lester, E. Investigation into the mechanisms by which microwave heating enhances separation of water-in-oil emulsions. Fuel 2014, 116, 516–521. [Google Scholar] [CrossRef]
- Hempoonsert, J.; Tansel, B.; Laha, S. Effect of temperature and pH on droplet aggregation and phase separation characteristics of flocs formed in oil-water emulsions after coagulation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 37–42. [Google Scholar] [CrossRef]
- Martinez-Palou, R.; Ceron-Camacho, R.; Chavez, B.; Vallejo, A.A.; Villanueva-Negrete, D.; Castellanos, J.; Karamath, J.; Reyes, J.; Aburto, J. Demulsification of heavy crude oil-in-water emulsions: A comparative study between microwave and thermal heating. Fuel 2013, 113, 407–414. [Google Scholar] [CrossRef]
- Zaki, N.N. Surfactant stabilized crude oil-in-water emulsions for pipeline transportation of viscous crude oils. Colloids Surf. A Physicochem. Eng. Asp. 1997, 125, 19–25. [Google Scholar] [CrossRef]
- Binks, B.P.; Rocher, A. Effects of temperature on water-in-oil emulsions stabilised solely by wax microparticles. J. Colloid Interface Sci. 2009, 335, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, X.; Rui, Z.; Xu, M.; Zhan, S. The role of shearing energy and interfacial Gibbs free energy in the emulsification mechanism of waxy crude oil. Energies 2017, 10, 721. [Google Scholar] [CrossRef] [Green Version]
- Djenouhat, M.; Hamdaoui, O.; Chiha, M.; Samar, M.H. Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane: Part 2. Permeation and stripping. Sep. Purif. Technol. 2008, 63, 231–238. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; G’omez, M.J.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Stability of oil-in-water paraffin emulsions prepared in a mixed ionic/nonionic surfactant system. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 222–229. [Google Scholar] [CrossRef]
- An, F.; Bai, J.; Qian, G.L. Study on the stability of W/O emulsion of Liu hua heavy oil. Petro-Chem. Equip. 2013, 16, 19–22. [Google Scholar]
- Yang, F.; Li, C.X.; Lin, M.Z.; Guo, G. Effect of emulsification conditions on rheology of O/W heavy oil emulsion. J. Petrochem. Univ. 2009, 22, 51–54. [Google Scholar]
- Yu, R.Y. Study on relationship between moisture content and stability of crude oil emulsion. Chem. Eng. Des. Commun. 2016, 42, 150. [Google Scholar]
- Duan, M.; Tao, J.; Fang, S.W.; Shi, P.; Li, K.; Song, X.; Tao, T. Effect of pH value on the stability of crude oil emulsion. Chem. Ind. Eng. Prog. 2015, 34, 1853–1857. [Google Scholar]






| Density | Viscosity | Saturates | Aromatics | Resins | Asphaltenes |
|---|---|---|---|---|---|
| (20 °C) | (50 °C) | (wt.%) | (wt.%) | (wt.%) | (wt.%) |
| (g/cm3) | (mPa·s) | ||||
| 0.92 | 1231 | 27.48 | 29.26 | 36.46 | 6.8 |
| Mineralization Degree (mg/L) | Ion Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| K+ | Na+ | Mg2+ | Ca2+ | HCO3− | Cl− | SO42− | |
| 7795.43 | 19.2 | 3404.7 | 13.6 | 52.2 | 314.45 | 3930.89 | 60.39 |
| Permeability Contrast | Permeability to Water (mD) | Porosity (%) | |
|---|---|---|---|
| ① | 2.03 | 124.36 | 19.29 |
| ② | 62.26 | 16.86 | |
| ③ | 3.24 | 121.34 | 18.92 |
| ④ | 37.45 | 15.65 | |
| ⑤ | 5.92 | 156.86 | 19.95 |
| ⑥ | 26.5 | 14.87 | |
| ⑦ | 8.34 | 119.2 | 17.28 |
| ⑧ | 14.29 | 12.15 |
| Item | Value | Item | Value |
|---|---|---|---|
| Reservoir buried depth (m) | 1800 | Porosity (%) | 21.08 |
| Thickness (m) | 21 | Oil saturation (%) | 60.1 |
| Formation temperature (°C) | 54 | Permeability (mD) | 63.9 |
| Oil viscosity (mPa·s) (50 °C) | 1231 | Permeability variation coefficient | 0.58 |
| Oil density (kg/m3) | 0.92 | Rock compressibility (1/MPa) | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Liu, P.; Wang, B.; Wang, C.; Liu, P.; Zhao, J.; Chen, J.; Zhang, J. Experimental Study and Numerical Simulation of W/O Emulsion in Developing Heavy Oil Reservoirs. Appl. Sci. 2022, 12, 11867. https://doi.org/10.3390/app122211867
Wang K, Liu P, Wang B, Wang C, Liu P, Zhao J, Chen J, Zhang J. Experimental Study and Numerical Simulation of W/O Emulsion in Developing Heavy Oil Reservoirs. Applied Sciences. 2022; 12(22):11867. https://doi.org/10.3390/app122211867
Chicago/Turabian StyleWang, Kaixuan, Pengcheng Liu, Bojun Wang, Chao Wang, Peng Liu, Jiu Zhao, Junwei Chen, and Jipeng Zhang. 2022. "Experimental Study and Numerical Simulation of W/O Emulsion in Developing Heavy Oil Reservoirs" Applied Sciences 12, no. 22: 11867. https://doi.org/10.3390/app122211867
APA StyleWang, K., Liu, P., Wang, B., Wang, C., Liu, P., Zhao, J., Chen, J., & Zhang, J. (2022). Experimental Study and Numerical Simulation of W/O Emulsion in Developing Heavy Oil Reservoirs. Applied Sciences, 12(22), 11867. https://doi.org/10.3390/app122211867







