Effect of a Conical Cellulose Structure on Horseradish Peroxidase Biomacromolecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Protein
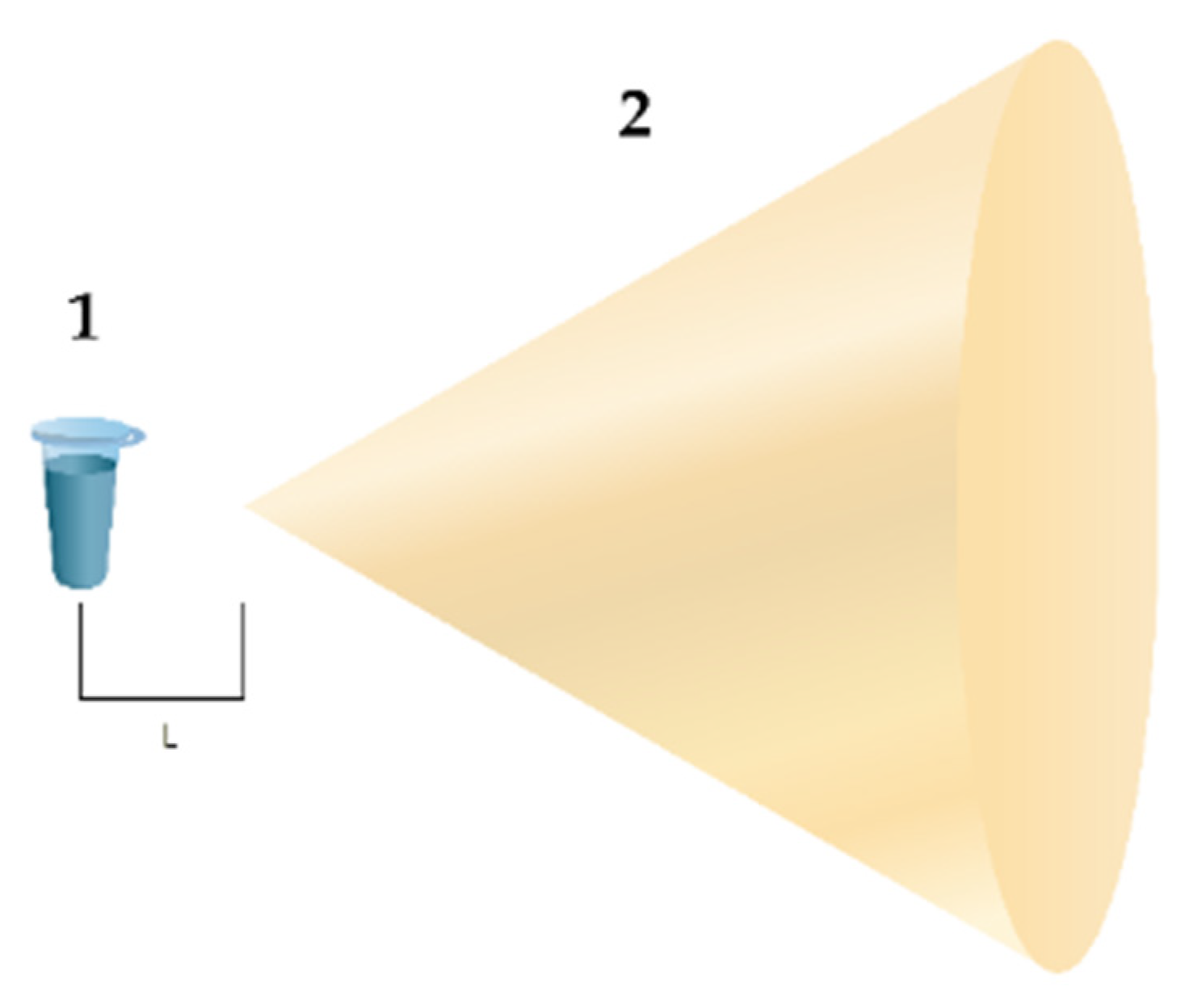
2.2. Experimental Setup
2.3. Atomic Force Microscopy
2.4. Spectrophotometry
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diab, K.A. The Impact of the Low Frequency of the Electromagnetic Field on Human. Adv. Exp. Med. Biol. Cell Biol. Trans. Med. 2019, 7, 135–149. [Google Scholar] [CrossRef]
- Warille, A.A.; Altun, G.; Elamin, A.A.; Kaplan, A.A.; Mohamed, H.; Yurt, K.K.; Elhaj, A.E. Skeptical approaches concerning the effect of exposure to electromagnetic fields on brain hormones and enzyme activities. J. Microsc. Ultrastruct. 2017, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Sun, J.; Sun, F.; Xu, B.; Gu, N. The quasi-one-dimensional assembly of horseradish peroxidase molecules in presence of the alternating magnetic field. Coll. Surf. A Physicochem. Eng. Aspects 2010, 360, 94–98. [Google Scholar] [CrossRef]
- Lopes, L.C.; Barreto, M.T.; Gonçalves, K.M.; Alvarez, H.M.; Heredia, M.F.; de Souza, R.O.M.; Cordeiro, Y.; Dariva, C.; Fricks, A.T. Stability and structural changes of horseradish peroxidase: Microwave versus conventional heating treatment. Enzym. Microb. Technol. 2015, 69, 10–18. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2023, 398, 133875. [Google Scholar] [CrossRef]
- Fortune, J.A.; Wu, B.-I.; Klibanov, A.M. Radio Frequency Radiation Causes No Nonthermal Damage in Enzymes and Living Cells. Biotechnol. Prog. 2010, 26, 1772–1776. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Balezin, M.; Baryshnikova, K.V.; Kapitanova, P.; Evlyukhin, A.B. Electromagnetic properties of the Great Pyramid: First multipole resonances and energy concentration. J. Appl. Phys. 2018, 124, 034903. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Ershova, M.O.; Tatur, V.Y.; Stepanov, I.N.; Repnikov, V.V.; et al. AFM study of changes in properties of horseradish peroxidase after incubation of its solution near a pyramidal structure. Sci. Rep. 2021, 11, 9907. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Repnikov, V.V.; et al. Effect of Spherical Elements of Biosensors and Bioreactors on the Physicochemical Properties of a Peroxidase Protein. Polymers 2021, 13, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; et al. The Effect of Incubation near an Inversely Oriented Square Pyramidal Structure on Adsorption Properties of Horseradish Peroxidase. Appl. Sci. 2022, 12, 4042. [Google Scholar] [CrossRef]
- Nakonechny, V.S.; Prisyazhny, A.E.; Poberezhny, A.A. Electrodynamic modeling using microwave anechoic chambers. Methodology for the anechoic factor estimation. Syst. Inf. Proc. 2005, 9, 116–123. [Google Scholar]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.I.; Lieber, C.M. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004, 4, 51–54. [Google Scholar] [CrossRef]
- Arjmand, M.; Saghafifar, H.; Alijanianzadeh, M.; Soltanolkotabi, M. A sensitive tapered-fiber optic biosensor for the label-free detection of organophosphate pesticides. Sens. Actuator B Chem. 2017, 249, 523–532. [Google Scholar] [CrossRef]
- Zitzmann, F.D.; Schmidt, S.; Naumann, M.; Belder, D.; Jahnke, H.-G.; Robitzki, A.A. Multielectrode biosensor chip for spatial resolution screening of 3D cell models based on microcavity arrays. Biosens. Bioelectr. 2022, 202, 114010. [Google Scholar] [CrossRef]
- Laskowski, D.; Strzelecki, J.; Pawlak, K.; Dahm, H.; Balter, A. Effect of ampicillin on adhesive properties of bacteria examined by atomic force microscopy. Micron 2018, 112, 84–90. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Malsagova, K.A.; Kaysheva, A.L.; Kopylov, A.T.; Tatur, V.Y.; Ziborov, V.S.; Ivanov, Y.D. Highly sensitive protein detection by biospecific AFM-based fishing with pulsed electrical stimulation. FEBS Open Bio. 2017, 7, 1186–1195. [Google Scholar] [CrossRef]
- Fraiwan, A.; Choi, S. Bacteria-powered battery on paper. Phys. Chem. 2014, 16, 26288–26293. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Zhang, M.; Jiang, S.; Farooq, A.; Li, L.; Liu, L.; Ge, A.; Liu, L. Recent Progress in the Application of Cellulose in Electromagnetic Interference Shielding Materials. Macromol. Mater. Eng. 2022, 307, 2100899. [Google Scholar] [CrossRef]
- Bordel, D.; Putaux, J.-L.; Heux, L. Orientation of Native Cellulose in an Electric Field. Langmuir 2006, 22, 4899–4901. [Google Scholar] [CrossRef]
- Gindl, W.; Emsenhuber, G.; Maier, G.; Keckes, J. Cellulose in Never-Dried Gel Oriented by an AC electric field. Biomacromolecules 2009, 10, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Pipliya, S.; Kumar, S.; Srivastav, P.P. Inactivation kinetics of polyphenol oxidase and peroxidase in pineapple juice by dielectric barrier discharge plasma technology. Innov. Food Sci. Emerg. Technol. 2022, 80, 103081. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Y.; Cui, B.; Fu, H.; Chen, X.; Wang, Y. Radio frequency energy inactivates peroxidase in stem lettuce at different heating rates and associate changes in physiochemical properties and cell morphology. Food Chem. 2021, 342, 128360. [Google Scholar] [CrossRef]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49, 69–86. [Google Scholar] [CrossRef]
- Yan, Q.; Tang, X.; Zhang, B.; Wang, C.; Deng, S.; Ma, X.; Wang, C.; Li, D.; Huang, S.; Dong, P. Biocatalytic oxidation of flavone analogues mediated by general biocatalysts: Horseradish peroxidase and laccase. RSC Adv. 2019, 9, 13325–13331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huddy, S.M.; Hitzeroth, I.I.; Meyers, A.E.; Weber, B.; Rybicki, E.P. Transient Expression and Purification of Horseradish Peroxidase C in Nicotiana benthamiana. Int. J. Mol. Sci. 2018, 19, 115. [Google Scholar] [CrossRef]
- Welinder, K.G. Amino Acid Sequence Studies of Horseradish Peroxidase. Amino and Carboxyl Termini, Cyanogen Bromide and Tryptic Fragments, the Complete Sequence, and Some Structural Characteristics of Horseradish Peroxidase C. JBIC J. Biol. Inorg. Chem. 1979, 96, 483–502. [Google Scholar] [CrossRef]
- Tams, J.W.; Welinder, K.G. Mild chemical deglycosylation of horseradish peroxidase yields a fully active, homogeneous enzyme. Anal. Biochem. 1995, 228, 48–55. [Google Scholar] [CrossRef]
- Gajhede, M.; Schuller, D.J.; Henriksen, A.; Smith, A.T.; Poulos, T.L. Crystal structure of horseradish peroxidase C at 2.15 Å resolution. Nat. Struct. Mol. Biol. 1997, 4, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ma, Y.; Li, Y.; Xiang, Q. Effect of dielectric barrier discharge (DBD) plasma on the activity and structural changes of horseradish peroxidase. Qual. Assur. Saf. Crops Foods 2021, 13, 92–101. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Li, J.; Zhang, Y.; Guo, Y.; Cheng, J.-H. Effects of dielectric barrier discharge cold plasma on the activity, structure and conformation of horseradish peroxidase (HRP) and on the activity of litchi peroxidase (POD). LWT Food Sci. Technol. 2021, 141, 111078. [Google Scholar] [CrossRef]
- Han, Y.-X.; Cheng, J.-H.; Sun, D.-W. Changes in activity, structure and morphology of horseradish peroxidase induced by cold plasma. Food Chem. 2019, 301, 125240. [Google Scholar] [CrossRef]
- Kiselyova, O.I.; Yaminsky, I.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Ornelas-González, A.; Rito-Palomares, M.; González-González, M. TMB vs ABTS: Comparison of multi-enzyme based approaches for the colorimetric quantification of salivary glucose. J. Chem. Technol. Biotechnol. 2022, 97, 2720–2727. [Google Scholar] [CrossRef]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7) 2,20-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) as a Substrate Sigma Prod. No. P-6782. Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzymeactivity-assays/enzymatic-assay-of-peroxidase-abts-as-substrate (accessed on 18 February 2022).
- Volume of a Pyramid or Cone. Available online: https://www.khanacademy.org/math/geometry/hs-geo-solids/xff63fac4:hs-geo-cavalieri-s-principle/a/volume-of-a-pyramid-or-cone (accessed on 17 November 2022).
- Uniform Solid Tetrahedron, Pyramid and Cone. Available online: https://phys.libretexts.org/Bookshelves/Classical_Mechanics/Classical_Mechanics_(Tatum)/01%3A_Centers_of_Mass/1.07%3A_Uniform_Solid_Tetrahedron%2C_Pyramid_and_Cone (accessed on 18 February 2022).
- Bunkin, A.F.; Nurmatov, A.A.; Pershin, S.M. Coherent four-photon spectroscopy of low-frequency molecular librations in a liquid. Phys. Uspekhi 2006, 49, 855–861. [Google Scholar] [CrossRef]
- Pershin, S.M. A New Conception of the Action of EMF on Water/Aqueous Solutions, Taking into Account the Quantum Differences of the Ortho/Para of Spin Isomers of H2O. Online Biophysical Blog. Available online: http://www.biophys.ru/archive/sarov2013/proc-p17.pdf (accessed on 3 October 2022).
- Pershin, S.M.; Bunkin, A.F.; Golo, V.L. H2O monomers in channels of icelike water structures. J. Exp. Theor. Phys. 2012, 115, 1008–1011. [Google Scholar] [CrossRef]
- Artmann, G.M.; Kelemen, C.; Porst, D.; Büldt, G.; Chien, S. Temperature Transitions of Protein Properties in Human Red Blood Cells. Biophys. J. 1998, 75, 3179–3183. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Evdokimov, A.N.; Tatur, V.Y.; et al. The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Appl. Sci. 2021, 11, 11677. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Tatur, V.Y.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; et al. Effect of a Conical Cellulose Structure on Horseradish Peroxidase Biomacromolecules. Appl. Sci. 2022, 12, 11994. https://doi.org/10.3390/app122311994
Ivanov YD, Tatur VY, Shumov ID, Kozlov AF, Valueva AA, Ivanova IA, Ershova MO, Ivanova ND, Stepanov IN, Lukyanitsa AA, et al. Effect of a Conical Cellulose Structure on Horseradish Peroxidase Biomacromolecules. Applied Sciences. 2022; 12(23):11994. https://doi.org/10.3390/app122311994
Chicago/Turabian StyleIvanov, Yuri D., Vadim Y. Tatur, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Nina D. Ivanova, Igor N. Stepanov, Andrei A. Lukyanitsa, and et al. 2022. "Effect of a Conical Cellulose Structure on Horseradish Peroxidase Biomacromolecules" Applied Sciences 12, no. 23: 11994. https://doi.org/10.3390/app122311994




