1. Introduction
Actinomycetes are a unique collection of aerobic unicellular, branching, and Gram-positive bacteria that can be found in an extensive variety of habitats, such as soil, warm water, and marine deposits, and they have hereditary genetic material with a 70% GC content [
1]. They include important taxa such as
Micromonospora, Nocardia, and
Streptomyces, which have large genomes and can produce a variety of secondary metabolites, antimicrobials, industrially relevant enzymes, anticancer materials, helpful metabolites, and insecticides. Furthermore,
Micromonospora,
Saccharopolyspora,
Amycolatopsis, and
Actinoplane all play a role in the delivery of commercially important compounds, including anti-infection agents supplied by
Streptomyces in over 80% of cases [
2,
3]. Hence,
Streptomycetes is a particularly well known basis of natural products, especially antibiotics. Accordingly, the
Streptomyces variety is given enormous importance since it is unique, has a high metabolic generation rate, and has the ability to recycle organic matter, as well as destroy chitin, lignocelluloses, and other materials [
4]. Generally, Streptomyces lives in groups with various microbes in different environments. This link could start a chain of events that lead to the formation of new natural products and, thus, chemical defense of the supplying bacteria and fungi [
5].
Microbial illnesses are spreading at an alarming rate, causing a severe risk to human health; following the rise in drug-resistant microbes, the mandate for new antibiotics is growing by the day [
6]. Despite the lavishness of potential sources of antimicrobial agents, soil-derived Streptomyces remains the most important repository, leaving other alternative bases such as the insect microbiome and marine microbial environments to produce novel antibiotics, pharmaceuticals, and bioactive compounds [
7]. Bioactive compounds are the microbe’s method of communication, regulating the interaction and resistance within and between species. The ecology and chemistry of bioactive substances describe the essential interplay among the microbial population, host, and environment within microbiomes [
8]. As a result, researching the chemistry of particular bioactive compounds found inside host microbial populations has become a new antimicrobial drug development paradigm that is quickly gaining traction [
9].
Topical antibiotics have recently been discovered in the microbes of a range of entities, including sea squirts and humans; however, defensive symbioses, in which microbial symbionts produce antibiotics to defend against opportunistic and specialized infections, are an auspicious source of new antimicrobials. This defensive symbiosis, which is well established in insects such as the solitary digger wasp, southern pine beetle, and fungus-growing ant systems, represents a potential solution to our need for antimicrobial compounds released by these taxa to prevent infectious disease [
10,
11,
12,
13]. Over five million insect species have been identified worldwide, with the orders of
Hymenoptera and
Coleoptera occupying practically every terrestrial niche. Insects produce chemical reactions that regulate and maintain the variety of their ecological partnerships. Hundreds of insect–Streptomyces interactions, for example, have created more than 10 unique natural antibacterial chemicals [
14].
However, very little information has been recorded on the occurrence of Streptomyces spp. that produce antimicrobial and antiproliferative compounds from the gut microbiome of the insect A. mellifera yemintica under the order of Hymenoptera. Streptomyces found in such uncharted territory can be considered wild species with natural diversity. Therefore, they may be a rich source of various beneficial metabolites. Moreover, due to nature’s limitless diversity, Streptomyces are predicted to continue being a source of natural compounds with features of interest in medicine and agriculture. Therefore, in this study, we were interested in investigating the morphological and molecular characteristics of Streptomyces spp. attained from the gut of A. mellifera yemintica and their capacity to develop natural products for therapeutic applications under various physiological circumstances.
2. Materials and Methods
2.1. Sample Collection
A. mellifera yemintica insects were collected from the botanical garden at King Saudi University’s agriculture college, Saudi Arabia. The target insect was identified by Prof. Mostafa Sharaf (professor of entomology) in a laboratory. The samples were preserved in sterile tubes in a deep freezer (−20 °C) until further processing.
2.2. Isolation of Actinomycetes from A. mellifera Yemintica
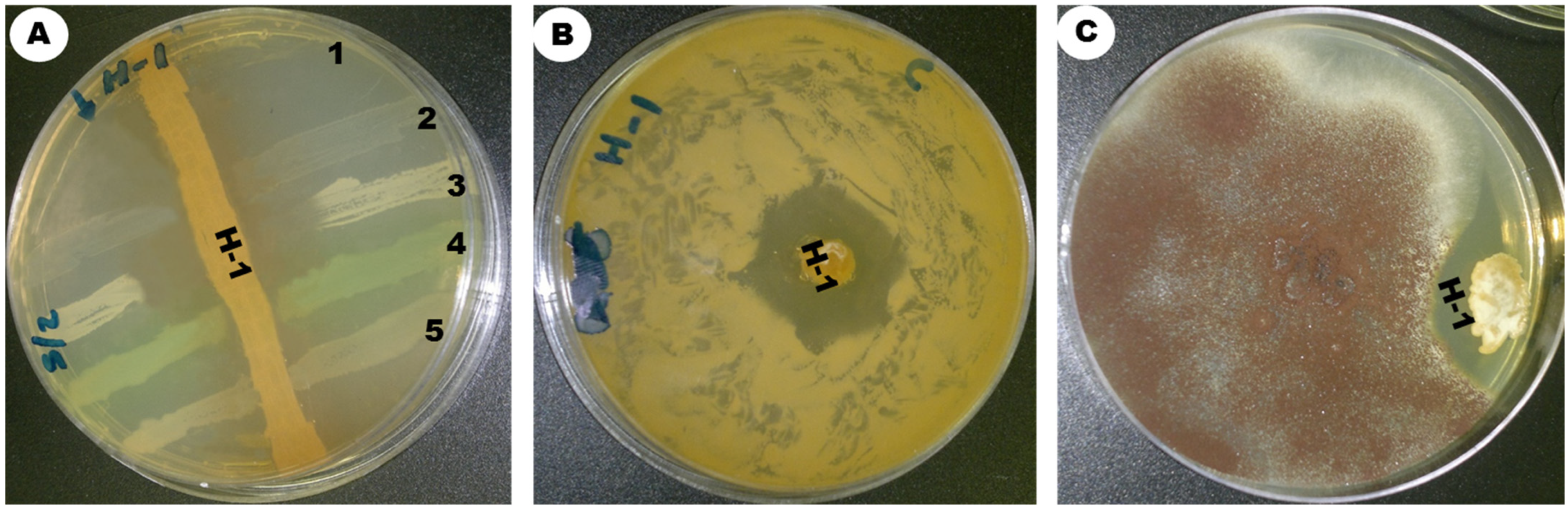
The insects were sterilized with ethanol alcohol 70% to kill all microbes on their surface. The gut was extracted from the insects using sterile tweezers and ground in 3 mL of normal saline solution, serially diluted from 10−9 to 10−1 in 0.85% NaCl, and shaken for 10 min with an orbital shaker at 250 rpm. Then, 50 µL of the dilution (10−5) was withdrawn from the insect-soaked saline solution, spread onto SCA (starch casein agar) treated with streptomycin at a concentration of 50 mg/L, and incubated at 30 °C in aerobic conditions until branched filamentous colonies and aerial mycelia developed. After the incubation period, particular colonies were sub-cultured and purified using the streaking method. Among the other strains, ess_amH1 was selected and further maintained on ISP-2 medium by periodical subculture. The culture was kept in glycerol stock for further studies.
2.3. Morphological Characteristics of Streptomyces sp. Strain ess_amH1
Morphological and physiological characterizations were based on International Streptomyces Project Methods [
15]. The strain ess_amH1 was inoculated on different standard culture media of ISP-3 (oatmeal agar), ISP-6 (peptone yeast extract iron agar), ISP-5 (glycerol asparagine agar), ISP-2 (yeast malt extract agar), ISP-4 (inorganic salt starch agar), ISP-7 (tyrosine agar), starch casein agar, and Czapek Dox agar for the observation of morphological characters [
16]. The mycelium structure, color, and arrangement of conidiophores and arthrospore on the mycelium were observed through an oil immersion lens (100×). Scanning electron microscopy was used to analyze the spore surface morphology. Using the coverslip culture method, the morphology of spore and sporulation structures was analyzed microscopically. The organism was identified on the basis of its perceived structure according to the project guidelines of Shirling and Gottlieb [
15].
2.4. Biochemical Characteristics of Streptomyces sp. Strain ess_amH1
The isolated strain was tested on ISP basal medium with 1% of different carbon and nitrogen sources. The findings of biochemical tests including nitrate and nitrite reduction matched Bergey’s manual of decisive bacteriology. Enzymatic activity of the strain was studied for the enzymes listed as Amylase, DNase, Catalase, H2S production (cystathionine β-synthetase), Hydrolysis of esculin (esculinase), Protease, Lecithin hydrolysis (Lecithinase), Lipid hydrolysis (lipase), Urea hydrolysis (urease), Nitrate reduction (nitrate reductases) and Chitinase according to methods of Holding and Collee (1971) [
17]. The development of the strain in different conditions (NaCl concentration (1%, 4%, 7%, 10%, and 14%), pH (5, 7, and 10), temperature (4 °C, 26 °C, 30 °C, 37 °C, and 45 °C)) and antibiotic resistance were tested.
2.5. Molecular Characterization of Strain
The isolated strain was cultured in ISP 2 broth, and complete DNA was isolated using Sambrook et al. (1989) [
18]. PCR amplification of the 16S rRNA gene was carried with forward primer 27F and reverse primer 1492R. Amplification was carried out for 32 cycles at 95 °C for 5 min, 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 2 min with a final extension at 72 °C for 10 min. nBLAST was used to compare the 16S rRNA gene with homologous sequences of the
Streptomyces genus obtained from NCBI. Later, MSA (multiple sequence alignment), and phylogenetic tree analyses were performed using the MEGA 7 program, with the maximum likelihood approach used to construct the phylogenetic tree.
2.6. Extraction of Secondary Metabolites from Streptomyces sp. Strain ess_amH1
In the ISP-2 broth, 2 L of culture was inoculated and maintained at 30 °C in a rotary shaker for 7 days. The cultured broth was centrifuged for 20 min at 5000 rpm at 4 °C. After collecting the supernatant, an equal amount of ethyl acetate was poured and stirred at periodic intervals. The solvent phase was extracted, and the solvent was evaporated using a rotary vacuum evaporator.
2.7. GC–MS Analysis
The EtoAc extract of
Streptomyces sp. strain ess_amH1 was completely dissolved in the organic solvent and analyzed by GC–MS on a GC-Clarus 500 (Perkin Elmer, column type-Elite-5 ms (30 m × 250), detector: mass detector: turbo mass gold-Perkin Elmer, software: Tubomass 5.4.2). The identification of chemical constituents was based on matching their recorded mass spectra with those attained from the NIST (National Institute and Standard Technology), version-year 2005 [
19].
2.8. Antimicrobial Activity of the Extract of Streptomyces sp. Strain ess_amH1 against Bacterial and Fungal Pathogens (Disc Diffusion Assay)
The disc diffusion assay was used to determine the antibacterial and antifungal activity of the solvent extract of
Streptomyces sp. strain ess_amH1 by following de Souza Constantino et al.’s (2018) method [
20]. Then, 1.0 mL of individual bacterial cultures such as
Streptococcus pneumoniae (ATCC 49619),
Staphylococcus aureus (ATCC 25923),
Salmonella typhi (ATCC 14028),
Klebsiella pneumoniae (ATCC 13883), and
Pseudomonas aeruginosa (ATCC 27853)
, as well as a fungal culture, i.e.,
Candida albicans, were mixed with 300 mL of nutrient agar medium and transferred into the 90 mm petri dishes. Then, sterilized Whatman no. 3 filter paper discs (6 mm size) were soaked in different beakers containing various concentrations of solvent extract of
Streptomyces sp. strain ess_amH1. The concentrations of 5, 10, 40, and 80 µg per disc and 20, 40, 80, 200, and 400 µg per disc were used for antibacterial activity and antifungal activity respectively. Soaked discs were taken out with sterilized forceps, air-dried, and located on the Petri dishes with different microbes. The plates were incubated at 37 °C for 24 h. The control was carried out by soaking the paper disc in ethyl acetate and placing it against each of the test microbes to ensure that ethyl acetate did not have any antibacterial/antifungal activity; streptomycin (50 µg/disc)/fluconazole (100 µg/disc) was used as a positive control. The assay was repeated three times. The zone of inhibition (mm) produced by the solvent extract was used to measure antibacterial and antifungal activity.
2.9. SEM Examination
The antibacterial effects of the EtOAc extract of
Streptomyces sp. strain ess_amH1 against the
K. pnemoniae morphology were observed by SEM. The bacterial samples were prepared following Shukla’s (2015) method [
21].
2.10. Antiproliferative Activity of the Crude Extract
The MTT assay was used to study the antiproliferative activity of solvent extract of
Streptomyces sp. on human breast cancer cells (MCF-7) and human liver cancer cells (Hep-G2) [
22]. The cancer cells (1 × 10
4 cells/well) seeded in 96-well plates and then treated for 24 h to seven different doses of crude extract (0, 3, 6, 12.5, 25, 50, and 100 µg/mL). Furthermore, for comparison, a negative/vehicle control and a positive control (doxorubicin 100 µg/mL) were used. After the required incubation time, 10 µL of MTT was added, before incubating for 3 h at room temperature. Finally, the solution was removed from the plate wells, and each well was completed with 100 µL of cell-culture-grade DMSO. The optical density (OD) of each well at 570 nm was measured in microplate reader (Synergy; BioTek, Winooski, VT, USA). The proportion of cytotoxicity was calculated in comparison to untreated cells. The assay was conducted in triplicate. The inhibitory concentration (IC
50) was determined using the following formula:
2.11. Statistical Analysis
The experiments were conducted using a completely randomized technique, with the findings presented as the mean ± SD. For statistical analysis, the SPSS software package 16.0 edition was employed (SPSS Inc., Chicago, IL, USA). One-way ANOVA (post hoc Tukey’s test) was performed to discern the statistical difference in antibacterial and anticancer studies.
4. Discussion
Insects have a remarkable ability to live in an extensive range of niches and are constantly threatened by predatory bugs, parasites, and entomopathogens. Symbiotic microorganisms play a critical part in the insect’s defense system against intruders, as well as their ability to survive in hard settings [
14]. Insect symbionts have recently been mentioned in a number of studies, and their compounds have been labeled as “potential antimicrobials”. The identified compounds involved in the defensive insect–microbe symbiosis highlight the antibacterial latency of insect-related
Streptomyces bacteria. As a result, researchers are becoming more attentive in insect symbionts as a basis of novel active chemicals [
41].
The Actinomycetales order, which includes
Streptomyces, has usually been identified for its production of antimicrobial compounds under active symbiotic conditions. So far, approximately <3% of its antimicrobial compounds have been identified, signifying the large undiscovered majority [
42]. These isolated
Streptomyces are claimed to have greater antibacterial activity than
Streptomyces strains isolated from soil or plant [
14]. These outcomes shed light on why
Streptomyces strains are so widely used in screening. Actinomycetes are found in the gut microbiome of insects and widely spread in nature. Many studies have paid attention to the synthesis of a range of compounds generated by gut Actinomycetes. Isolation of Actinomycetes from the insect gut habitat is more difficult than isolation from normal environments, due to the availability of limited bacterial communities. Most commonly, the microbiome of insects under the order of Hymenoptera has been examined for antibacterial activity. Fungus-eating ants have received more attention for the search of microbes producing antibiotics, although the order of Hymenoptera includes ants, bees, and wasps. Bees, which contain some of the smallest bacterial populations of any insect, are frequently the subject of research [
43], and the knowledge that honey has antibacterial properties has driven microbiota studies. Isolating bioactive metabolite-producing actinomycetes from competitive habitats such as the gut of
A. mellifera yemintica is a vital arena of investigation in view of the existing drug resistance dilemma and the hunt for novel antimicrobials. The strain ess_amH1 from this investigation was categorized in the genus
Streptomyces on the basis of the improvement of aerial or reproductive mycelia, as well as sporulation on solid media, according to macroscopic and microscopic examination. It was coenocytic in the vegetative mycelium. These species develop filamentous, densely branched mycelia with a net-like shape.
Streptomyces species can be identified from the existence of characteristic aerial mycelium on top of substrate growth. The size of the aerial hyphae was discovered to be variable.
Additionally, growth conditions and biochemical properties of the isolate ess_amH1 assist in the identification of biotechnological characteristics. The carbon and nitrogen supplies in the medium most likely influence the antimicrobial production. According to a previous study, 0.55% glucose and 0.835% yeast extract in the medium produced the most secondary metabolites with the greatest antibacterial activity in
Streptomyces sp. strain ess_amH1 [
44,
45]. In this study, the optimum medium for antibacterial production of strain ess_amH1 was confirmed as ISP-2 broth. ISP-2 medium is made up of simple carbon bases such as glucose, as well as organic nitrogen sources such as malt and yeast extract, which can help with growth, pigment formation, and antibacterial agent production [
26]. Physiological characteristics of the strain ess_amH1 at 30 °C, pH 7, and 1% NaCl exhibited optimal growth, which is similar to other reported
Streptomyces strains isolated from soil [
42,
45,
46]. The enzymatic activity of strain ess_amH1 (
Table 3) showed that the strain is capable of producing a variety of enzymes that catalyze macromolecules such as starch, protein, lipid, and nucleic acid. Furthermore, the strain can produce hydrolytic enzymes against esculin, nitrate, and urea, whereas the production of catalase enzymes proves the capability of the strain to produce antioxidant enzymes. The utilization of carbon and nitrogen sources, as well as the development of antimicrobials and industrially significant enzymes, provides interesting leads for further research of the isolate ess_amH1. The significance of incorporating various antibiotics into the medium in the selective isolation of the strain has been identified [
4,
45,
46,
47]. Furthermore, the 16S rRNA sequence analysis showed that the strain ess_amH1 was related to the genus
Streptomyces. The phylogenetic analysis revealed 90% similarity of the strain ess_amH1 with antibiotics producing
Streptomyces sp. such as
Streptomyces griseus isolate AT1NF9 and
Streptomyces avermitilis isolate HL3RP7. Although typical phenotypic characteristics of our strain ess_amH1 were similar, other aspects differed such as the color characteristics of the aerial mycelium and nitrate reduction [
48].
GC–MS analysis of ethyl acetate extract of ess_amH1 strain revealed its antibacterial, antifungal, antitumor, and antioxidant activities, as shown in
Table 6 [
33,
34,
35,
36,
37,
38,
39,
40]. As
Actinomycetes produce chemicals that are useful against multidrug-resistant infections under a variability of development settings, the crude extract extracted from the strain ess_amH1 also demonstrated broad-spectrum antibacterial activity against Gram-positive (
S. aureus and
S. pneumoniae) and Gram-negative bacteria (
P. aeruginosa and
K. pneumoniae). Despite the differences in cell-wall properties, the compounds in the solvent extract could act equally against Gram-positive and -negative bacteria. The low-molecular-weight antimicrobial compounds present in the extract help hydrophilic, low-molecular-weight molecules diffuse into the outer layer of Gram-negative bacteria, because the hydrophilic channels produced by specific porin proteins help the hydrophilic low-molecular-weight molecules diffuse into the outer layer of Gram-negative bacteria, whereas peptidoglycan polymers are relatively inert [
47].
Furthermore, it was discovered that the crude extract had considerable activity against
Candida albicans. Since antibiotics are notoriously produced by
Streptomyces griseus and
Streptomyces avermitilis, along with an extensive array of microbes, it appears that the comparable strain ess_amH1 is also capable of producing an extensive variety of bioactive molecules to overcome bacterial and fungal growth [
1,
46]. The antiproliferative activity of the EtOAc extract against the breast cell line and hepatocarcinoma cell lines proved that the extract might possess strong anticancer properties. The cytotoxic properties of the extract might increase the destructive signals that alter cell endurance and lead to death via apoptosis. Antitumor compounds existing in the EtOAc extract were accountable for the decrease in cancer cell viability [
31,
32,
33,
34,
35,
36]. As a result, they could be developed as anticancer therapies for human use, as cytotoxicity testing is an important stage in developing new therapeutic medications for clinical use. Further studies on purification of natural compounds from the extract can elaborate the specific role and mechanism behind the antimicrobial and antitumor activity of the specific compounds identified from the crude extract.