Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties
Abstract
1. Introduction
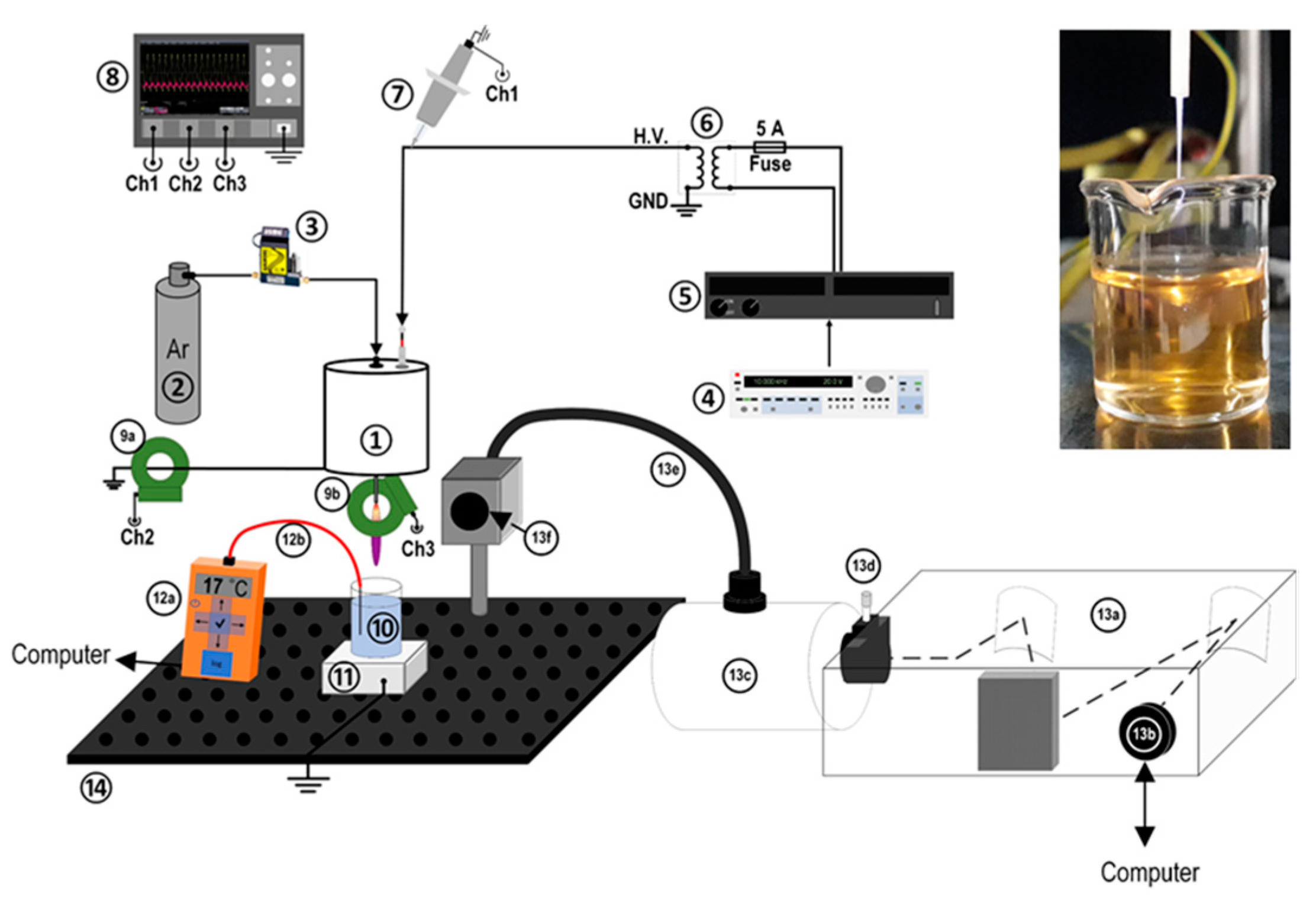
2. Experimental Setup and Materials
2.1. Plasma Setup and Diagnostics
2.2. Basic Characterization of the Liquids
2.3. ROS Detection in the Liquids
- -
- Hydroethidine (HE; Fluka, Buchs, Switzerland; cat. no. 37291).
- -
- Dimethyl sulfoxide (DMSO, 99.9% or 14 M; ChemLab, Zedelgem, Belgium; cat. no. CL00.0422).
- -
- Hydrochloric acid (HCl, 37% w/w or 12 M; ChemLab, Zedelgem, Belgium; cat. no. CL00.0310).
- -
- 2-hydroxy terephthalate (2-OH-TPA, 98%; TCI, Portland, USA; cat. no. H1385).
- -
- 2-hydroxy ethidium (2-OH-E+, synthesized as described elsewhere [33]).
- -
- Sodium hydroxide (NaOH, pellets; Merck, New Jersey, USA; cat. no. 106498).
- -
- Phosphate Buffered Saline (PBS powder, for preparing 1 L, pH 7.4; Sigma-Aldrich, Burlington, MA, USA; P3813).
- -
- Terephthalic acid (TPA, 98+%; Alfa Aesar, Kandel, Germany; cat. no. A12527).
- -
- DDW, purified by a Milli-Q system (Millipore, Massachusetts, USA).
- -
- Acetonitrile (ACN, HPLC grade; Sigma-Aldrich, Burlington, MA, USA; cat. no. 34851).
- -
- Chloroform (CHCl3; Merck, New Jersey, USA; cat. no. 1.02445).
- -
- Methanol (MetOH, ≥99.9%; Merck, New Jersey, USA; cat. no. 106009).
- -
- 5 mM TPA, pH 7: Dissolve 4.15 mg TPA in 1 mL 0.1 M NaOH and ddH2O is added to 5 mL final volume. Adjust pH to 7, by dropwise addition of 0.5 M HCl. Long storage at −20 °C.
- -
- 1 M DMSO: prepare 1 mL by mixing 71.4 μL 14 M DMSO with 928.6 μL DDW.
- -
- 0.4 M HCl: prepare 2 mL by mixing 66.7 μL 12 M HCl with 1933.3 μL DDW.
- -
- 5 M NaOH: dissolve 0.4 g in DDW to final 2 mL.
- -
- 20 mM HE: dissolve 6.3 mg HE in 1 mL 0.4 M HCl. Long storage at −20 °C.
- -
- PBS, pH 7: mix the content of a PBS bag with 1 L DDW.
- -
- 1 M DMSO in PBS, pH 7: prepare 1 mL by mixing 71.4 μL 14 M DMSO with 928.6 μL PBS.
- -
- 5 mM 2-OH-TPA, pH 7: dissolve 1.4 mg HTPA in 0.3 mL 0.1 M NaOH and add DDW to final 1.5 mL. The pH is neutralized to 7, by addition of about 10 μL 1 M HCl. Long storage at −20 °C.
- -
- 0.2 mM 2-OH-E+ (in 0.4 M HCl) prepared from synthetic 2-OH-E+ as described elsewhere [33]. Long storage at −20 °C.
- -
- 50 mM Pi buffer, pH 7.8: dissolve 0.45 g Νa2HPO4.2H2O in about 48 mL DDW, final 50 mL DDW, adjust to pH 7.8 (dropwise with 1 M HCl) and bring to final 50 mL with DDW.
- -
- 100 μM TPA, pH 7: prepare 8 mL by mixing 0.16 mL 5 mM TPA with 7.84 mL DDW, and if needed adjust pH to 7.
- -
- 100 μM TPA + 50 mM DMSO, pH 7: prepare 8 mL by mixing 0.16 mL 5 mM TPA with 0.4 mL 1 M DMSO and 7.44 mL DDW and, if needed, adjust pH to 7.
- -
- 100 μM TPA in PBS, pH 7: prepare 8 mL for each experiment by mixing 0.16 mL 5 mM TPA with 7.84 mL PBS and, if needed, adjust pH to 7.
- -
- 100 μM TPA + 50 mM DMSO in PBS, pH 7: prepare 8 mL for each experiment by mixing 0.16 mL 5 mM TPA with 0.4 mL 1 M DMSO and 7.44 mL PBS and, if needed, adjust pH to 7.
- -
- 100 μM HE, pH 7: prepare 8 mL for each experiment by mixing 40 μL 20 mM HE with 7.96 mL DDW. Adjust pH to 7 by dropwise addition of 5 M NaOH.
- -
- 100 μM HE in PBS, pH 7: prepare 8 mL for each experiment by mixing 40 μL 20 mM HE with 7.96 mL PBS and, if needed, adjust pH to 7.
3. Results and Discussion
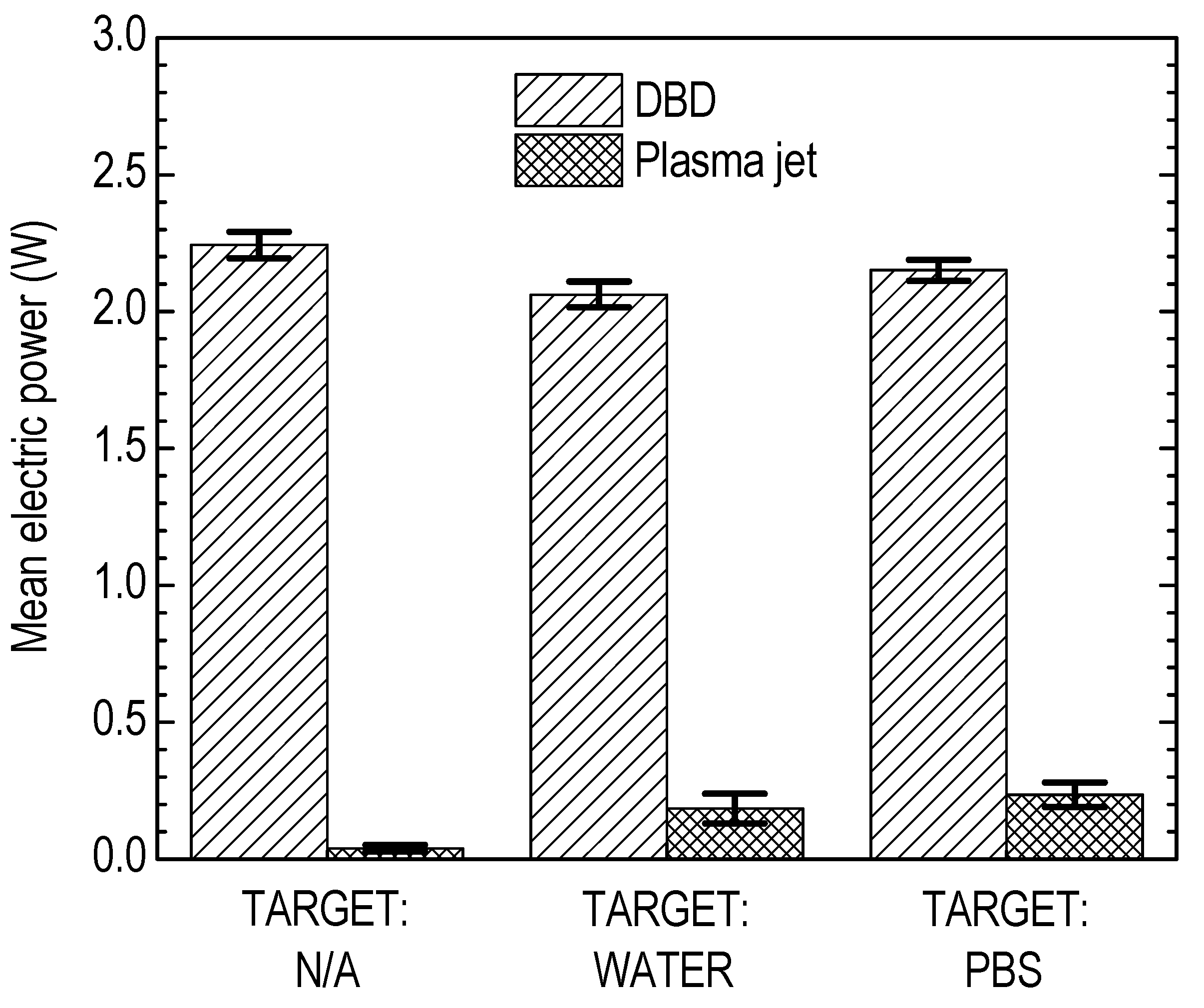
3.1. Plasma Jet Operational Window
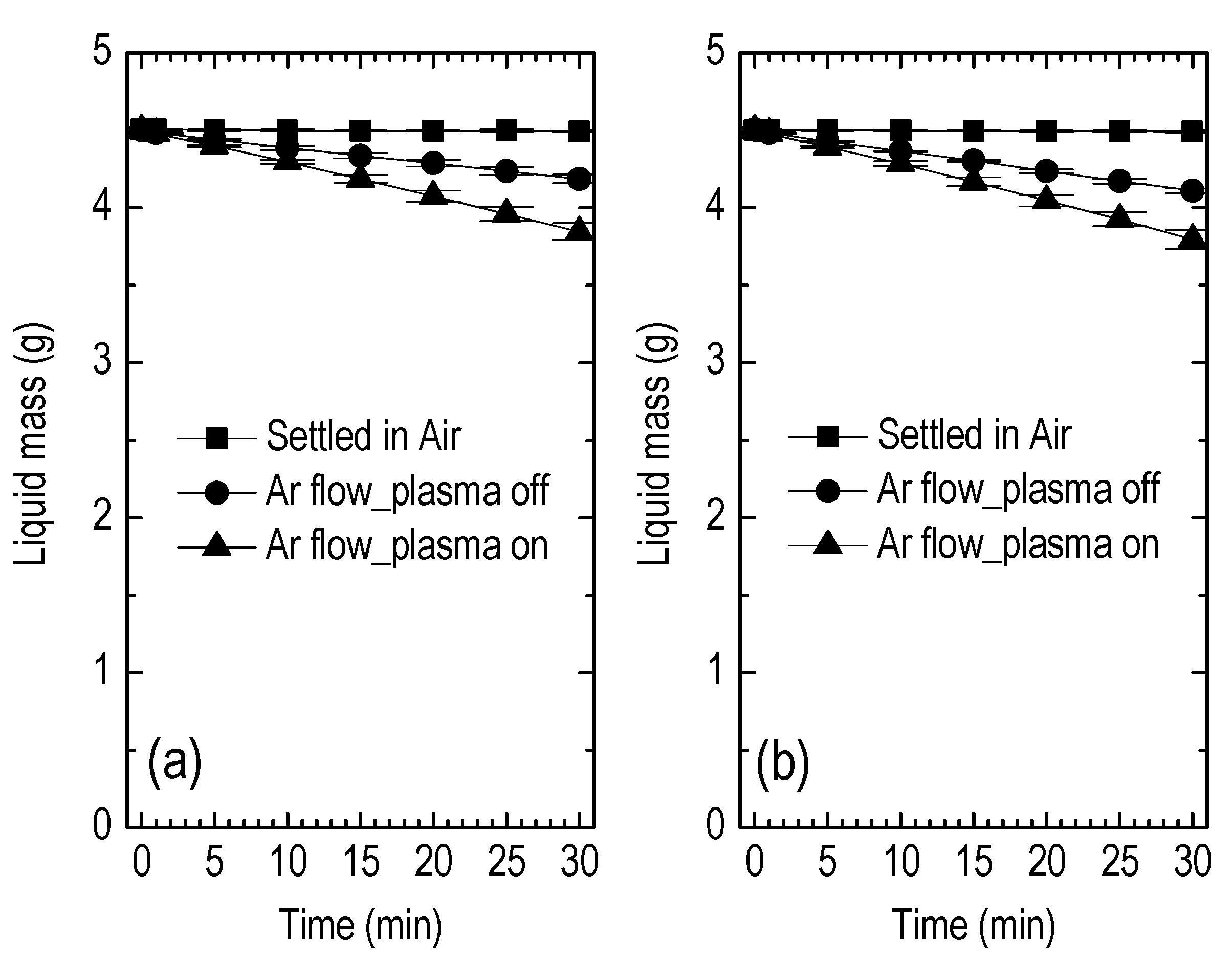
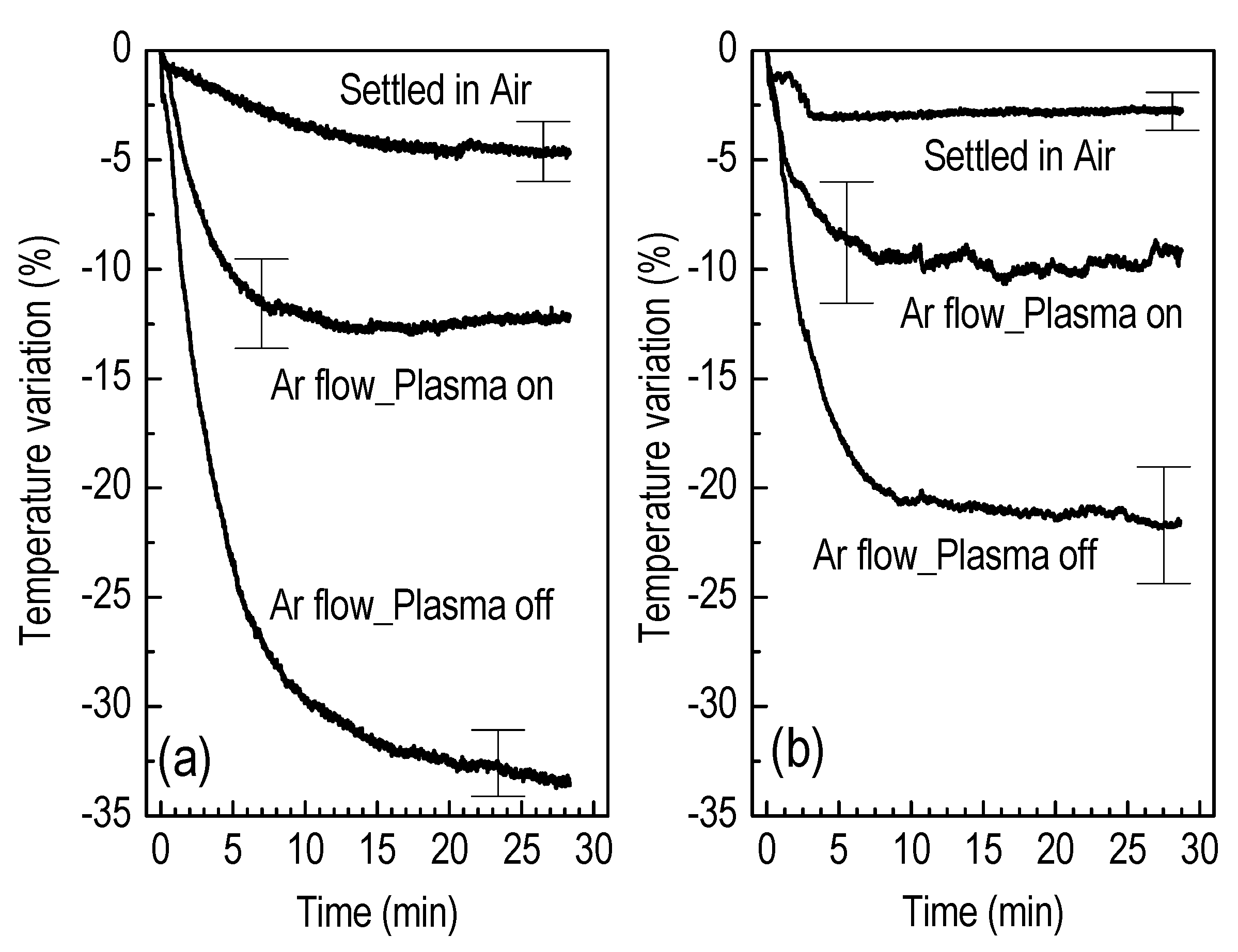
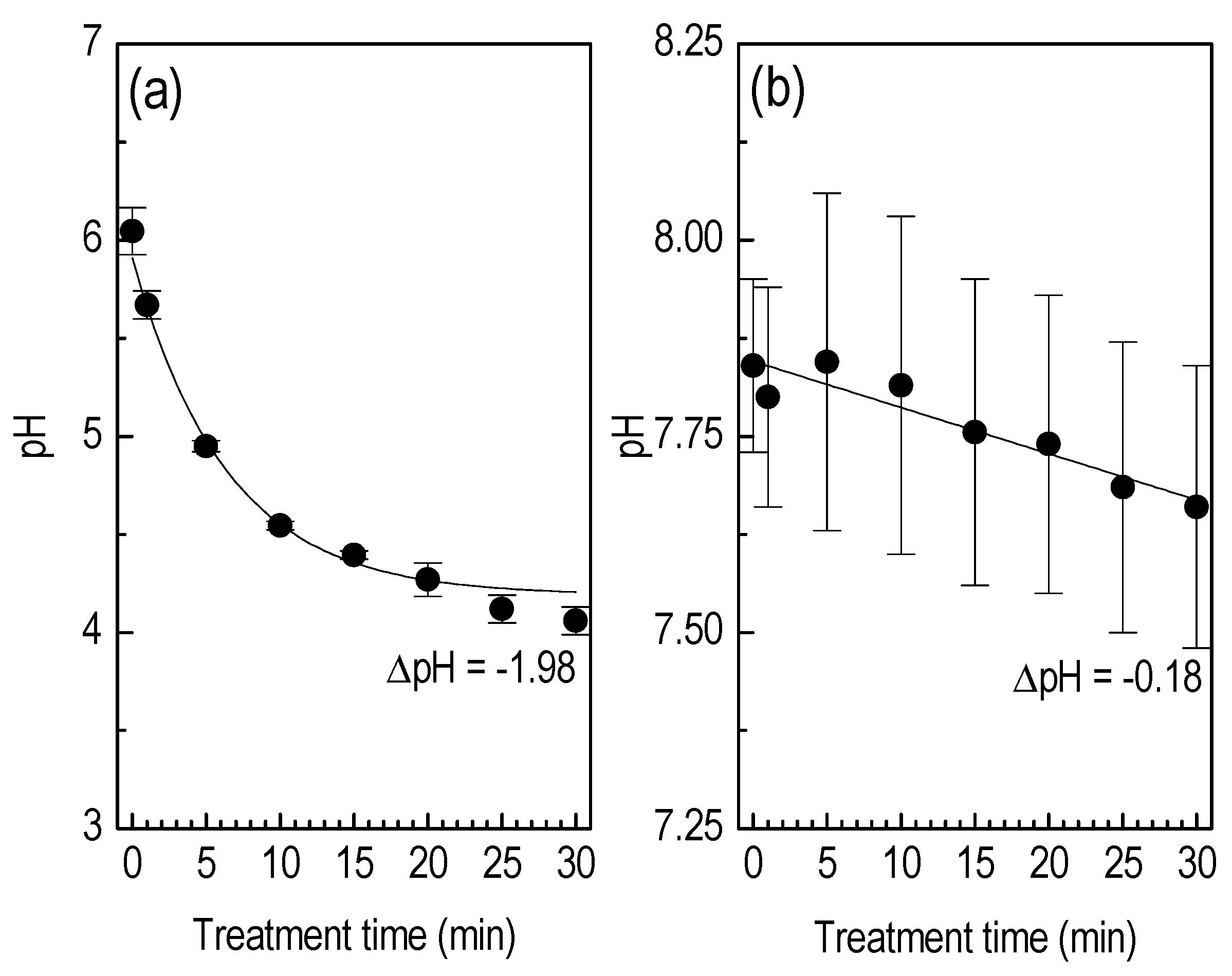
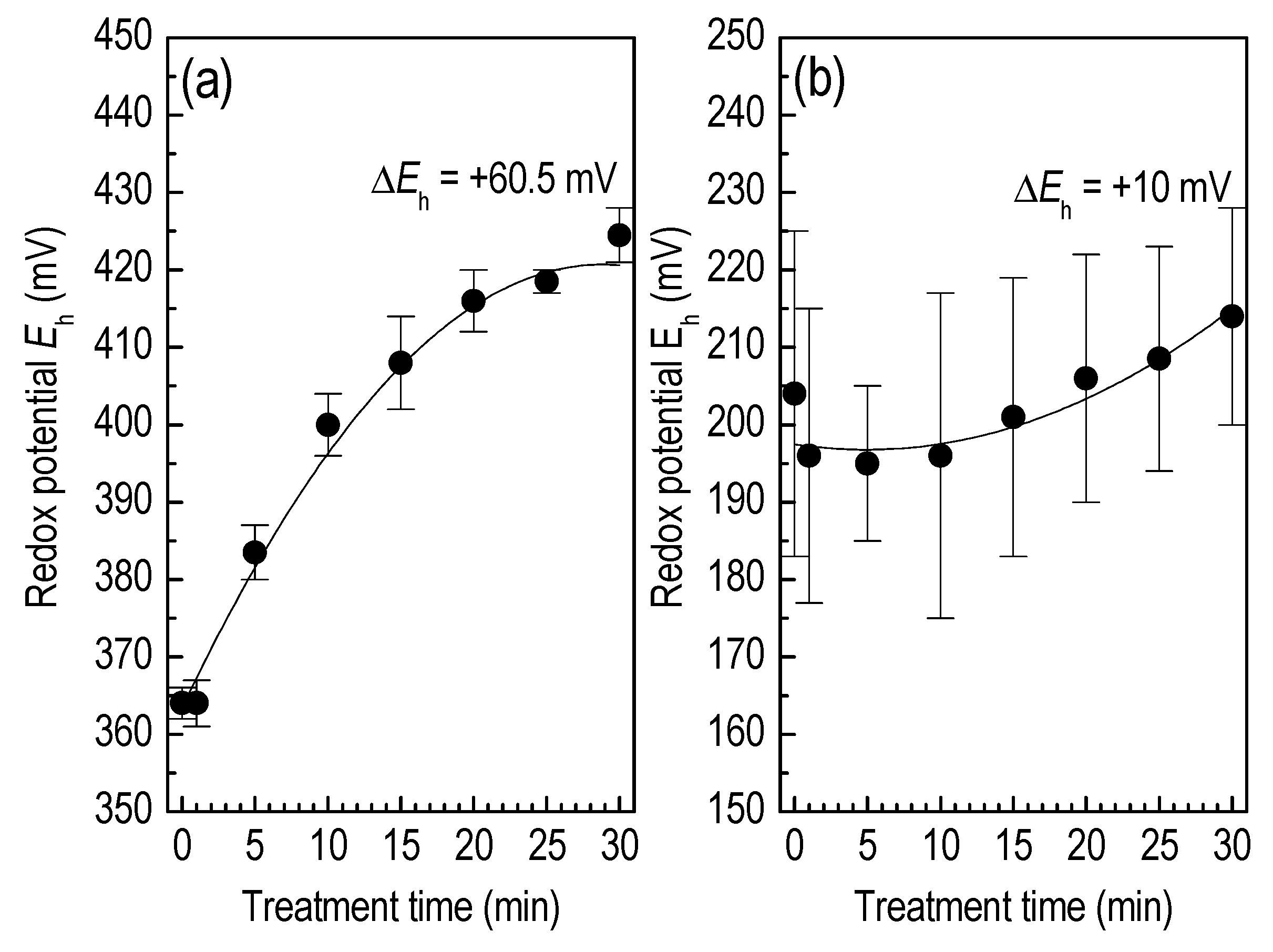
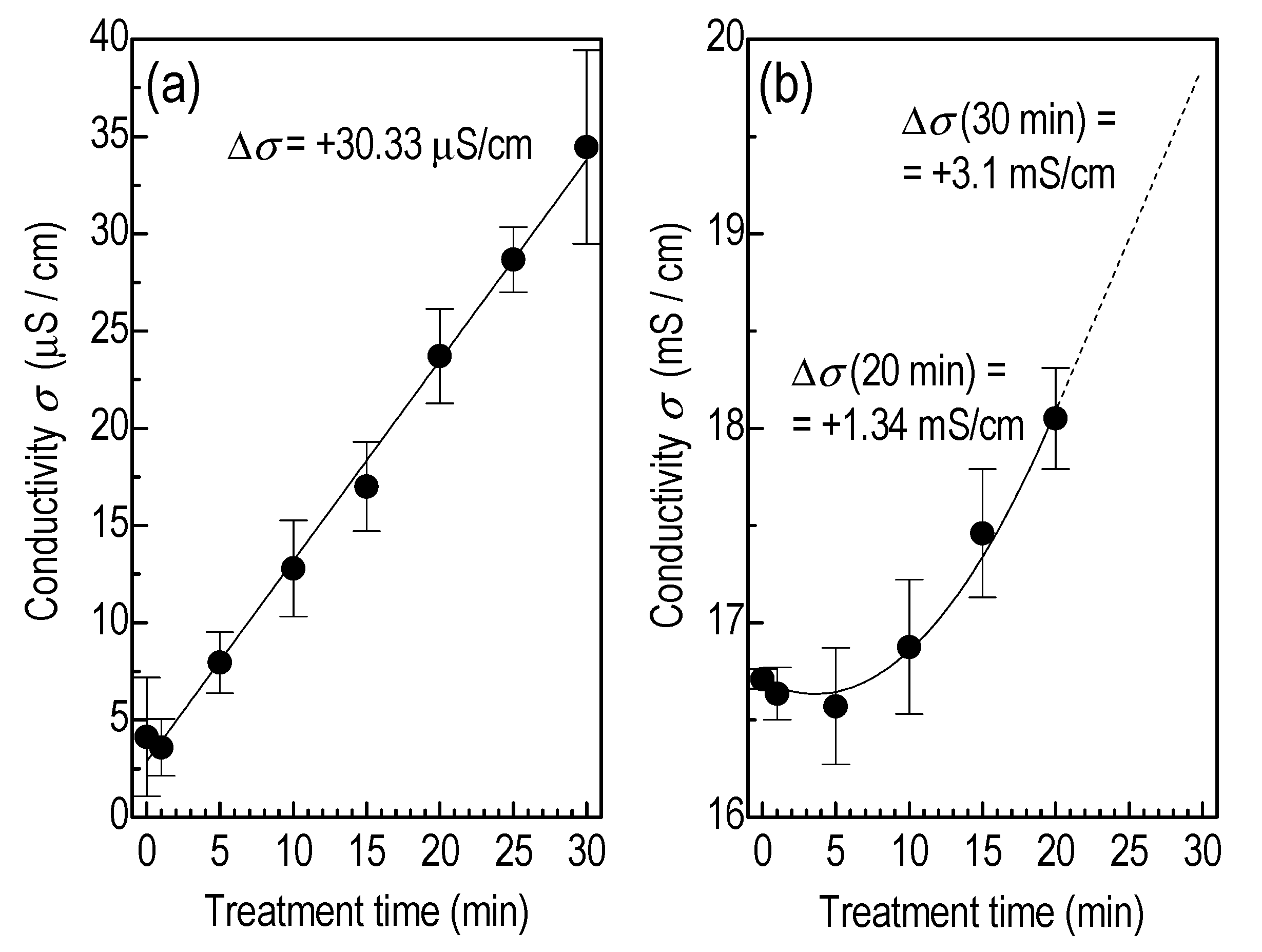
3.2. Liquid Treatment—Basic Characteristics
3.3. Liquid Treatment—ROS Detection
| H2O + hf | → | H2O+ + e− | (01) |
| H2O+ | → | •OH + H+ | (02) |
| UV + H2O | → | H2O• | (03) |
| UV + H2O• | → | H+ + OH− | (04) |
| OH− | → | •OH + e− | (05) |
| e− + O2 | → | (06) | |
| e− + O2 + H+ | → | (07) | |
| e− + H2O | → | •OH + •H + e− | (08) |
| e− + H2O | → | •OH + H+ + 2e− | (09) |
| e− + H2O | → | •H + •OH | (10) |
| •H + O2 | → | (11) | |
| + 2H+ | → | H2O2 + O2 | (12) |
| + H2O | → | O2 + H2O2 + OH− | (13) |
| → | H2O2 + O2 | (14) | |
| • | → | H2O + 1O2 | (15) |
| • | → | OH− + O2 | (16) |
| H2O2 + •H | → | •OH + H2O | (17) |
| •OH + H2O2 | → | + H2O | (18) |
| •OH + •OH | → | H2O2 | (19) |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Becker, K.H.; Kogelschatz, U.; Schoenbach, K.H.; Barker, R.J. Non-Equilibrium Air Plasmas at Atmospheric Pressure. Institute of Physics; Series in Plasma Physics; CRC Press: Bristol, UK, 2005. [Google Scholar]
- NATO Report. In Plasma Based Flow Control for Performance and Control of Military Vehicles. AC/323(AVT-190)TP/866; NATO: Neuilly-sur-Seine, France, 2021.
- Shafique, A.; Rangasamy, V.S.; Vanhulsel, A.; Safari, M.; Gross, S.; Adriaensens, P.; Van Bael, M.K.; Hardy, A.; Sallard, S. Dielectric barrier discharge (DBD) plasma coating of sulfur for mitigation of capacity fade in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 28072–28089. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Nie, X.; Jin, Z.; Huang, Z.; Wang, X.; Zhang, X. Dual dielectric barrier discharge plasma treatments for synthesis of Ag–TiO2 functionalized polypropylene fabrics. Ind. Eng. Chem. Res. 2019, 58, 7734–7741. [Google Scholar] [CrossRef]
- King, B.; Patel, D.; Chen, J.Z.; Drapanauskaite, D.; Handler, R.; Nozaki, T.; Baltrusaitis, J. Comprehensive process and environmental impact analysis of integrated DBD plasma steam methane reforming. Fuel 2021, 304, 121328. [Google Scholar] [CrossRef]
- Liu, Y.; Kolbakir, C.; Hu, H.; Hu, H. A comparison study on the thermal effects in DBD plasma actuation and electrical heating for aircraft icing mitigation. Int. J. Heat Mass Transf. 2018, 124, 319–330. [Google Scholar] [CrossRef]
- Kuchenbecker, M.; Bibinov, N.; Kaemlimg, A.; Wandke, D.; Awakowicz, P.; Viöl, W. Characterization of DBD plasma source for biomedical applications. J. Phys. D Appl. Phys. 2009, 42, 045212. [Google Scholar] [CrossRef]
- Yagyu, Y.; Hatayama, Y.; Hayashi, N.; Mishima, T.; Nishioka, T.; Sakudo, A.; Ihara, T.; Ohshima, T.; Kawasaki, H.; Suda, Y. Direct plasma disinfection of green mold spore on citrus by atmospheric pressure dielectric barrier discharge for agricultural applications. Trans. Mater. Res. Soc. 2016, 41, 127–130. [Google Scholar] [CrossRef]
- Roy, S.; Choudhury, B.; Johnson, J.; Schindler-Tyka, A. Application of dielectric barrier discharge for improving food shelf life and reducing spoilage. Sci. Rep. 2021, 11, 19200. [Google Scholar] [CrossRef]
- Rumbach, P.; Clarke, J.P.; Go, D.B. Electrostatic Debye layer formed at a plasma-liquid interface. Phys. Rev. E 2017, 95, 053203. [Google Scholar] [CrossRef]
- Akishev, Y.; Arefi-Khonsari, F.; Demir, A.; Grushin, M.; Karalnik, V.; Petryakov, A.; Trushkin, N. The interaction of positive streamers with bubbles floating on a liquid surface. Plasma Sources Sci. Technol. 2015, 24, 065021. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Yue, Y.; Santosh, V.S.; Kondeti, K.; Sadeghi, N.; Bruggeman, P.J. Plasma dynamics, instabilities and OH generation in a pulsed atmospheric pressure plasma with liquid cathode: A diagnostic study. Plasma Sources Sci. Technol. 2022, 31, 025008. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2019, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of plasma-liquid systems: A review. Materials 2019, 2, 2751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Patange, A.; Sun, D.W.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Jiang, H.; Feng, J. Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. J. Saudi Chem. Soc. 2017, 21, 545–557. [Google Scholar] [CrossRef]
- Bai, N.; Sun, P.; Zhou, H.; Wu, H.; Wang, R.; Liu, F.; Zhu, W.; Lopez, J.L.; Zhang, J.; Fang, J. Inactivation of Staphylococcus aureus in water by a cold, He/O2 atmospheric pressure plasma microjet. Plasma Process. Polym. 2011, 8, 424–431. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; Chiappim, W.; da Graça Sampaio, A.; da Cruz Vegian, M.R.; Pessoa, R.S.; Koga-Ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.S.; Islam, R.; Rana, R.; Sayem, A.S.M.; As Sad, M.A.; Matin, A.; Raposo, A.; Zandonadi, R.P.; Han, H.; et al. Plasma-activated water for food safety and quality: A review of recent developments. Int. J. Environ. Res. Public Health 2022, 19, 6630. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lee, G.J.; Choi, E.H. Influences of plasma plume length on structural, optical and dye degradation properties of citrate-stabilized silver nanoparticles synthesized by plasma-assisted reduction. Nanomaterials 2022, 12, 2367. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Vanraes, P.; Bogaerts, A. Plasma physics of liquids—A focused review. Appl. Phys. Rev. 2018, 5, 031103. [Google Scholar] [CrossRef]
- Svarnas, P.; Spiliopoulou, A.; Koutsoukos, P.G.; Gazeli, K.; Anastassiou, E.D. Acinetobacter baumannii deactivation by means of DBD-based helium plasma jet. Plasma 2019, 2, 77–90. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisaris, S.G. Synergistic effect of cold atmospheric pressure plasma and free or liposomal doxorubicin on melanoma cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef]
- Athanasopoulos, D.; Svarnas, P.; Ladas, S.; Kennou, S.; Koutsoukos, P. On the wetting properties of human stratum corneum epidermidis surface exposed to cold atmospheric-pressure pulsed plasma. Appl. Phys. Lett. 2018, 112, 213703. [Google Scholar] [CrossRef]
- Svarnas, P.; Matrali, S.H.; Gazeli, K. Aleiferis, S.; Clément, F.; Antimisiaris, S.G. Atmospheric-pressure guided streamers for liposomal membrane disruption. Appl. Phys. Lett. 2012, 101, 264103. [Google Scholar] [CrossRef]
- Gazeli, K.; Svarnas, P.; Vafeas, P.; Papadopoulos, P.K.; Gkelios, A.; Clément, F. Investigation on streamers propagating into a helium jet in air at atmospheric pressure: Εlectrical and optical emission analysis. J. Appl. Phys. 2013, 114, 103304. [Google Scholar] [CrossRef]
- Cardoso, R.P.; Belmonte, T.; Keravec, P.; Kosior, F.; Henrion, G. Influence of impurities on the temperature of an atmospheric helium plasma in microwave resonant cavity. J. Phys. D Appl. Phys. 2007, 40, 1394–1400. [Google Scholar] [CrossRef]
- Roland, U.; Renschen, C.P.; Lippik, D.; Stallmach, F.; Holzer, F. A new fiber optical thermometer and its application for process control in strong electric, magnetic, and electromagnetic fields. Sens. Lett. 2003, 1, 93–98. [Google Scholar] [CrossRef]
- Papadopoulos, P.K.; Athanasopoulos, D.; Sklias, K.; Svarnas, P.; Mourousias, N.; Vratsinis, K.; Vafeas, P. Generic residual charge based model for the interpretation of the electrohydrodynamic effects in cold atmospheric pressure plasmas. Plasma Sources Sci. Technol. 2019, 28, 065005. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Papapostolou, I.; Grintzalis, K. Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils. Nat. Prot. 2008, 3, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Linxiang, L.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chromatogr. 2004, 18, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Skipitari, M.; Kalaitzopoulou, E.; Papadea, P.; Varemmenou, A.; Gavriil, V.E.; Sarantopoulou, E.; Cefalas, A.-C.; Tsakas, S.; Rosmaraki, E.; Margiolaki, I.; et al. Titanium dioxide nanoparticle-based hydroxyl and superoxide radical production for oxidative stress biological simulations. J. Photochem. Photobiol. A Chem. 2022, 435, 114290. [Google Scholar] [CrossRef]
- Grintzalis, K.; Zisimopoulos, D.; Grune, T.; Weber, D.; Georgiou, C.D. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic. Biol. Med. 2013, 59, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zisimopoulos, D.N.; Kalaitzopoulou, E.; Skipitari, M.; Papadea, P.; Panagopoulos, N.T.; Salahas, G.; Georgiou, C.D. Detection of superoxide radical in all biological systems by Thin Layer Chromatography. Arch. Biochem. Biophys. 2022, 716, 109110. [Google Scholar] [CrossRef]
- Gazeli, K.; Svarnas, P.; Held, B.; Marlin, L.; Clément, F. Possibility of controlling the chemical pattern of He and Ar “guided streamers” by means of N2 or O2 additives. J. Appl. Phys. 2015, 117, 093302. [Google Scholar] [CrossRef]
- NIST, Atomic Spectra Database Lines Form (Online). Available online: https://physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 20 November 2022).
- Zhao, G.B.; Argyle, M.D.; Radosz, M. Optical emission study of nonthermal plasma confirms reaction mechanisms involving neutral rather than charged species. J. Appl. Phys. 2007, 101, 033303. [Google Scholar] [CrossRef]
- Xiong, Q.; Nikiforov, A.Y.; Lu, X.P.; Leys, C. High-speed dispersed photographing of an open-air argon plasma plume by a grating–ICCD camera system. J. Phys. D Appl. Phys. 2010, 43, 415201. [Google Scholar] [CrossRef]
- Gazeli, K.; Svarnas, P.; Lazarou, C.; Anastassiou, C.; Georghiou, G.E.; Papadopoulos, P.K.; Clément, F. Physical interpretation of a pulsed atmospheric pressure plasma jet following parametric study of the UV–to–NIR emission. Phys. Plasmas 2020, 27, 123503. [Google Scholar] [CrossRef]
- Papadopoulos, P.K.; Vafeas, P.; Svarnas, P.; Gazeli, K.; Hatzikonstantinou, P.M.; Gkelios, A.; Clément, F. Interpretation of the gas flow field modification induced by guided streamer (“plasma bullet”) propagation. J. Phys. D Appl. Phys. 2014, 47, 425203. [Google Scholar] [CrossRef]
- Logothetis, D.K.; Papadopoulos, P.K.; Svarnas, P.; Vafeas, P. Numerical simulation of the interaction between helium jet flow and an atmospheric-pressure “plasma-jet”. Comput. Fluids 2016, 140, 11–18. [Google Scholar] [CrossRef]
- Slikboer, E.; Walsh, J.L. Characterization of a kHz sinusoidal Argon plasma jet impinging on water using Thomson scattering and fast imaging. J. Phys. D Appl. Phys. 2021, 54, 325201. [Google Scholar] [CrossRef]
- Svarnas, P.; Papadopoulos, P.K.; Athanasopoulos, D.; Sklias, K.; Gazeli, K.; Vafeas, P. Parametric study of thermal effects in a capillary dielectric-barrier discharge related to plasma jet production: Experiments and numerical modelling. J. Appl. Phys. 2018, 124, 064902. [Google Scholar] [CrossRef]
- Babbs, C.F.; Steiner, M.G. Detection and quantitation of hydroxyl radical using dimethyl sulfoxide as molecular probe. Meth. Enzymol. 1990, 186, 137–147. [Google Scholar]
- Klein, M.S.; Cohen, G.; Cederbaum, I.A. The interaction of hydroxyl radicals with dimethylsulfoxide. FEBS Lett. 1980, 116, 220–222. [Google Scholar] [CrossRef]
- Papapostolou, I.; Patsoukis, N.; Georgiou, C.D. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme-proteins. Anal. Biochem. 2004, 332, 290–298. [Google Scholar] [CrossRef]
- Patsoukis, N.; Papapostolou, I.; Georgiou, C.D. Interference of non-specific peroxidases in the fluorescence detection of superoxide radical by hydroethidine oxidation: A new assay for H2O2. Anal. Bioanal. Chem. 2005, 381, 1065–1072. [Google Scholar] [CrossRef]
- Zielonka, J.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nat. Prot. 2008, 3, 8–21. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.; Park, D.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.-N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Verlackt, C.C.W.; Van Boxem, W.; Bogaerts, A. Transport and accumulation of plasma generated species in aqueous solution. Phys. Chem. Chem. Phys. 2018, 20, 6845–6859. [Google Scholar] [CrossRef]
- Heirman, P.; Van Boxem, W.; Bogaerts, A. Reactivity and stability of plasma-generated oxygen and nitrogen species in buffered water solution: A computational study. Phys. Chem. Chem. Phys. 2019, 21, 12881–12894. [Google Scholar] [CrossRef]
- Casado, E.; Garcia, M.C.; Krawczyk, D.A.; Romero-Salguero, F.-J.; Rodero, A. Study of the plasma–liquid interaction for an argon nonthermal microwave plasma jet from the analysis of benzene degradation. Plasma Process. Polym. 2020, 17, 2000030. [Google Scholar] [CrossRef]
- Oinuma, G.; Nayak, G.; Du, Y.; Bruggeman, P.J. Controlled plasma–droplet interactions: A quantitative study of OH transfer in plasma–liquid interaction. Plasma Sources Sci. Technol. 2020, 29, 14. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J. Appl. Phys. 2021, 129, 084901. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/ radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Richmonds, C.; Witzke, M.; Bartling, B.; Lee, S.W.; Wainright, J.; Liu, C.C.; Sankaran, R.M. Electron-transfer reactions at the plasma-liquid interface. J. Am. Chem. Soc. 2011, 133, 17582–17585. [Google Scholar] [CrossRef]
- Witzke, M.; Rumbach, P.; Go, D.B.; Sankaran, R.M. Evidence for the electrolysis of water by atmospheric-pressure plasmas formed at the surface of aqueous solutions. J. Phys. D Appl. Phys. 2012, 45, 442001. [Google Scholar] [CrossRef]
- Girard, F.; Badets, V.; Blanc, S.; Gazeli, K.; Marlin, L.; Authier, L.; Svarnas, P.; Sojic, N.; Clément, F.; Arbault, S. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv. 2016, 6, 78457–78467. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of short-lived reactive species in plasma-air-water systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, H.; Schmidt-Bleker, A.; Weltmann, K.-D.; von Woedtkeb, T.; Wende, K. Non-touching plasma–liquid interaction—Where is aqueous nitric oxide generated? Phys. Chem. Chem. Phys. 2018, 20, 25387–25398. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jeon, H.; Yi, C.; Park, S.; Ryu, S.; Kim, S.B. Mutual interaction between plasma characteristics and liquid properties in AC-driven pin-to-liquid discharge. Sci. Rep. 2018, 8, 12037. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, X.; Chen, Q.; Xiong, Q.; Li, J.; Xin, W.; Guolong, C.; Liu, Q.H.; Ostrikov, K. The formation mechanism of aqueous hydrogen peroxide in a plasma-liquid system with liquid as the anode. Eur. Phys. J. D 2020, 74, 80. [Google Scholar] [CrossRef]
- Ng, S.W.; Slikboer, E.; Dickenson, A.; Walsh, J.L.; Lu, P.; Boehm, D.; Bourke, P. Characterization of an atmospheric pressure air plasma device under different modes of operation and their impact on the liquid chemistry. J. Appl. Phys. 2021, 129, 123303. [Google Scholar] [CrossRef]
- Volkov, A.G.; Bookal, A.; Hairston, J.S.; Roberts, J.; Taengwa, G.; Patel, D. Mechanisms of multielectron reactions at the plasma/water interface: Interfacial catalysis, RONS, nitrogen fixation, and plasma activated water. Electrochim. Acta 2021, 385, 138441. [Google Scholar] [CrossRef]
- Wartel, M.; Faubert, F.; Dirlau, I.D.; Rudz, S.; Pellerin, N.; Astanei, D.; Burlica, R.; Hnatiuc, B.; Pellerin, S. Analysis of plasma activated water by gliding arc at atmospheric pressure: Effect of the chemical composition of water on the activation. J. Appl. Phys. 2021, 129, 233301. [Google Scholar] [CrossRef]
- Lamichhane, P.; Acharya, T.R.; Kaushik, N.; Nguyen, L.N.; Lim, J.S.; Hessel, V.; Kaushik, N.K.; Choi, E.H. Non-thermal argon plasma jets of various lengths for selective reactive oxygen and nitrogen species production. J. Environ. Chem. Eng. 2022, 10, 107782. [Google Scholar] [CrossRef]

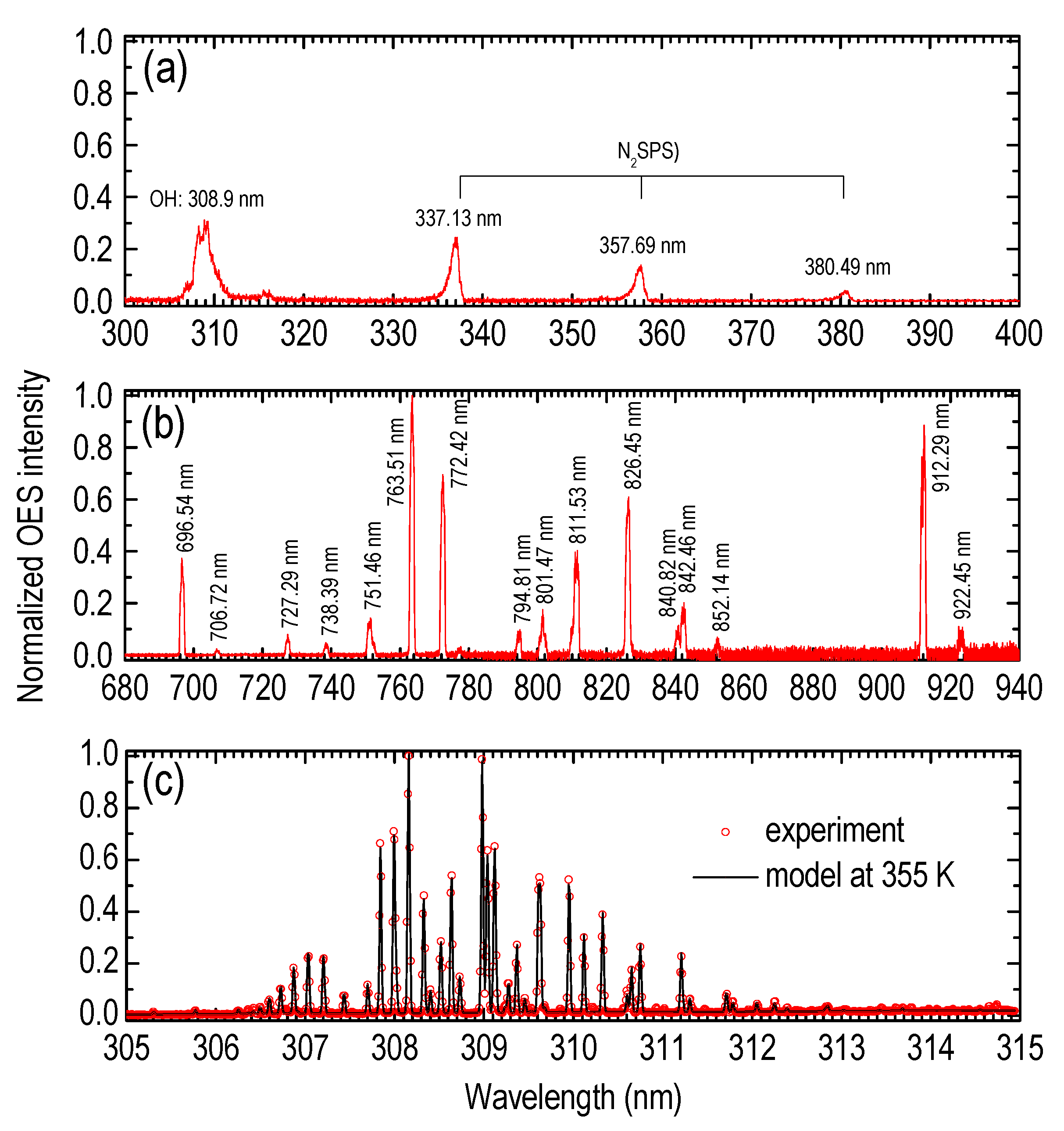
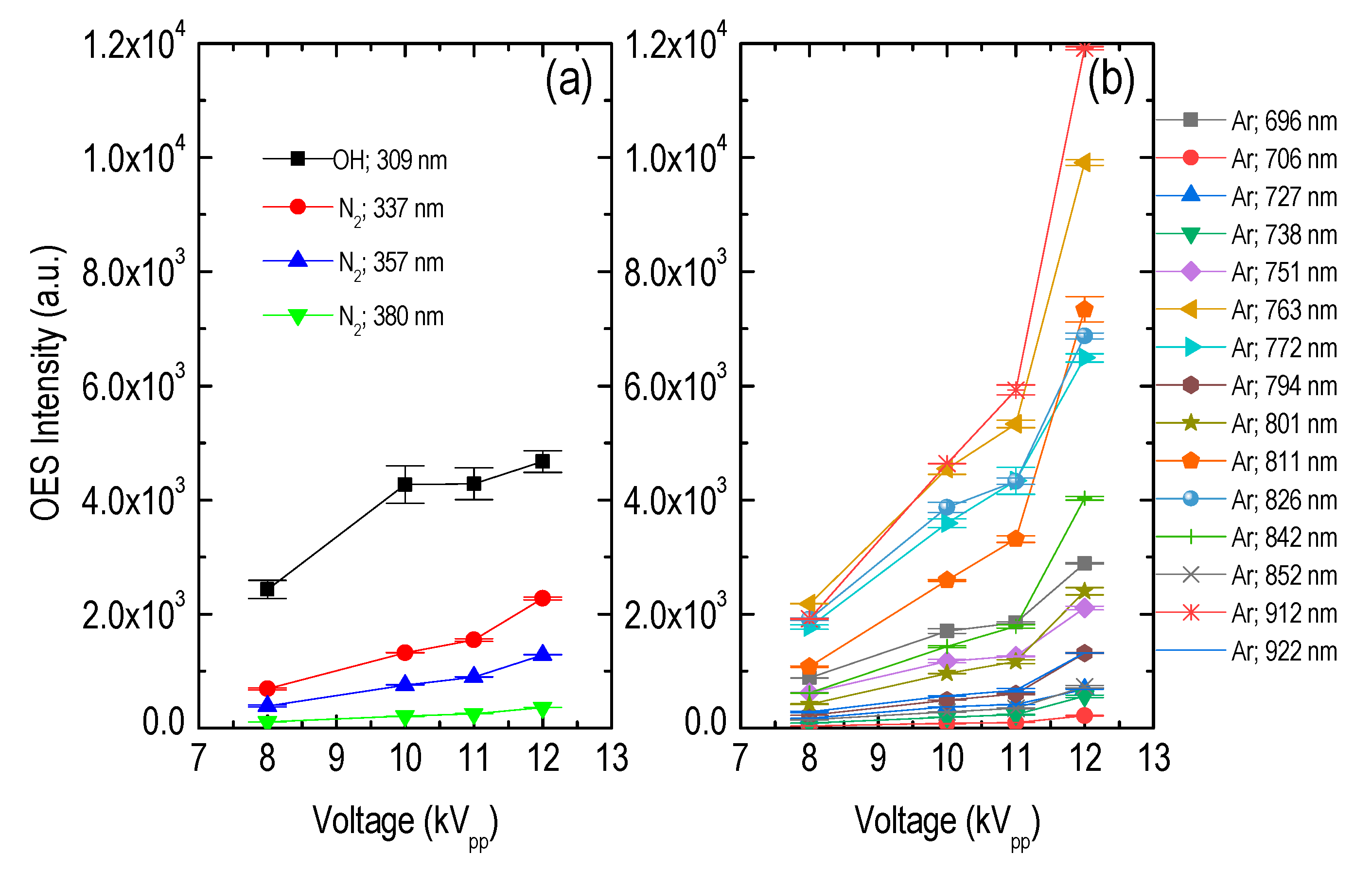

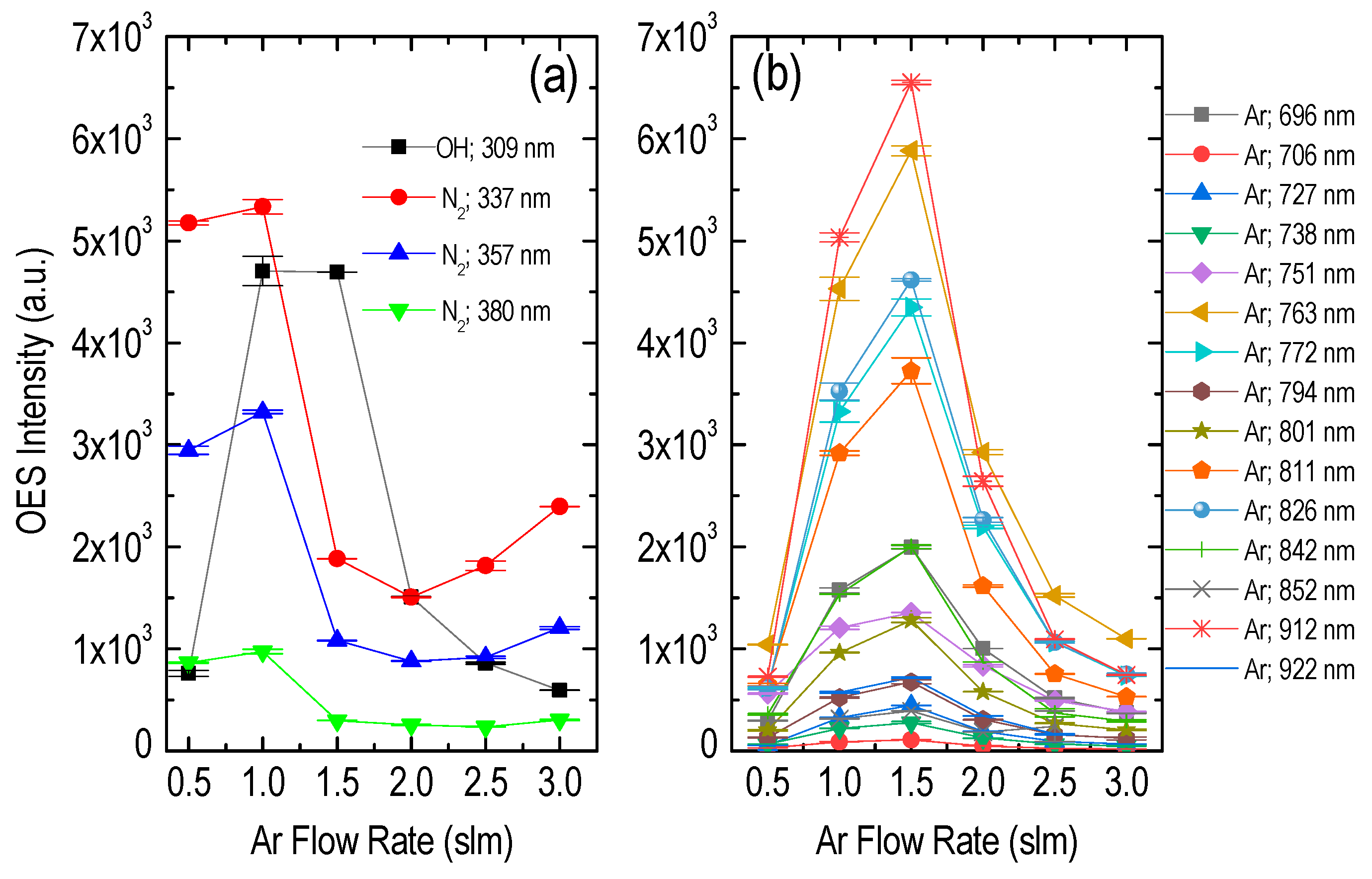
| Species | λ (nm) | Vibrational Transition [38] v′–v″ (Δv) | Lower Level El (eV) | Upper Level Eu (eV) | Radiative Lifetime τ (ns) |
|---|---|---|---|---|---|
| X2Π | 309 [38] | 0–0(0) | - | 4 [38] | 690 [38] |
| B3Πg | 337.13 [38] | 0–0(0) | - | 36.6–42 ± 2 [38] | |
| B3Πg | 357.69 [38] | 0–1(−1) | - | - | |
| B3Πg | 380.49 [38] | 0–2(−2) | - | - | |
| Ar I: (2P01/2)4p → (2P03/2)4s | 696.54 [39] | - | 11.54 [39] | 13.32 [39] | - |
| Ar I: (2P01/2)4p → (2P03/2)4s | 706.72 [39] | - | 11.54 [39] | 13.30 [39] | 29 [38,40] |
| Ar I: (2P01/2)4p → (2P03/2)4s | 727.29 [39] | - | 11.62 [39] | 13.32 [39] | 28.3 [38,40] |
| Ar I: (2P01/2)4p → (2P03/2)4s | 738.39 [39] | - | 11.62 [39] | 13.30 [39] | 29 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 751.46 [39] | - | 11.62 [39] | 13.27 [39] | 24.4 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 763.51 [39] | - | 11.54 [39] | 13.17 [39] | 29.4 [38,40] |
| Ar I: (2P01/2)4p → (2P01/2)4s | 772.42 [39] | - | 11.72 [39] | 13.32 [39] | 28.3 [38,40] |
| Ar I: (2P01/2)4p → (2P01/2)4s | 794.81 [39] | - | 11.72 [39] | 13.28 [39] | 29.3 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 801.47 [39] | - | 11.54 [39] | 13.09 [39] | 30.6 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 811.53 [39] | - | 11.54 [39] | 13.07 [39] | 30.7 [38,40] |
| Ar I: (2P01/2)4p → (2P01/2)4s | 826.45 [39] | - | 11.82 [39] | 13.32 [39] | 28.3 [38,40] |
| Ar I: (2P01/2)4p → (2P01/2)4s | 840.82 [39] | - | 11.82 [39] | 13.30 [39] | 29 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 842.46 [39] | - | 11.62 [39] | 13.09 [39] | 30.6 [38,40] |
| Ar I: (2P01/2)4p → (2P01/2)4s | 852.14 [39] | - | 11.82 [39] | 13.28 [39] | 29.3 [38,40] |
| Ar I: (2P03/2)4p → (2P03/2)4s | 912.29 [39,40] | - | 11.54 [39,40] | 12.90 [39,40] | - |
| Ar I: (2P03/2)4p → (2P01/2)4s | 922.45 [39,40] | - | 11.82 [39,40] | 13.17 [39,40] | 29.4 [40] |
| •OH (2-OH-TPA) (nM) | H2O2 (μM) | ΔpH | |
|---|---|---|---|
| Plasma-exposed: TPA in DDW | 565 (565) ± 27 | 359 ± 17 | 2 ± 0.02 |
| Plasma-exposed: TPA + DMSO (•OH scavenger) in DDW | 0 (0) | 336 ± 15 | 2 ± 0.01 |
| Plasma-non-exposed: TPA in DDW | 0 (0) | 0 (0) | 0 ± 0.02 |
| Plasma-exposed: TPA in PBS | 481 (481) ± 23 | 255 ± 11 | 0 ± 0.02 |
| Plasma-exposed: TPA + DMSO (•OH scavenger) in PBS | 0 (0) | 263 ± 13 | 0 ± 0.02 |
| Plasma-non-exposed: TPA in PBS | 0 (0) | 0 (0) | 0 ± 0.01 |
| ΔpH | ||
|---|---|---|
| Plasma-exposed: HE in DDW | 873 ± 40 (1745 ± 80) | 3 ± 0.02 |
| Plasma-non-exposed: HE in DDW | 0 (0) | 0 ± 0.02 |
| Plasma-exposed: HE in PBS | 80 ± 4 (160 ± 8) | 0 ± 0.02 |
| Plasma-non-exposed: HE in PBS | 0 (0) | 0 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svarnas, P.; Poupouzas, M.; Papalexopoulou, K.; Kalaitzopoulou, E.; Skipitari, M.; Papadea, P.; Varemmenou, A.; Giannakopoulos, E.; Georgiou, C.D.; Georga, S.; et al. Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties. Appl. Sci. 2022, 12, 11950. https://doi.org/10.3390/app122311950
Svarnas P, Poupouzas M, Papalexopoulou K, Kalaitzopoulou E, Skipitari M, Papadea P, Varemmenou A, Giannakopoulos E, Georgiou CD, Georga S, et al. Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties. Applied Sciences. 2022; 12(23):11950. https://doi.org/10.3390/app122311950
Chicago/Turabian StyleSvarnas, Panagiotis, Michael Poupouzas, Konstantia Papalexopoulou, Electra Kalaitzopoulou, Marianna Skipitari, Polyxeni Papadea, Athina Varemmenou, Evangelos Giannakopoulos, Christos D. Georgiou, Stavroula Georga, and et al. 2022. "Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties" Applied Sciences 12, no. 23: 11950. https://doi.org/10.3390/app122311950
APA StyleSvarnas, P., Poupouzas, M., Papalexopoulou, K., Kalaitzopoulou, E., Skipitari, M., Papadea, P., Varemmenou, A., Giannakopoulos, E., Georgiou, C. D., Georga, S., & Krontiras, C. (2022). Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties. Applied Sciences, 12(23), 11950. https://doi.org/10.3390/app122311950








