The Molecular Docking and Inhibition Kinetics of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Soft-Shelled Turtle Yolk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of ACE Inhibitory Activity and Measurement of IC50 Value
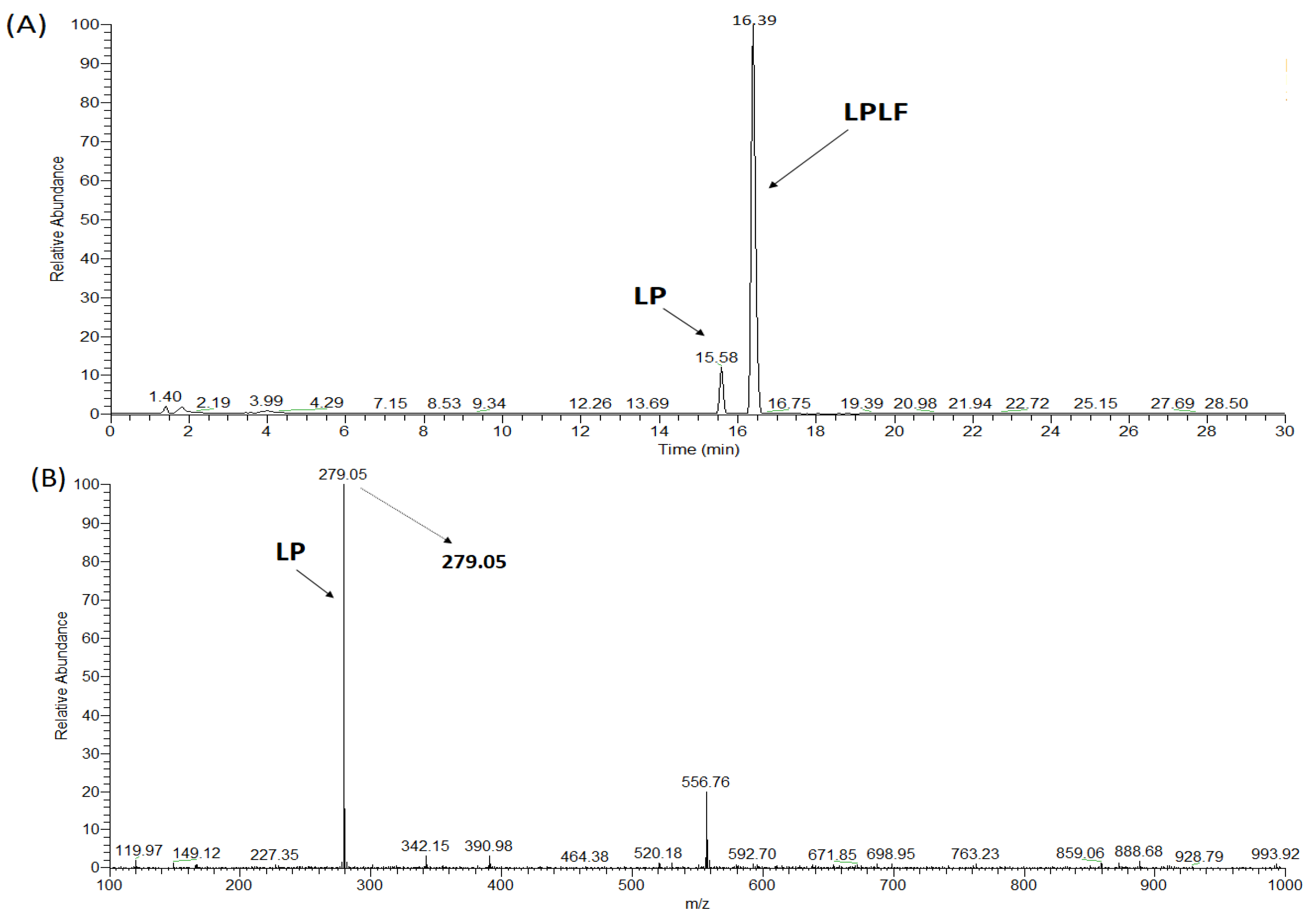
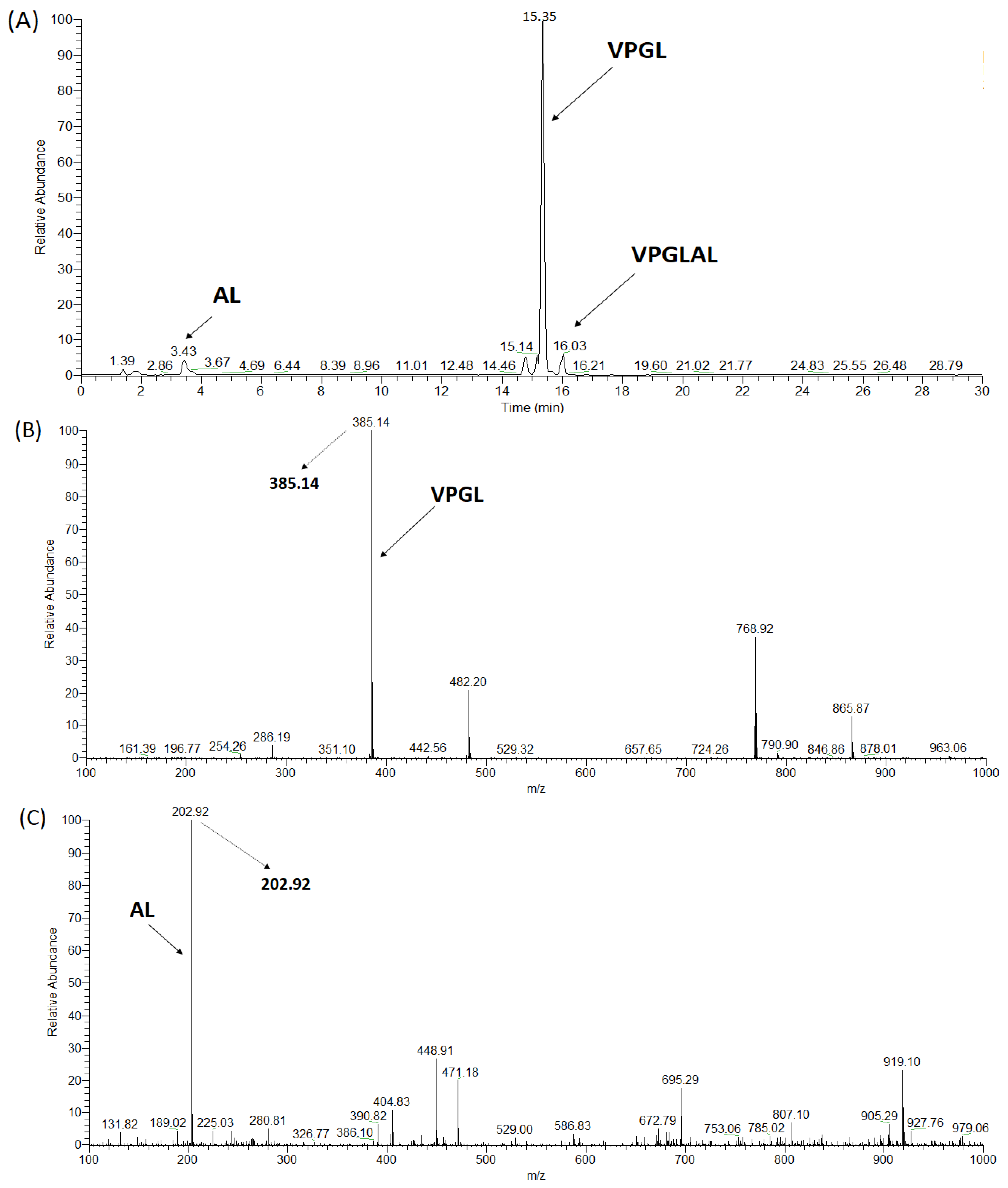
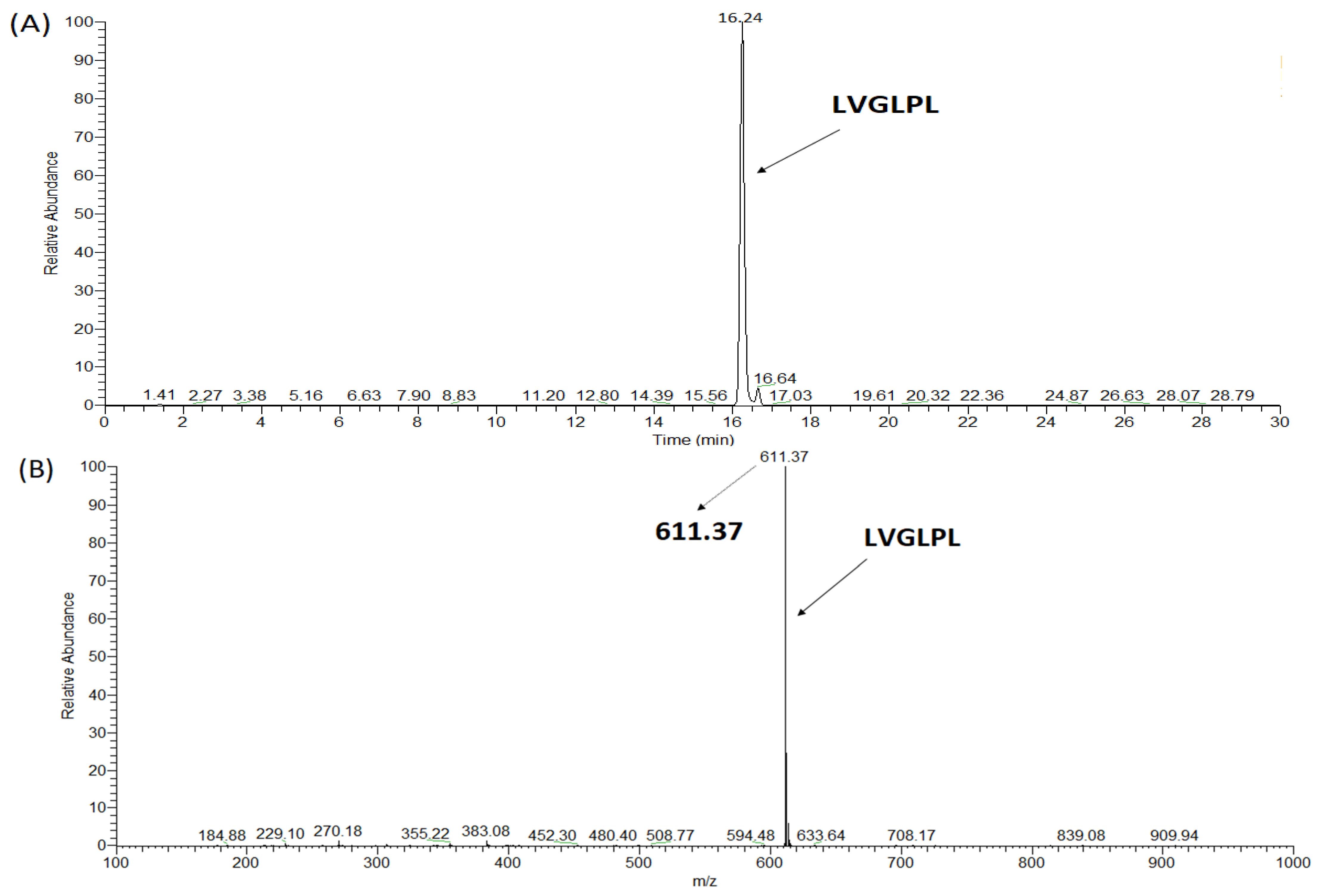
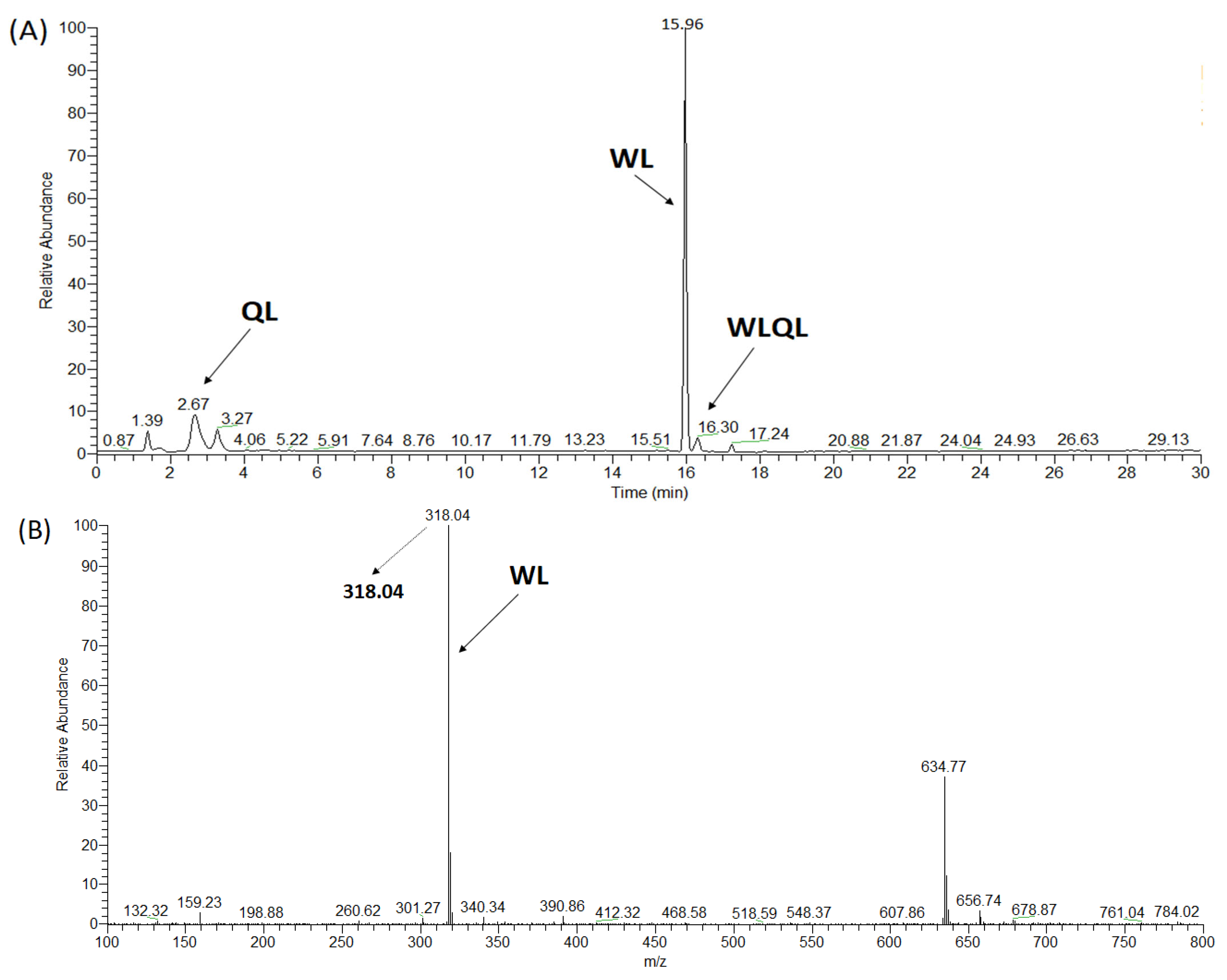
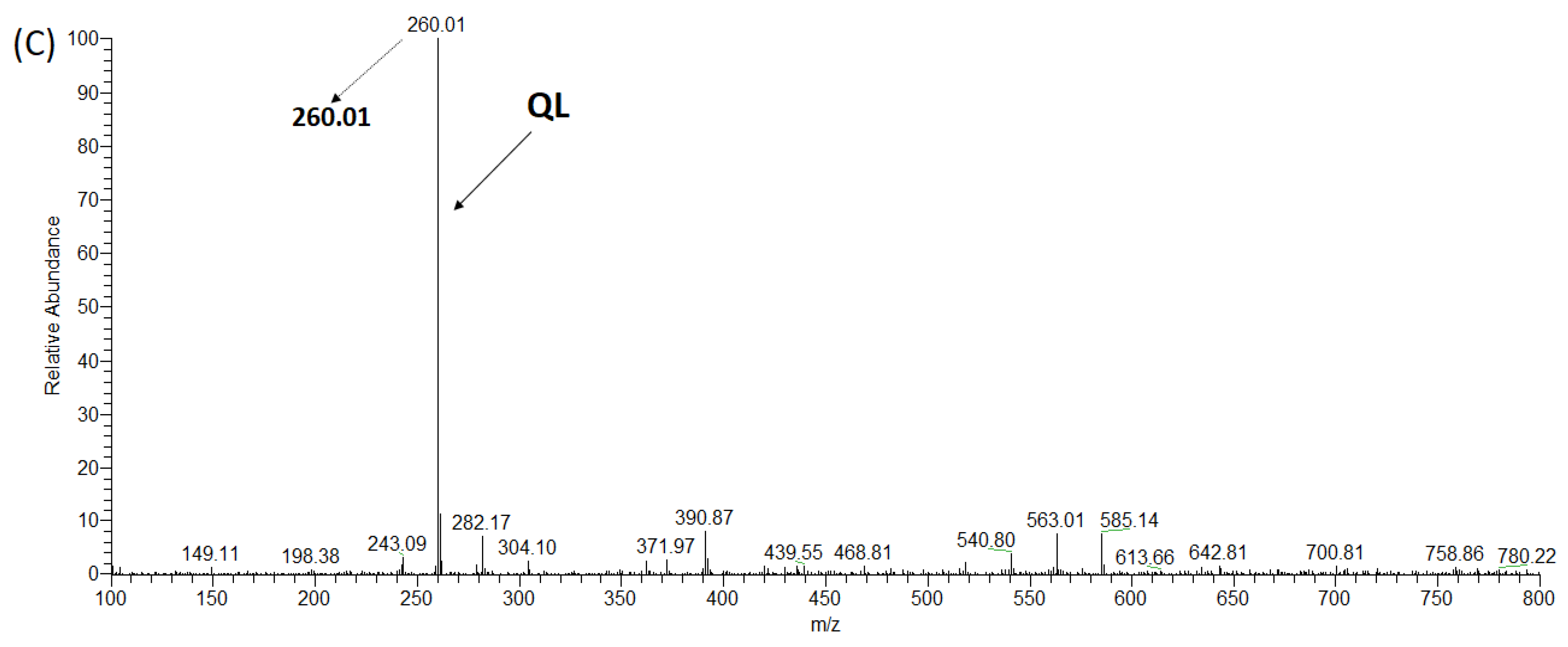
2.3. Synthesis of ACE Peptides
2.4. Determination of Inhibitory Patterns of Synthetic Peptides for ACE
2.5. Stability of ACEI Peptides against ACE
2.6. Molecular Docking to ACE
2.7. Statistical Analysis
3. Results
3.1. Identification of Angiotensin-Converting Enzyme (ACE) Inhibitory Activity
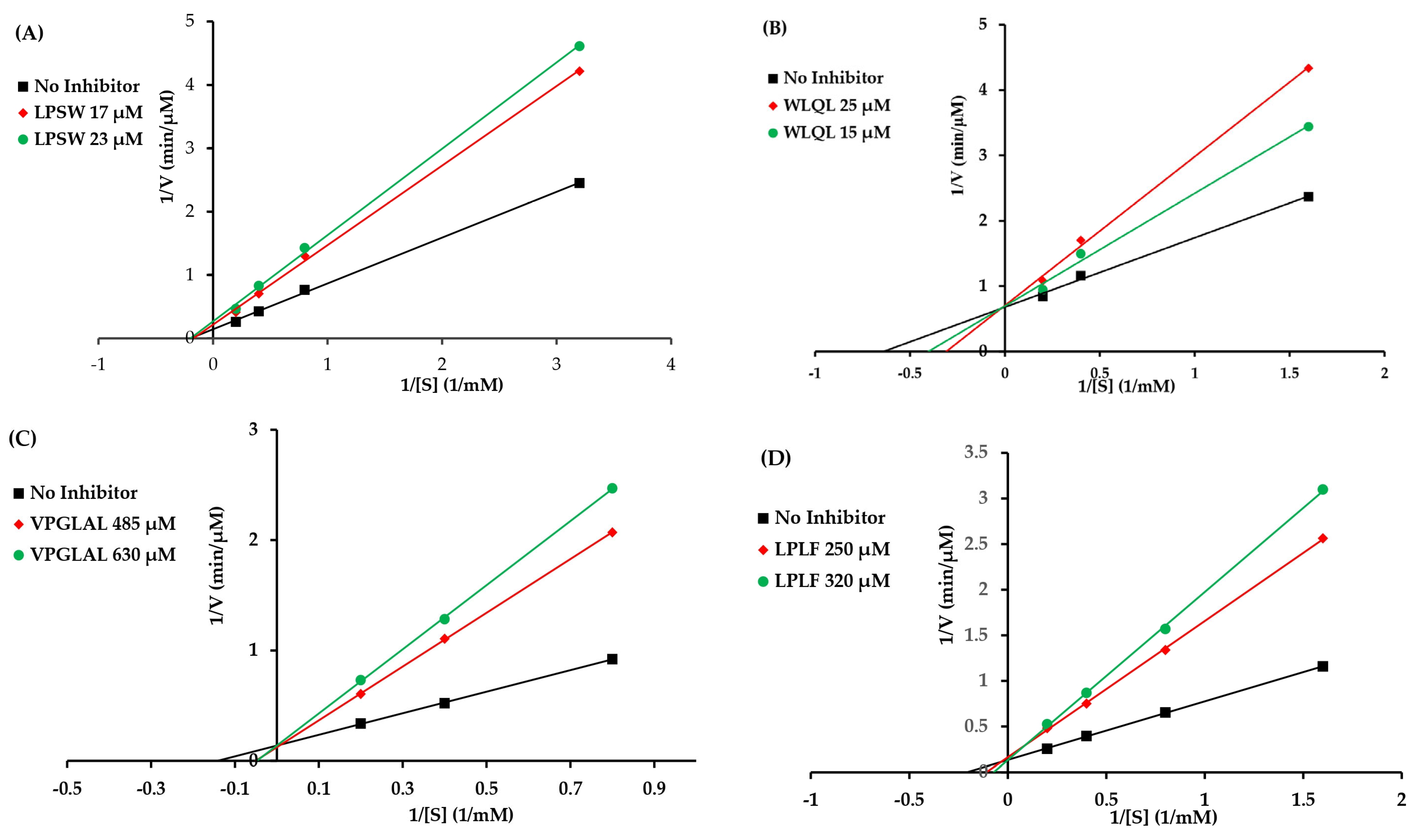
3.2. Kinetic Study with ACE
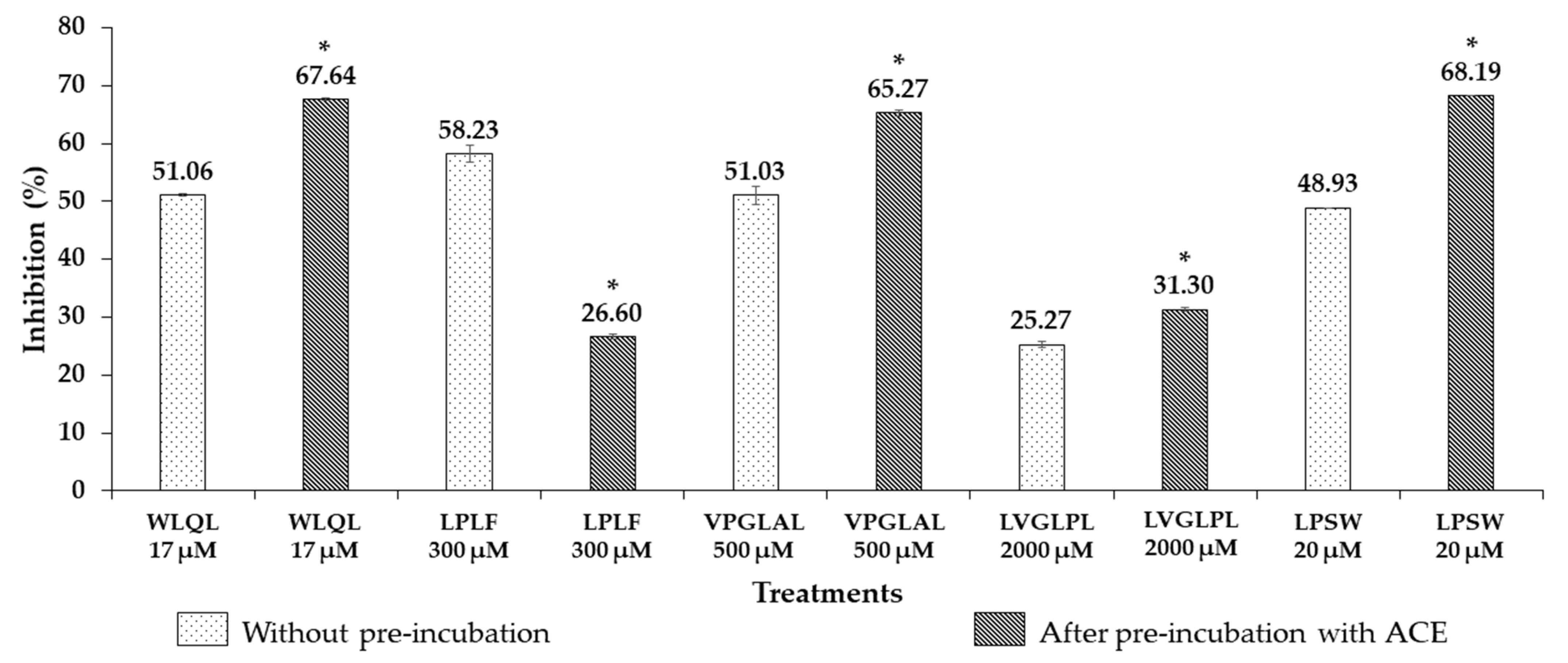
3.3. Stability of ACEI Peptides against ACE
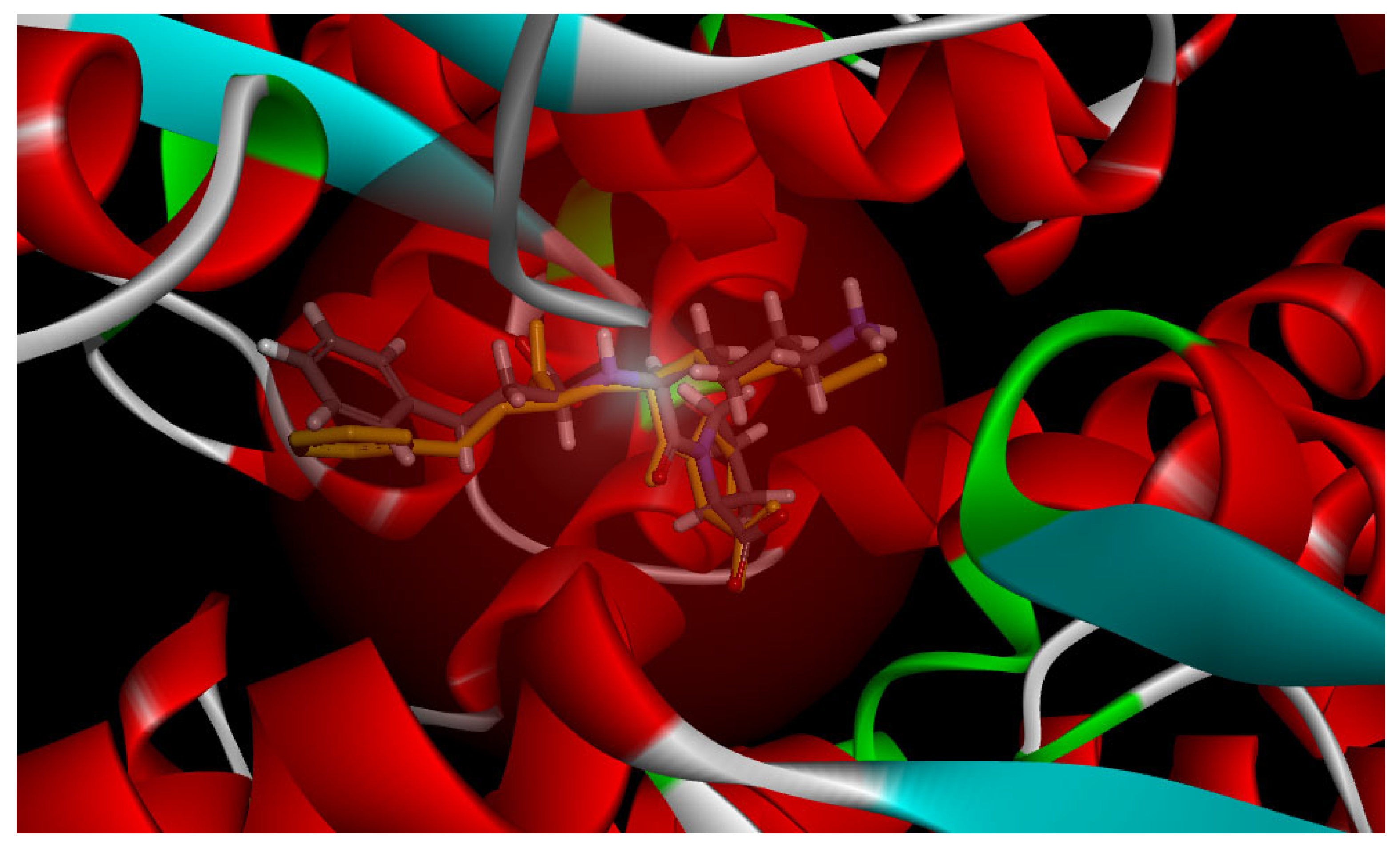
3.4. Molecular Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Majumder, K.; Wu, J. Molecular targets of antihypertensive peptides: Understanding the mechanisms of action based on the pathophysiology of hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Mendys, P.M.; Kelly, S.P.; Chen, L.Y.; Soliman, E.Z. Relationship between high blood pressure, atrial cardiopathy, and mortality in the general population. Am. J. Hypertens. 2022. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Zhuang, Y.; Li, Y.; Shi, P.; Tian, H.; Li, X.; Chen, X. Isolation of novel ACE-inhibitory peptide from naked oat globulin hydrolysates in silico approach: Molecular docking, in vivo antihypertension and effects on renin and intracellular endothelin-1. J. Food Sci. 2020, 85, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. In vitro antioxidant and angiotensin-converting enzyme inhibitory activity of fermented milk with different culture combinations. J. Dairy Sci. 2020, 103, 1120–1130. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Flack, J.M.; Ito, S.; Muntner, P.; Webb, R.C. Hypertension and COVID-19. Am. J. Hypertens. 2022, 33, 373–374. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chen, F.-A.; Wang, L.-F.; Hsu, J.-L. Discovery and study of novel antihypertensive peptides derived from Cassia obtusifolia seeds. J. Agric. Food Chem. 2019, 67, 7810–7820. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Chamata, Y.; Watson, K.A.; Jauregi, P. Whey-derived peptides interactions with ACE by molecular docking as a potential predictive tool of natural ACE inhibitors. Int. J. Mol. Sci. 2020, 21, 864. [Google Scholar] [CrossRef]
- Salim, M.A.S.M.; Gan, C.-Y. Dual-function peptides derived from egg white ovalbumin: Bioinformatics identification with validation using in vitro assay. J. Funct. Foods 2020, 64, 103618. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Liao, W.; Jiang, X.; Wu, J. Identification and characterization of gastrointestinal-resistant angiotensin-converting enzyme inhibitory peptides from egg white proteins. J. Agric. Food Chem. 2019, 67, 7147–7156. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, B.; Gong, L.; Wu, X.; Zhang, Y. Studies on bioactive peptide from Chinese soft-shelled turtle (Pelodiscus sinensis) with functionalities of ACE inhibition and antioxidation. Afr. J. Biotechnol. 2012, 11, 6723–6729. [Google Scholar]
- Huanling, Y.; Yong, L.; Junbo, W.; Liping, Z.; Weixing, Y. Chinese soft-shelled turtle egg powder lowers serum cholesterol, increases faecal neutral steroids and bile acid excretion, and up-regulates liver cytochrome P450 mRNA level in rats. Br. J. Nutr. 2005, 94, 315–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiu, L.H.; Hsu, G.S.W.; Lu, Y.F. Antihypertensive capacity of defatted soft-shelled turtle powder after hydrolysis by gastrointestinal enzymes. J. Food Biochem. 2006, 30, 589–603. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Chen, S.-H.; Chang, C.-I.; Shih, W.-L.; Huang, T.-C.; Liao, M.-H.; Hsu, J.-L. Isolation and characterization of a novel angiotensin-converting enzyme-inhibitory tripeptide from enzymatic hydrolysis of soft-shelled turtle (Pelodiscus sinensis) egg white: In vitro, in vivo, and in silico study. J. Agric. Food Chem. 2014, 62, 12178–12185. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Chang, C.-I.; Chen, H.-H.; Huang, T.-C.; Hsu, J.-L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteomics 2013, 94, 359–369. [Google Scholar] [CrossRef]
- Pujiastuti, D.Y.; Shih, Y.-H.; Chen, W.-L.; Hsu, J.-L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from soft-shelled turtle yolk using two orthogonal bioassay-guided fractionations. J. Funct. Foods 2017, 28, 36–47. [Google Scholar] [CrossRef]
- Nong, N.T.P.; Chen, Y.-K.; Shih, W.-L.; Hsu, J.-L. Characterization of Novel Dipeptidyl Peptidase-IV Inhibitory Peptides from Soft-Shelled Turtle Yolk Hydrolysate Using Orthogonal Bioassay-Guided Fractionations Coupled with In Vitro and In Silico Study. Pharmaceuticals 2020, 13, 308. [Google Scholar] [CrossRef]
- Abouelkheir, M.; El-Metwally, T.H. Dipeptidyl peptidase-4 inhibitors can inhibit angiotensin converting enzyme. Eur. J. Pharmacol. 2019, 862, 172638. [Google Scholar] [CrossRef]
- Gomez, H.L.R.; Peralta, J.P.; Tejano, L.A.; Chang, Y.-W. In silico and in vitro assessment of portuguese oyster (Crassostrea angulata) proteins as precursor of bioactive peptides. Int. J. Mol. Sci. 2019, 20, 5191. [Google Scholar] [CrossRef]
- Sasaki, C.; Tamura, S.; Tohse, R.; Fujita, S.; Kikuchi, M.; Asada, C.; Nakamura, Y. Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochem. 2019, 77, 137–142. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE inhibitory peptides derived from simulated gastrointestinal digestion in vitro of sesame (Sesamum indicum L.) protein and molecular docking study. Int. J. Mol. Sci. 2020, 21, 1059. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.; Sabo, E.; Ondetti, M. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Joel, C.H.; Sutopo, C.C.; Prajitno, A.; Su, J.-H.; Hsu, J.-L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef] [PubMed]
- Sutopo, C.C.; Sutrisno, A.; Wang, L.-F.; Hsu, J.-L. Identification of a potent angiotensin-I converting enzyme inhibitory peptide from black cumin seed hydrolysate using orthogonal bioassay-guided fractionations coupled with in silico screening. Process Biochem. 2020, 95, 204–213. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.; Sturrock, E.; Acharya, K. Crystal Structure of Human Angiotensin Converting Enzyme in complex with lisinopril. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, characterization and evaluation of inhibitory mechanism of ACE inhibitory peptides from pearl oyster (Pinctada fucata martensii) meat protein hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Purification and identification of novel ACE inhibitory and ACE2 upregulating peptides from spent hen muscle proteins. Food Chem. 2021, 345, 128867. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- Cheung, B.M.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Nong, N.T.P.; Hsu, J.-L. Characteristics of Food Protein-Derived Antidiabetic Bioactive Peptides: A Literature Update. Int. J. Mol. Sci. 2021, 22, 9508. [Google Scholar] [CrossRef]
- Chen, M.; Pan, D.; Zhou, T.; Gao, X.; Dang, Y. Novel Umami Peptide IPIPATKT with Dual Dipeptidyl Peptidase-IV and Angiotensin I-Converting Enzyme Inhibitory Activities. J. Agric. Food Chem. 2021, 69, 5463–5470. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, D.; Chen, X.; Tang, Y.-J.; Wang, L.; Xie, J.; Wei, D. Inhibitory mechanism of a substrate-type angiotensin I-converting enzyme inhibitory peptide. Process Biochem. 2019, 79, 97–104. [Google Scholar] [CrossRef]
- Lunow, D.; Kaiser, S.; Rückriemen, J.; Pohl, C.; Henle, T. Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme. Food Chem. 2015, 166, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lunow, D.; Kaiser, S.; Brückner, S.; Gotsch, A.; Henle, T. Selective release of ACE-inhibiting tryptophan-containing dipeptides from food proteins by enzymatic hydrolysis. Eur. Food Res. Technol. 2013, 237, 27–37. [Google Scholar] [CrossRef]
- Rao, S.-Q.; Liu, S.; Ju, T.; Xu, W.-Q.; Mei, G.-M.; Xu, Y.-S.; Yang, Y.-J. Design of substrate-type ACE inhibitory pentapeptides with an antepenultimate C-terminal proline for efficient release of inhibitory activity. Biochem. Eng. J. 2012, 60, 50–55. [Google Scholar] [CrossRef]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Li, J.; Su, J.; Chen, M.; Chen, J.; Ding, W.; Li, Y.; Yin, H. Two novel potent ACEI peptides isolated from Pinctada fucata meat hydrolysates using in silico analysis: Identification, screening and inhibitory mechanisms. RCS Adv. 2021, 11, 12172–12182. [Google Scholar] [CrossRef]
- Ruiz, J.Á.G.; Ramos, M.; Recio, I. Angiotensin converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int. Dairy J. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Salmaso, V.; Moro, S. Bridging molecular docking to molecular dynamics in exploring ligand-protein recognition process: An overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Tian, C.; Huo, J.; Ding, Z.; Xu, R.; Zhu, J.; Yu, L.; Villarreal, O.D. Design and evaluation of four novel tripeptides as potent angiotensin converting enzyme (ACE) inhibitors with anti-hypertension activity. Peptides 2019, 122, 170171. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Hao, Y.; Zhang, W.; Zhou, G. Antihypertensive effects in vitro and in vivo of novel angiotensin-converting enzyme inhibitory peptides from bovine bone gelatin hydrolysate. J. Agric. Food Chem. 2019, 68, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, L.; Min, W.; Liu, J.; Li, H. Exploration of the molecular interactions between angiotensin-I-converting enzyme (ACE) and the inhibitory peptides derived from hazelnut (Corylus heterophylla Fisch.). Food Chem. 2018, 245, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Wang, M.; Zhang, B.; Wang, E.; Liu, B.; Zhang, T. Construction and application of membrane-bound angiotensin-I converting enzyme system: A new approach for the evaluation of angiotensin-I converting enzyme inhibitory peptides. J. Agric. Food Chem. 2020, 68, 5723–5731. [Google Scholar] [CrossRef]
- Khedr, S.; Martin, M.; Deussen, A. Inhibitory efficacy and biological variability of tryptophan containing dipeptides on human plasma angiotensin converting enzyme activity. J. Hypertens. 2015, 4. [Google Scholar] [CrossRef]
- Chen, Y.-C. Beware of docking! Trends Pharmacol. Sci. 2015, 36, 78–95. [Google Scholar] [CrossRef]

| Peptide | Molecular Mass (Da) | Length | DPP-IV IC50 (µM) [18] | ACE IC50 (µM) | Mode of ACE Inhibition | Stability of Peptide with ACE | ACEI IC50 (µM) after Pre-Incubation with ACE |
|---|---|---|---|---|---|---|---|
| WLQL | 559 | 4 | 432.5 ± 11.85 | 16.87 ± 0.54 | Competitive | Hydrolyzed | 8.5 ± 0.86 |
| LPSW | 502 | 4 | 269.7 ± 15.91 | 20.80 ± 0.79 | Non-competitive | Hydrolyzed | 13.39 ± 0.88 |
| LPLF | 489 | 4 | 463.6 ± 5.52 | 300.08 ± 17.30 | Competitive | Hydrolyzed | nd |
| VPGLAL | 569 | 6 | 289.2 ± 11.85 | 573.00 ± 54.10 | Competitive | Hydrolyzed | nd |
| LVGLPL | 611 | 6 | >2000 | >2000 | nd | Not hydrolyzed | nd |
| Peptide | Catalytic Parameter * | Concentration (µM) | ||
|---|---|---|---|---|
| LPSW | 0 µM | 17 µM | 23 µM | |
| Vmax | 6.99 | 4.65 | 3.76 | |
| Km | 5.06 | 5.84 | 5.13 | |
| WLQL | 0 µM | 15 µM | 25 µM | |
| Vmax | 1.47 | 1.44 | 1.42 | |
| Km | 1.57 | 2.48 | 3.24 | |
| VPGLAL | 0 µM | 485 µM | 630 µM | |
| Vmax | 7.19 | 8.06 | 7.17 | |
| Km | 7.02 | 19.64 | 20.83 | |
| LPLF | 0 µM | 250 µM | 320 µM | |
| Vmax | 7.52 | 6.15 | 7.59 | |
| Km | 4.83 | 9.19 | 13.99 | |
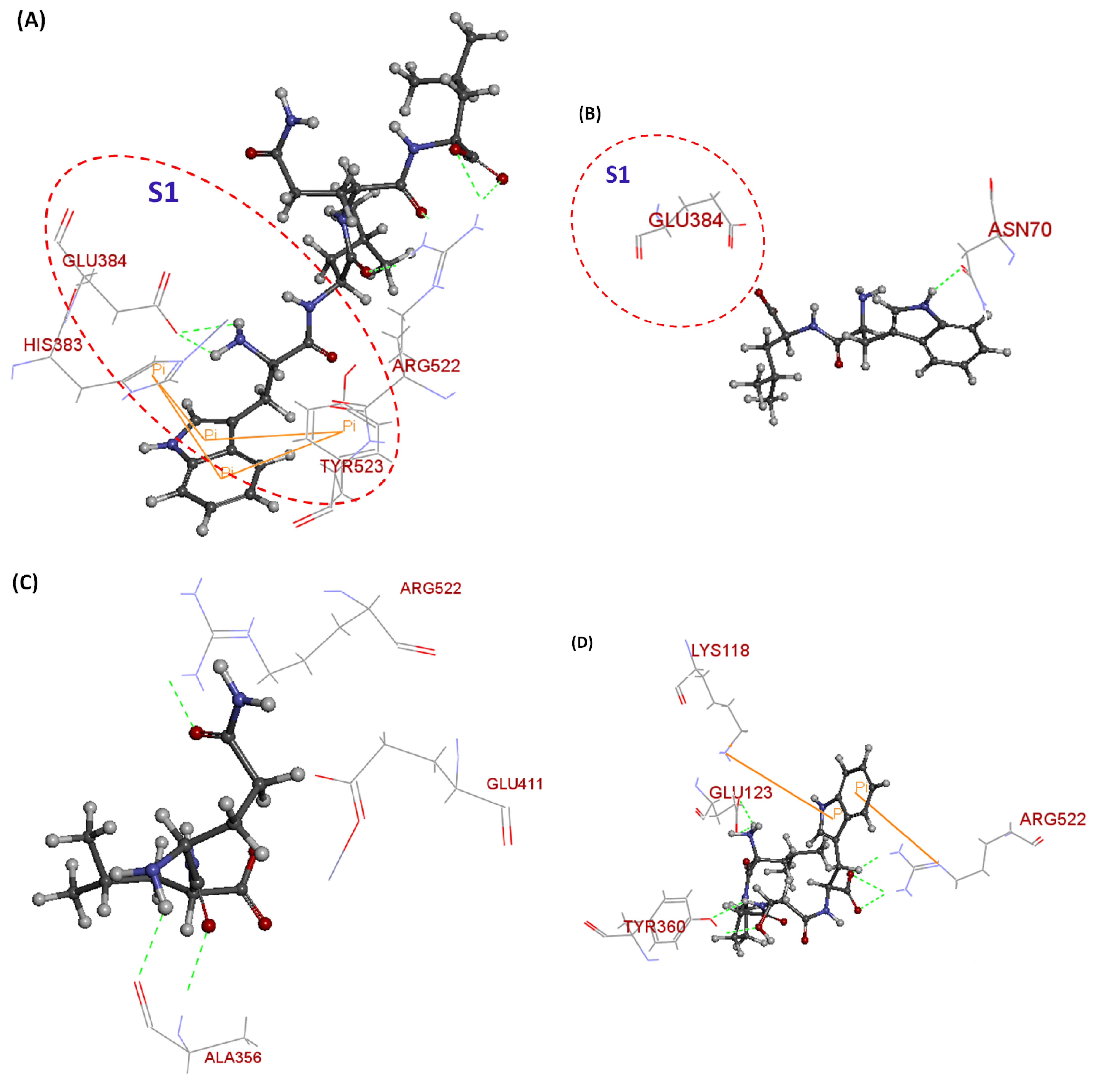
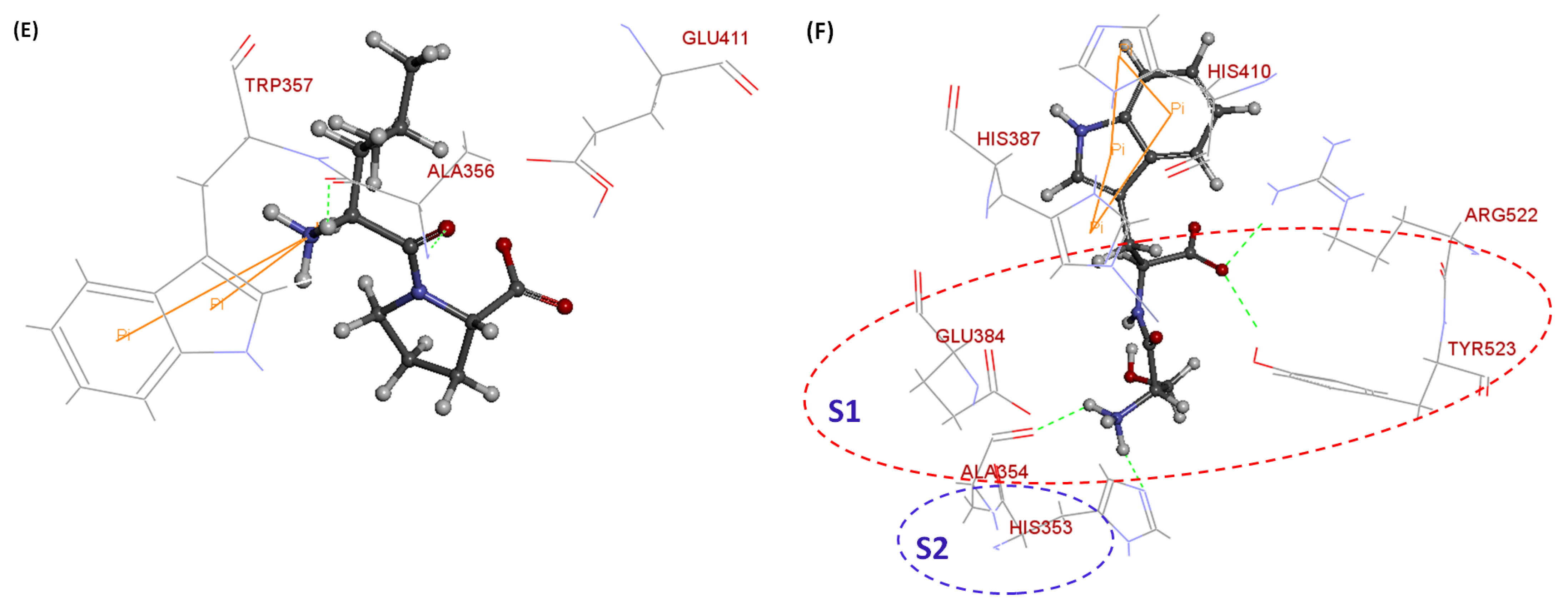
| Sequences | Potential Binding Site | Total Binding Sites | Number of H-Bonds | Number of Pi-Pi Bonds | CDOCKER Energy (kJ/mol) |
|---|---|---|---|---|---|
| WLQL | Tyr523, Arg522, Glu384, His383 | 9 | 5 | 4 | 80.4321 |
| LPSW | Tyr360, Lys118, Glu123, Arg522 | 8 | 6 | 2 | 55.1801 |
| WL | Glu384, Asn70 | 2 | 2 | 0 | 58.8494 |
| QL | Arg522, Ala356, Glu411 | 4 | 4 | 0 | 62.3901 |
| LP | Trp357, Ala356, Glu411 | 4 | 3 | 1 | 53.3389 |
| SW | Glu384, His353, Tyr523, Arg522, His387, His410, Ala354 | 10 | 6 | 4 | 61.9276 |
| tACE Residues Involved in H-Bonding b | No. of H-Bonds and Corresponding Distance (Å) | |||||
|---|---|---|---|---|---|---|
| WLQL | LPSW | WL | QL | LP | SW | |
| Arg522: O | 2; 2; 2.2 | 1.9; 2.1 | − | 2.4 | − | 2.1; 2.5 |
| Glu384: H | 2.2; 2.4 | − | − | − | − | − |
| Glu384: O | − | − | 3.3 | − | − | − |
| Glu384: N | − | − | − | − | − | 3.7 |
| Asn70: H | − | − | 2.0 | − | − | − |
| Glu411: O | − | − | − | 2.9 | 3 | − |
| Ala356: OH | − | − | 2; 2.1 | 1.7; 1.9 | − | |
| Glu123: NH | − | 2; 2.7 | − | − | − | − |
| Tyr360: H | − | 2.2; 2.2 | − | − | − | − |
| Ala354: H | − | − | − | − | − | 1.9 |
| His353: H | − | − | − | − | − | 2.2 |
| Tyr523: O | − | − | − | − | − | 2.2 |
| Total | 5 | 6 | 2 | 4 | 3 | 6 |
| tACE Residues | No. of pi-pi Bonds and Corresponding Distance (Å) | |||||
|---|---|---|---|---|---|---|
| WLQL | LPSW | WL | QL | LP | SW | |
| Tyr523: H | 5; 6.3 | − | − | − | − | − |
| His383: H | 4; 4.3 | − | − | − | − | − |
| Arg522: H | − | 6.8 | − | − | − | − |
| Lys118: H | − | 6.8 | − | − | − | − |
| Trp357: H | − | − | − | − | 4.9 | |
| His387: H | − | − | − | − | − | 4.4; 5.4 |
| His410: H | − | − | − | − | − | 4.2; 4.7 |
| Total | 4 | 2 | 0 | 0 | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nong, N.T.P.; Sutopo, C.C.Y.; Hung, W.-T.; Wu, P.-H.; Hsu, J.-L. The Molecular Docking and Inhibition Kinetics of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Soft-Shelled Turtle Yolk. Appl. Sci. 2022, 12, 12340. https://doi.org/10.3390/app122312340
Nong NTP, Sutopo CCY, Hung W-T, Wu P-H, Hsu J-L. The Molecular Docking and Inhibition Kinetics of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Soft-Shelled Turtle Yolk. Applied Sciences. 2022; 12(23):12340. https://doi.org/10.3390/app122312340
Chicago/Turabian StyleNong, Nhung Thi Phuong, Christoper Caesar Yudho Sutopo, Wei-Ting Hung, Ping-Hsun Wu, and Jue-Liang Hsu. 2022. "The Molecular Docking and Inhibition Kinetics of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Soft-Shelled Turtle Yolk" Applied Sciences 12, no. 23: 12340. https://doi.org/10.3390/app122312340
APA StyleNong, N. T. P., Sutopo, C. C. Y., Hung, W.-T., Wu, P.-H., & Hsu, J.-L. (2022). The Molecular Docking and Inhibition Kinetics of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Soft-Shelled Turtle Yolk. Applied Sciences, 12(23), 12340. https://doi.org/10.3390/app122312340









