Dry Test Methods for Micropumps
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
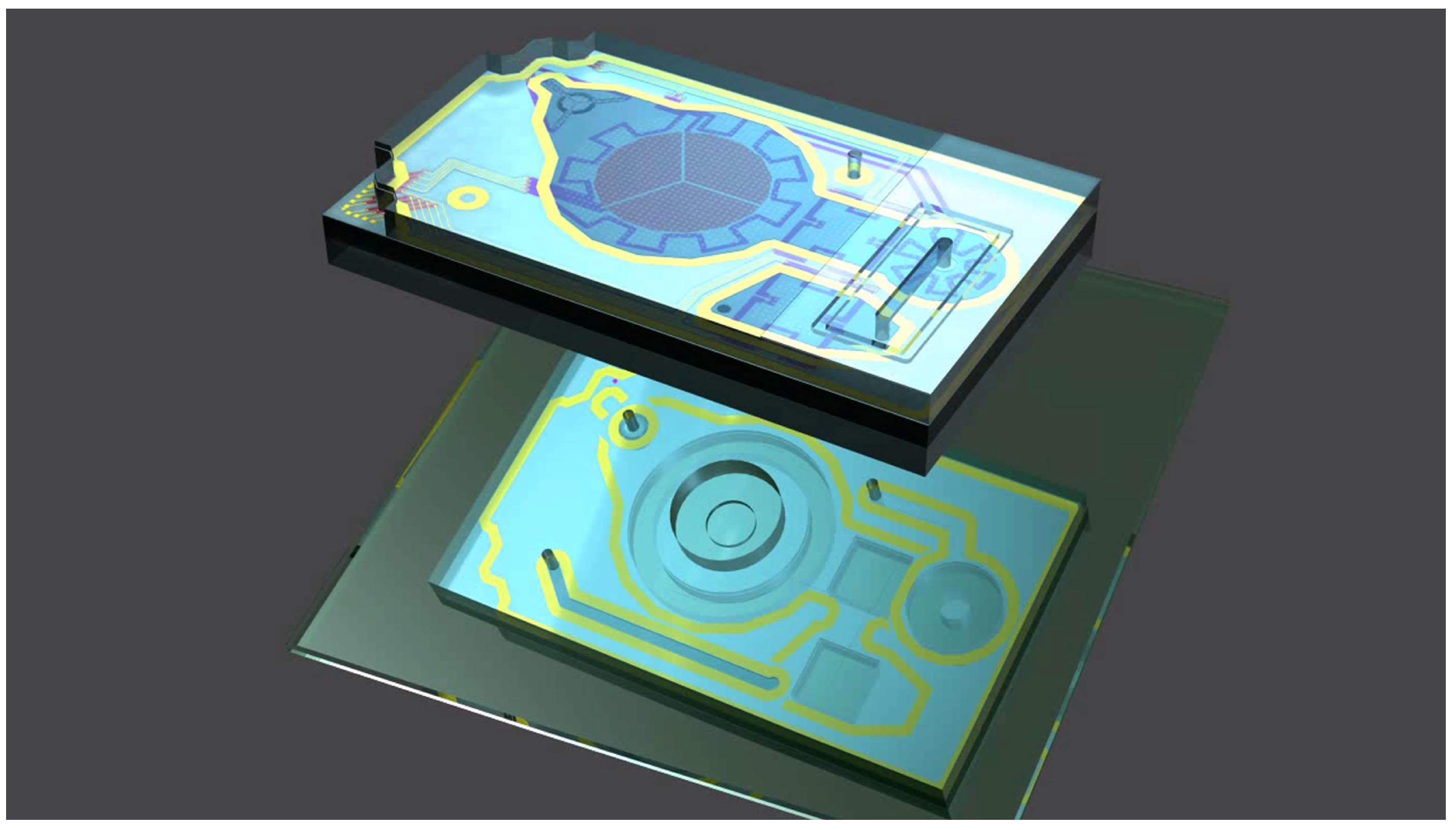
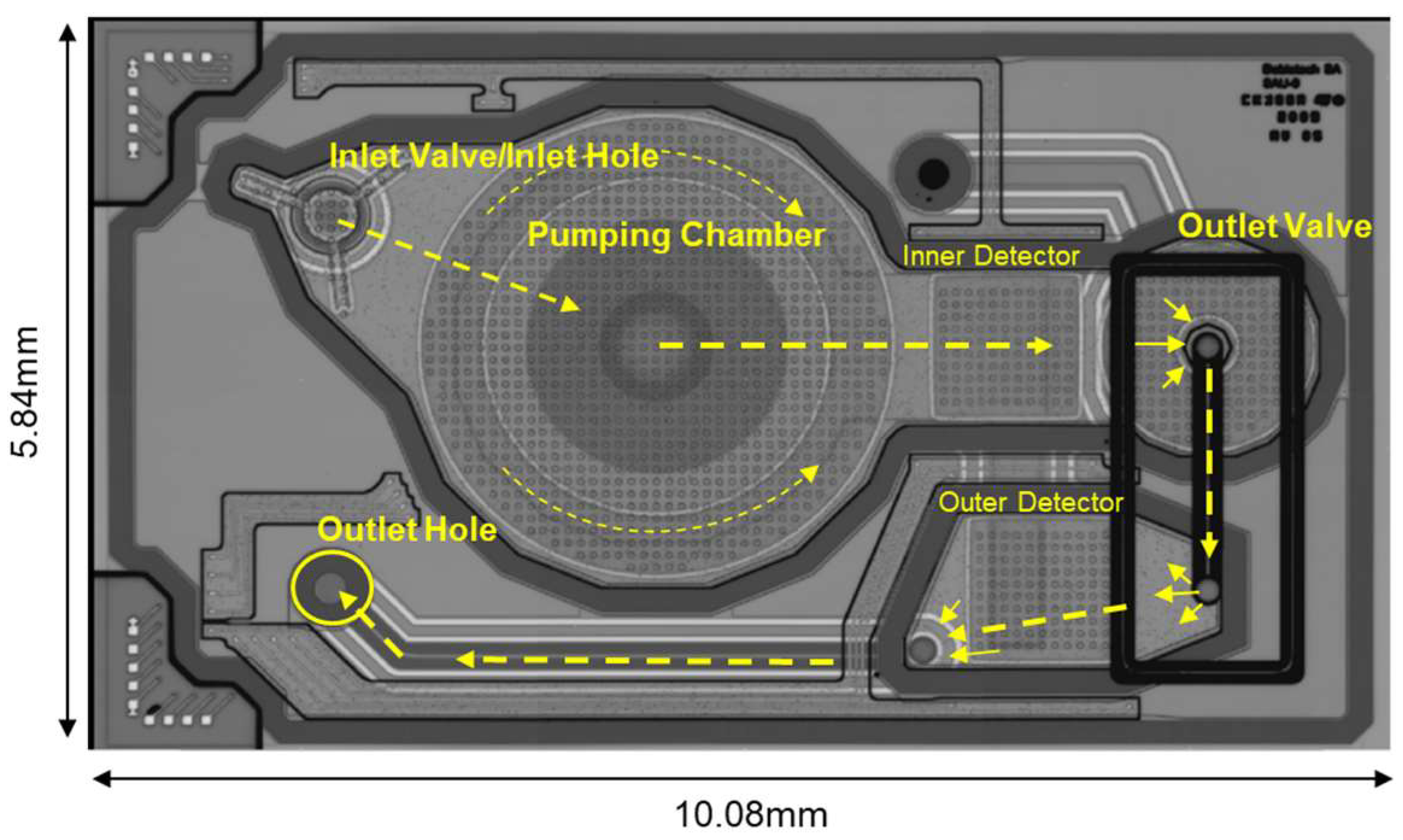
2.1. Design Description
2.1.1. MEMS Membrane Micropump
- Supply phase: the upward movement of the membrane leads to an increase in the pumping chamber volume and a lowering of the pressure; thus, liquid enters from the inlet toward the pumping chamber.
- Infusion phase: the downward movement of the membrane lowers the pumping chamber volume and increases the pressure; thus, liquid flows from the pumping chamber toward the outlet.
2.1.2. Critical-to-Quality Parameters and Basic Outputs
- Compression ratio;
- Stroke volume;
- Pumping cavity tightness;
- Valve tightness;
- Valve pretension (opening threshold);
- Pressure sensor functionality (calibration/offset);
- Outlet fluid path tightness;
- Maximum pumping pressure;
- Actuator functionality.
- Membrane offset;
- Pumping mechanism stiffness;
- Actuator blocking force.
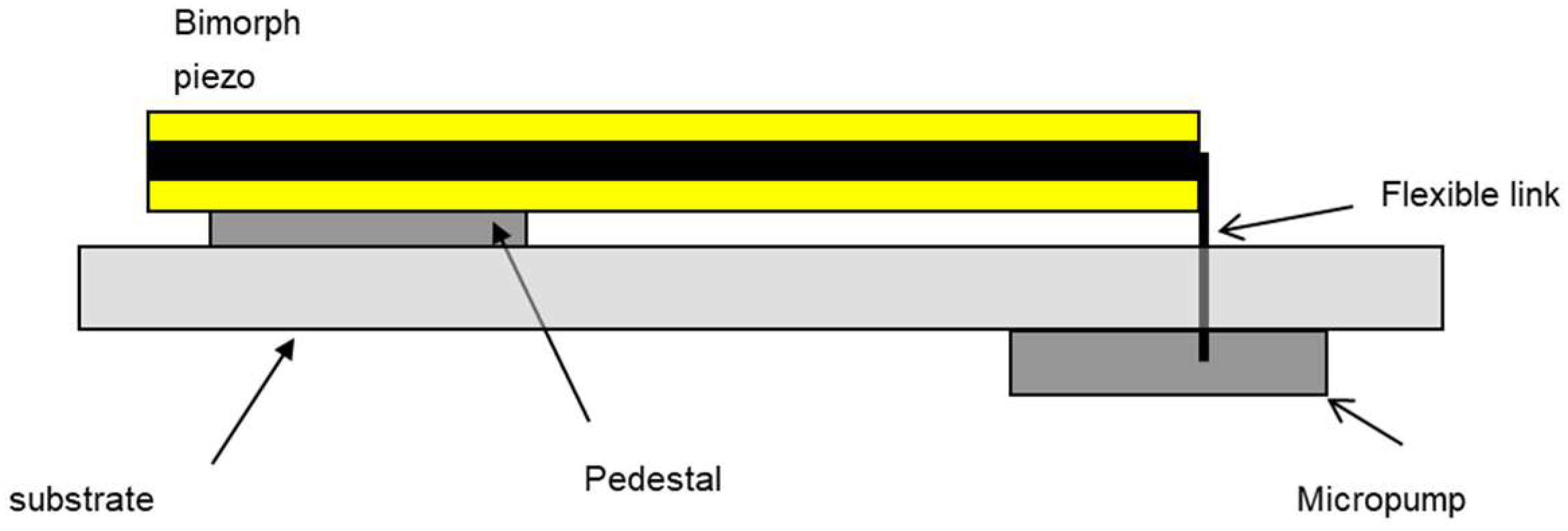
2.1.3. Design-for-Testability
2.2. Methods
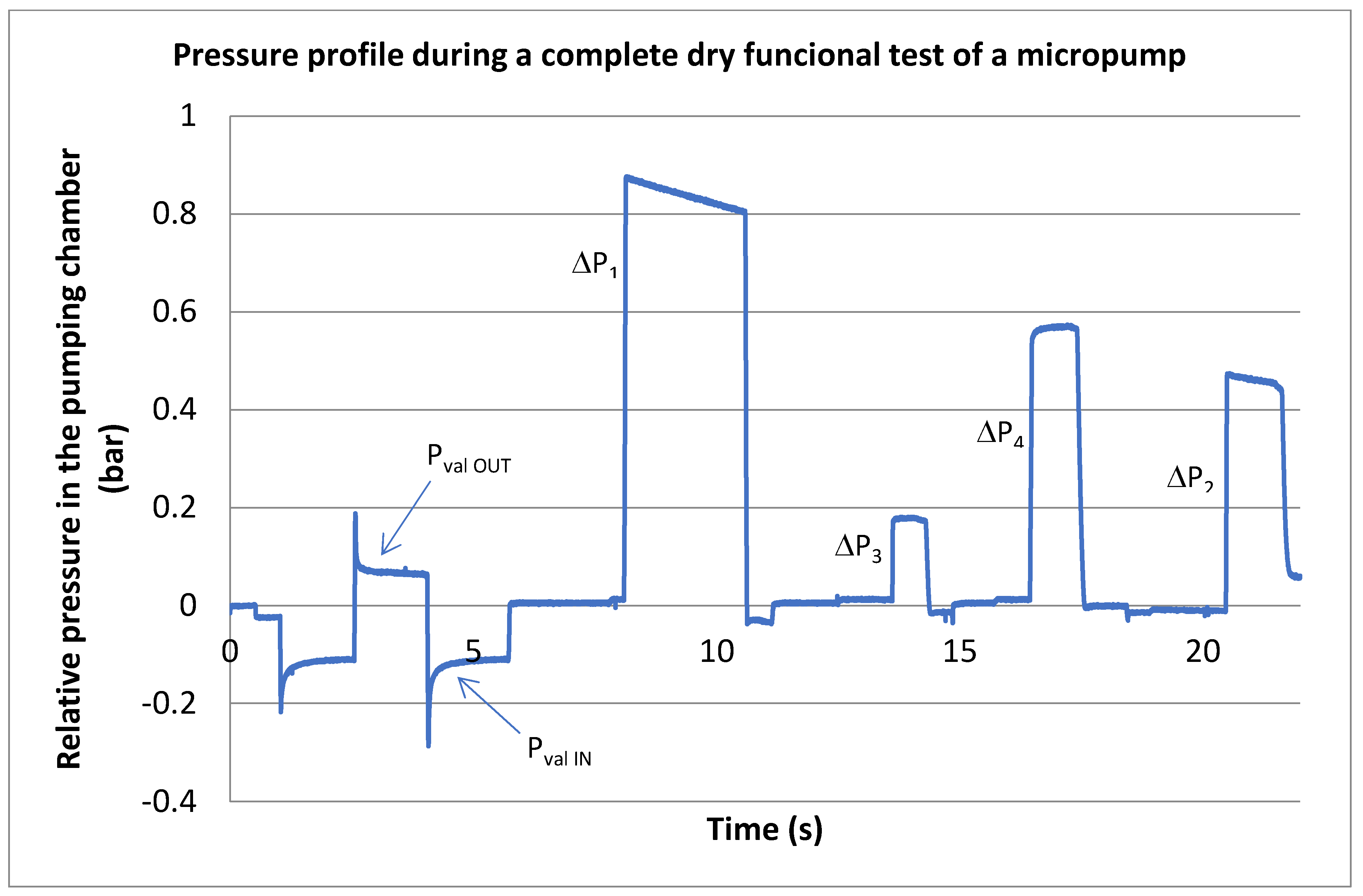
2.2.1. Compression Ratio and Stroke Volume
- Admission phase
- ⚬
- Pull down the pumping membrane;
- ⚬
- Set at low pressure (e.g., <0.3 bar or −0.7 barg) to widely open the outlet valve and thus fill the pumping chamber with air at the pressure .
- Compression phase:
- ⚬
- Set at high pressure (e.g., >2.1 bar or +1.1 barg) to keep the outlet closed during the compression of the air in the pumping chamber;
- ⚬
- Push up the pumping membrane and compress the gas in the pumping chamber.
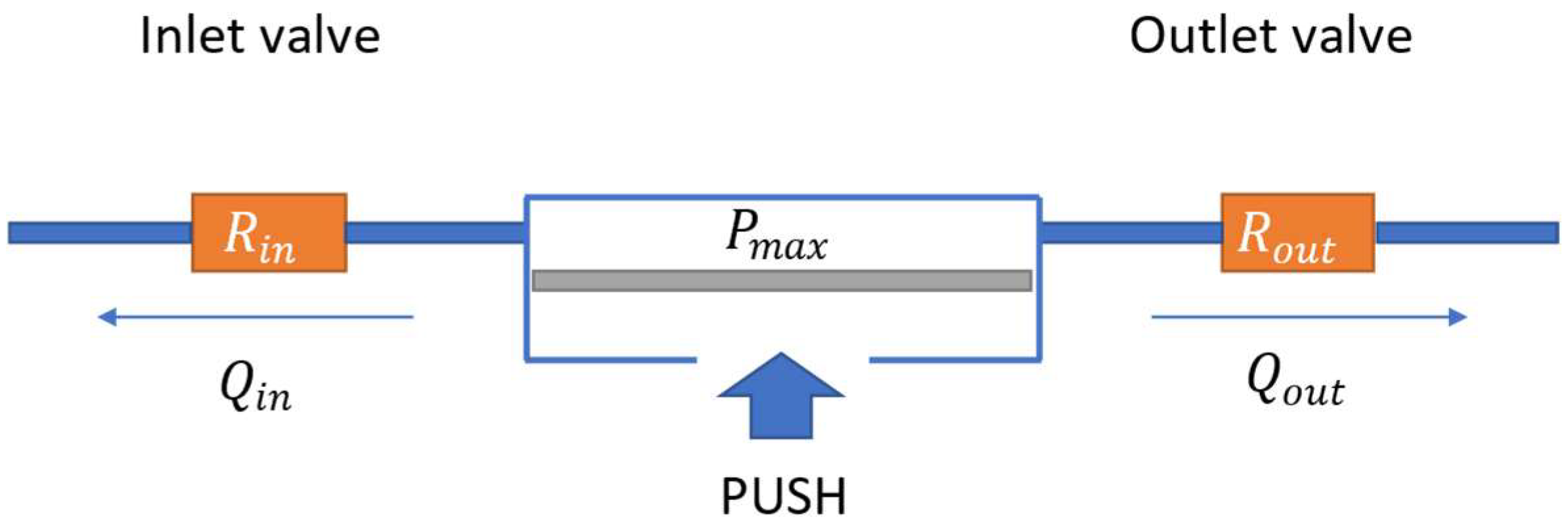
2.2.2. Valve Leakage
2.2.3. Maximum Free Flow
- An open fluid path between the reservoir and the delivery site;
- A pressure gradient to generate the flow.
- In the normal condition of use, if the micropump is connected to the patient with a catheter. As an example, a 1 m connection line can generate a hydrostatic pressure of up to 100 mbar.
- In a faulty condition of use, if the patient overfills the reservoir or leaves air bubbles in a rigid reservoir. Air bubbles can indeed expand and pressurize the reservoir in the case of a temperature increase or change in atmospheric pressure [15].
- Large generated pressure (up to +1 bar);
- Small cavity (typical dead volume of 200 nL);
- Low viscosity (e.g., 0.0175 mPa.s);
- High-pressure sensor sensitivity (better than 1 mbar).
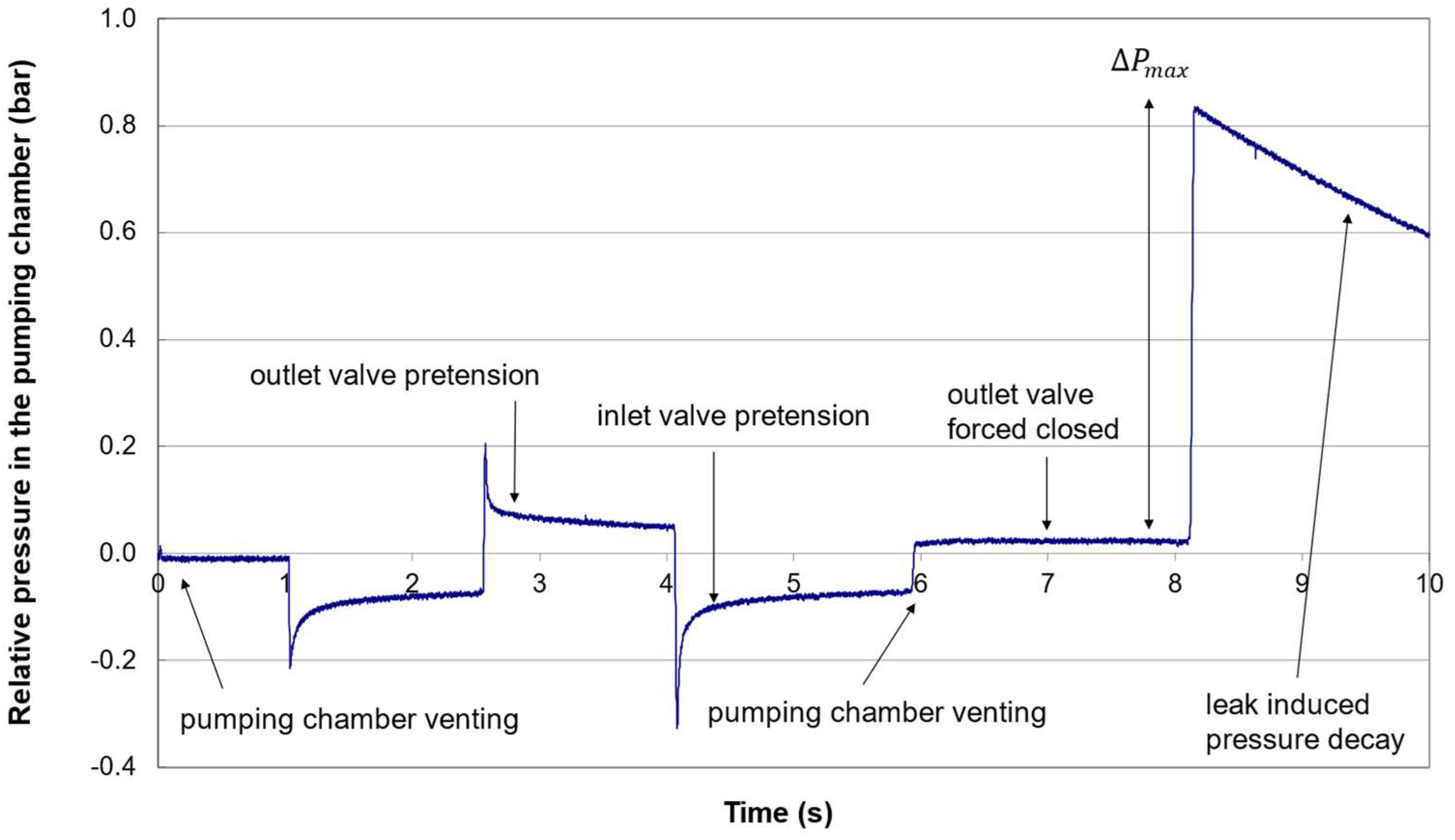
2.2.4. Valve Pretensions
2.2.5. Membrane Offset
2.2.6. Pumping Mechanism Stiffness
- Activate the piezo to pull down the pumping membrane up to (PULL position);
- Apply a vacuum to the outlet valve port to vent the pumping chamber;
- Apply a large pressure (>+ 1 barg) to the outlet valve port to force the valve closed;
- Set the piezo voltage to to release the actuator and compress the air in the pumping chamber; the pumping membrane moves from position to .
- Activate the piezo to pull down the pumping membrane up to (PULL position);
- Apply a vacuum to the outlet valve port to vent the pumping chamber;
- Apply a large pressure (>+ 1 barg) to the outlet valve port to force the valve closed;
- Set the piezo voltage to a value to move the pumping chamber up to the position .
2.2.7. Actuator Blocking Force
2.2.8. Maximum Pumping Pressure
2.2.9. Test Setup
3. Results
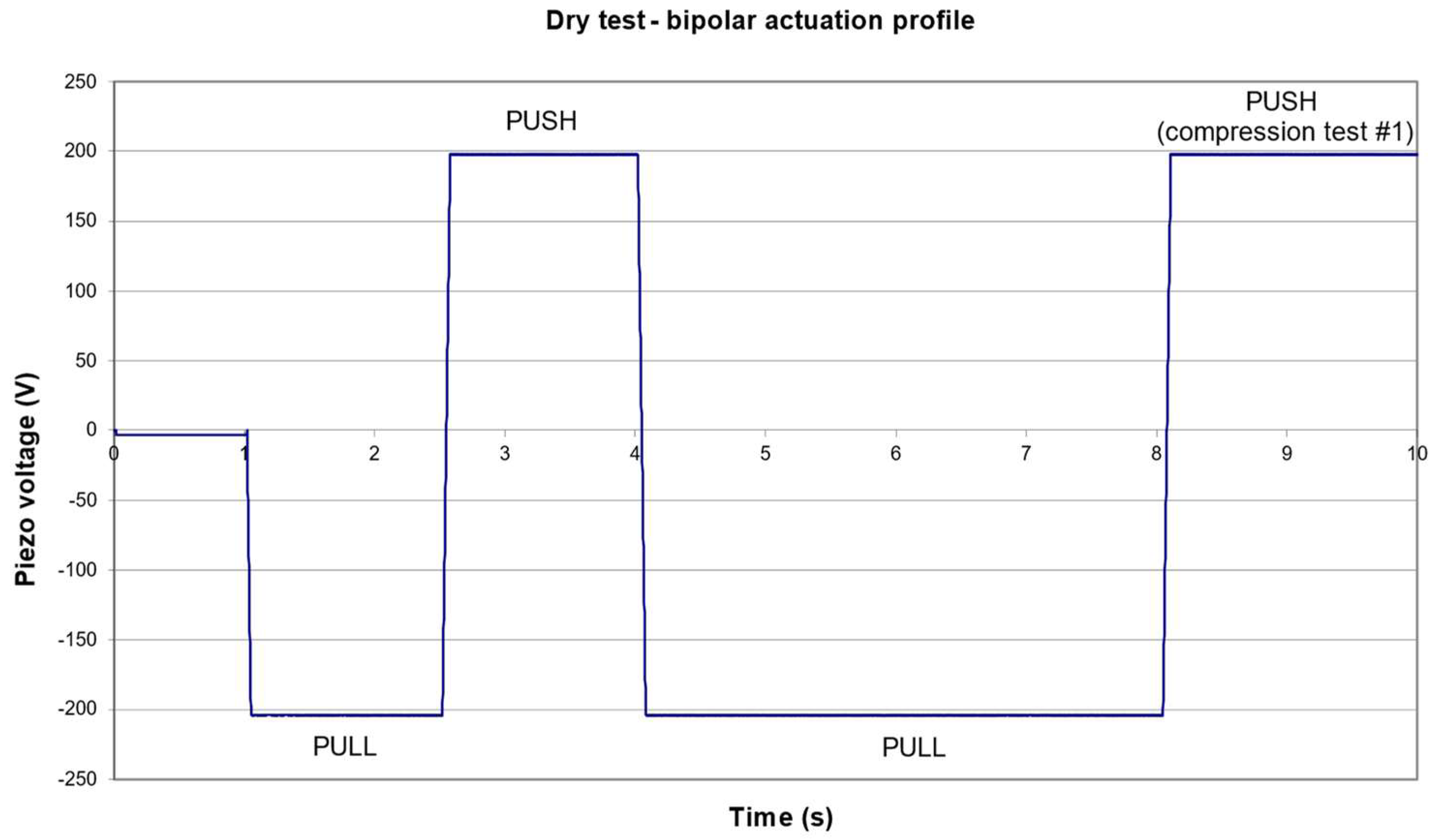
3.1. Nominal Pressure Profile
3.2. Failure Detection
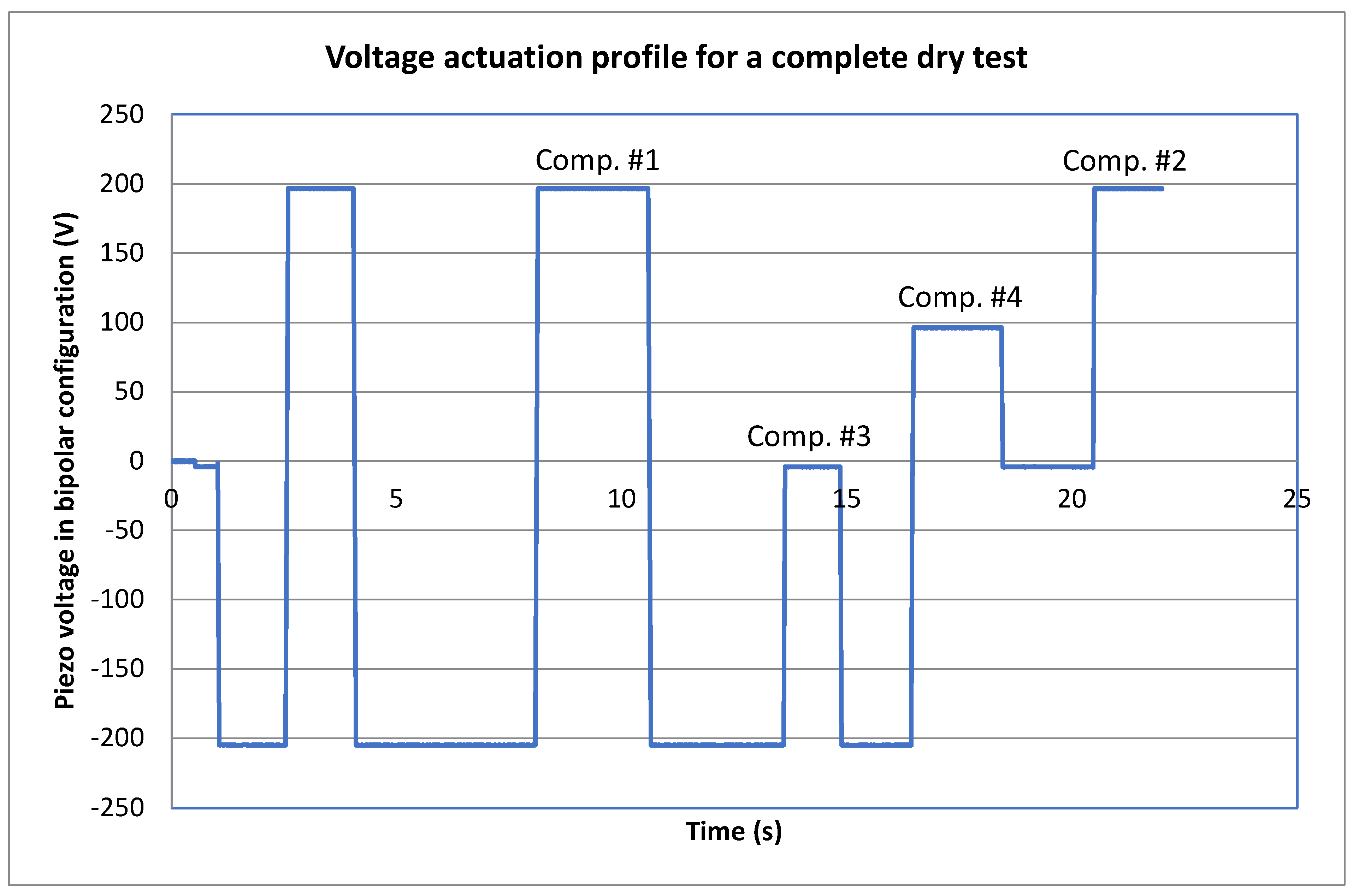
3.3. Extended Dry Tests for Piezo Actuator Assembly Characterization
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lawes, R. MEMS Cost Analysis: From Laboratory to Industry; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Vranes, M. Cost of MEMS testing: A strategic perspective. In Proceedings of the 3rd Annual MTR Conference, Shanghai, China, 15–16 September 2011. [Google Scholar]
- Hantos, G.; Flynn, D.; Desmulliez, M.P.Y. Built-In Self-Test (BIST) Methods for MEMS: A Review. Micromachines 2021, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, H.G. Testing of MEMS-based microsystems. In Proceedings of the European Test Symposium (ETS’05), Tallinn, Estonia, 22–25 May 2005; pp. 223–228. [Google Scholar] [CrossRef]
- Shoaib, M.; Hamid, N.H.; Malik, A.F.; Zain Ali, N.B.; Tariq Jan, M. A review on key issues and challenges in devices level MEMS testing. J. Sens. 2016, 2016, 1639805. [Google Scholar] [CrossRef] [Green Version]
- Tanner, D.M. MEMS reliability: Where are we now? Microelectron. Reliab. 2009, 49, 937–940. [Google Scholar] [CrossRef]
- Olbrich, T.; Richardson, A.M.D.; Bradley, D.A. Built-in self-test and diagnostic support for safety critical microsystems. Microelectron. Reliab. 1996, 36, 1125–1136. [Google Scholar] [CrossRef]
- Ramadoss, R.; Dean, R.; Xiong, X. MEMS testing. In System-on-Chip Test Architectures; Elsevier: Amsterdam, The Netherlands, 2008; pp. 591–651. [Google Scholar] [CrossRef]
- Clark, J.R.; Hsu, W.T.; Nguyen, C.T.C. Measurement techniques for capacitively-transduced VHF-to-UHF micromechanical resonators. In Transducers’ 01 Eurosensors XV; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1090–1093. [Google Scholar] [CrossRef]
- Puers, R.; De Bruyker, D.; Cozma, A. A novel combined redundant pressure sensor with self-test function. Sens. Actuators A Phys. 1997, 60, 68–71. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Li, Y.; Cima, M.J.; Langer, R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc. IEEE 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Mao, Z.; Yoshida, K.; Kim, J.W. Active sorting of droplets by using an ECF (Electro-conjugate Fluid)micropump. Sens. Actuators A Phys. 2020, 303, 111702. [Google Scholar] [CrossRef]
- Medtronic plc. Medtronic SynchroMed®II Infusion System Implant Manual; Medtronic PLC.: Minneapolis, MN, USA, 2017. [Google Scholar]
- Chappel, E. Implantable Drug Delivery Devices. In Drug Delivery Devices and Therapeutic Systems; Chappel, E., Ed.; Academic Press: New York, NY, USA, 2020; pp. 129–156. [Google Scholar] [CrossRef]
- Combination Products. Available online: https://www.fda.gov/combination-products (accessed on 12 October 2022).
- Chappel, E. A Review of Passive Constant Flow Regulators for Microfluidic Applications. Appl. Sci. 2020, 10, 8858. [Google Scholar] [CrossRef]
- Bußmann, A.; Leistner, H.; Zhou, D.; Wackerle, M.; Congar, Y.; Richter, M.; Hubbuch, J. Piezoelectric Silicon Micropump for Drug Delivery Applications. Appl. Sci. 2021, 11, 8008. [Google Scholar] [CrossRef]
- Dumont-Fillon, D.; Chappel, E. Micropumps for drug delivery. In Drug Delivery Devices and Therapeutic Systems; Chappel, E., Ed.; Academic Press: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Rebordão, G.; Palma, S.I.C.J.; Roque, A.C.A. Microfluidics in Gas Sensing and Artificial Olfaction. Sensors 2020, 20, 5742. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huo, D.; Zhang, J. Gas Recognition in E-Nose System: A Review. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chen, C.P.; Chang, C.M.; Lin, C.-P.; Lin, C.-H.; Fu, L.-M.; Lee, C.-Y. MEMS-based gas flow sensors. Microfluid Nanofluid 2009, 6, 333. [Google Scholar] [CrossRef]
- Cho, M.-O.; Jang, W.; Lim, S.-H. Fabrication and Evaluation of a Flexible MEMS-Based Microthermal Flow Sensor. Sensors 2021, 21, 8153. [Google Scholar] [CrossRef]
- Xu, T.; Chakrabarty, K. Fault modeling and functional test methods for digital microfluidic biochips. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past. present and future. Int. J. Pharm. Investig. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hovorka, R. Closed-loop insulin delivery: From bench to clinical practice. Nat. Rev. Endocrinol. 2011, 7, 385–395. [Google Scholar] [CrossRef]
- Schneeberger, N.; Allendes, R.; Bianchi, F.; Chappel, E.; Conan, C.; Gamper, S.; Schlund, M. Drug delivery micropump with built-in monitoring. Procedia Chem. 2009, 1, 1339–1342. [Google Scholar] [CrossRef] [Green Version]
- Dumont-Fillon, D.; Tahriou, H.; Conan, C.; Chappel, E. Insulin Micropump with Embedded Pressure Sensors for Failure Detection and Delivery of Accurate Monitoring. Micromachines 2014, 5, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Fournier, S.; Chappel, E. Modeling of a Piezoelectric MEMS Micropump Dedicated to Insulin Delivery and Experimental Validation Using Integrated Pressure Sensors: Application to Partial Occlusion Management. J. Sens. 2017, 2017, 3719853. [Google Scholar] [CrossRef]
- Richter, M.; Linnemann, R.; Woias, P. Robust design of gas and liquid micropumps. Sens. Actuators A Phys. 1998, 68, 480–486. [Google Scholar] [CrossRef]
- Bissig, H.; Petter, H.T.; Lucas, P.; Batista, E.; Filipe, E.; Almeida, N.; Ribeiro, L.F.; Gala, J.; Martins, R.; Savanier, B.; et al. Primary standards for measuring flow rates from 100 nl/min to 1 ml/min–gravimetric principle. Biomed. Eng./Biomed. Tech. 2015, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Chappel, E.; Proennecke, S.; Neftel, F. Method and System for Detecting Malfunction of a MEMS Micropump. U.S. Patent No 10.286.144, 14 May 2019. [Google Scholar]
- Gastinger, K.; Haugholt, K.H.; Kujawinska, M.; Jozwik, M.; Schaeffel, C.; Beer, S. Optical mechanical and electro-optical design of an interferometric test station for massive parallel inspection of MEMS and MOEMS. In Proceedings of the Optical Measurement Systems for Industrial Inspection VI (SPIE), Munich, Germany, 17 June 2009; Volume 7389, pp. 487–498. [Google Scholar] [CrossRef]
- Krauter, J.; Osten, W. Nondestructive surface profiling of hidden MEMS using an infrared low-coherence interferometric microscope. Surf. Topogr. Metrol. Prop. 2019, 6, 015005. [Google Scholar] [CrossRef]
- Emery, Y.; Cuche, E.; Marquet, F.; Aspert, N.; Marquet, P.; Kühn, J.; Botkine, M.; Colomb, T.; Montfort, F.; Charrière, F.; et al. Digital holographic microscopy (DHM) for metrology and dynamic characterization of MEMS and MOEMS. In Proceedings of the SPIE 6185, Mems, Moems, and Micromachining II, Strasbourg, France, 21 April 2006; pp. 205–209. [Google Scholar] [CrossRef]
- Singh, V.R.; Asundi, A. In-line digital holography for dynamic metrology of MEMS. Chin. Opt. Lett. 2009, 7, 1117–1122. [Google Scholar] [CrossRef]
- Singh, V.R.; Miao, J.; Wang, Z.; Hegde, G.; Asundi, A. Dynamic characterization of MEMS diaphragm using time averaged in-line digital holography. Opt. Commun. 2007, 280, 285–290. [Google Scholar] [CrossRef]
- Krehl, P.; Engemann, S.; Rembe, C.; Hofer, E.P. High-speed visualization. a powerful diagnostic tool for microactuators–retrospect and prospect. Microsyst. Technol. 1999, 5, 113–132. [Google Scholar] [CrossRef]
- Puers, R. Capacitive Sensors: When and How to use them. Sens. Actuators A Phys. 1993, 37, 93–105. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Atmospheric Pressure (QFE, QFF, QNH). Available online: https://www.meteoswiss.admin.ch/home/weather/wetterbegriffe/atmospheric-pressure-qfe-qff-qnh.html (accessed on 31 October 2022).
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1&subpartNode=21:8.0.1.1.12.6 (accessed on 31 October 2022).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chappel, E. Dry Test Methods for Micropumps. Appl. Sci. 2022, 12, 12258. https://doi.org/10.3390/app122312258
Chappel E. Dry Test Methods for Micropumps. Applied Sciences. 2022; 12(23):12258. https://doi.org/10.3390/app122312258
Chicago/Turabian StyleChappel, Eric. 2022. "Dry Test Methods for Micropumps" Applied Sciences 12, no. 23: 12258. https://doi.org/10.3390/app122312258
APA StyleChappel, E. (2022). Dry Test Methods for Micropumps. Applied Sciences, 12(23), 12258. https://doi.org/10.3390/app122312258





