Development of Polyamide 6 (PA6)/Polycaprolactone (PCL) Thermoplastic Self-Healing Polymer Blends for Multifunctional Structural Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Techniques
2.3.1. Rheological Properties
2.3.2. Microstructural Properties
2.3.3. Chemical Properties
2.3.4. Thermal Properties
2.3.5. Mechanical Properties
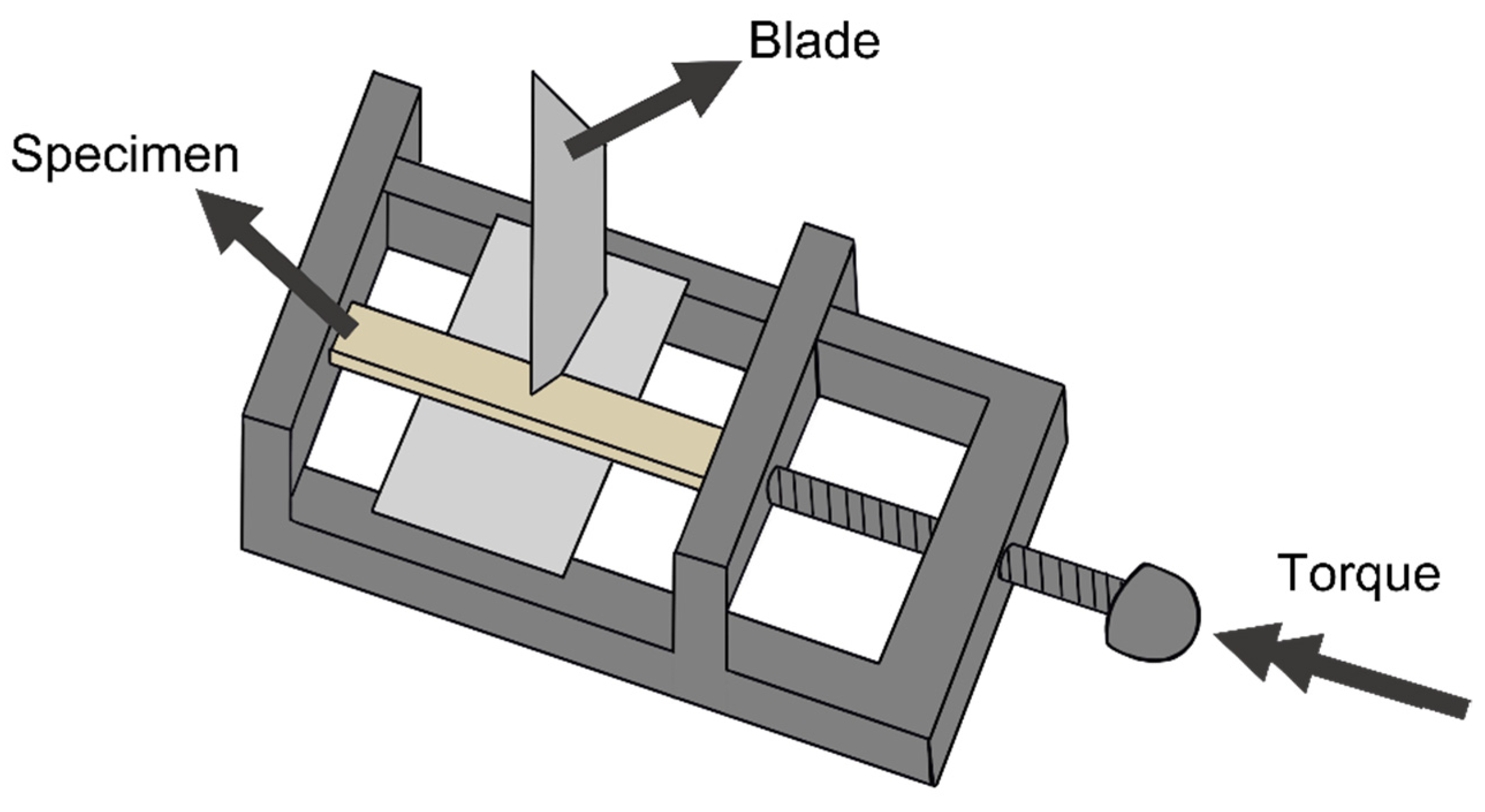
2.3.6. Evaluation of the Healing Efficiency
3. Results and Discussions
3.1. Rheological Properties
3.2. Microstructural Properties
3.3. Chemical Properties
3.4. Thermal Properties
3.5. Mechanical Properties
3.6. Evaluation of Healing Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, T.K.; Ghosh, P.; Das, N.C. Preparation, development, outcomes, and application versatility of carbon fiber-based polymer composites: A review. Adv. Compos. Hybrid Mater. 2019, 2, 214–233. [Google Scholar] [CrossRef]
- Fafenrot, S.; Korger, M.; Ehrmann, A. 20-Mechanical properties of composites from textiles and three-dimensional printed materials. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 409–425. [Google Scholar]
- Papadopoulos, G.A.; Papanicolaou, G.C. Dynamic crack propagation in rubber-modified composite models. J. Mater. Sci. 1988, 23, 3421–3434. [Google Scholar] [CrossRef]
- Katnam, K.B.; Comer, A.J.; Roy, D.; da Silva, L.F.M.; Young, T.M. Composite Repair in Wind Turbine Blades: An Overview. J. Adhes. 2015, 91, 113–139. [Google Scholar] [CrossRef]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Self-healing composites: A state-of-the-art review. Compos. Part A Appl. Sci. Manuf. 2019, 121, 474–486. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, N.; Rabnawaz, M. Covalent Adaptable Network and Self-Healing Materials: Current Trends and Future Prospects in Sustainability. Polymers 2020, 12, 2027. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, S.; Brinkman, E. Self Healing Materials: Pioneering Research in the Netherlands; IOS Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Mozafari, M. Basics of self-healing composite materials. In Self-Healing Composite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–31. [Google Scholar]
- Idumah, C.I. Recent advancements in self-healing polymers, polymer blends, and nanocomposites. Polym. Polym. Compos. 2020, 29, 246–258. [Google Scholar] [CrossRef]
- Billiet, S.; Hillewaere, X.K.D.; Teixeira, R.F.A.; Du Prez, F.E. Chemistry of Crosslinking Processes for Self-Healing Polymers. Macromol. Rapid Commun. 2013, 34, 290–309. [Google Scholar] [CrossRef]
- Bekas, D.G.; Tsirka, K.; Baltzis, D.; Paipetis, A.S. Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques. Compos. Part B Eng. 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Luterbacher, R.; Coope, T.S.; Trask, R.S.; Bond, I.P. Vascular self-healing within carbon fibre reinforced polymer stringer run-out configurations. Compos. Sci. Technol. 2016, 136, 67–75. [Google Scholar] [CrossRef]
- Wang, Y.; Pham, D.T.; Ji, C. Self-healing composites: A review. Cogent Eng. 2015, 2, 1075686. [Google Scholar] [CrossRef] [Green Version]
- Banshiwal, J.K.; Tripathi, D.N. Self-healing polymer composites for structural application. In Functional Materials; Sahu, D.R., Ed.; Intechopen: London, UK, 2019. [Google Scholar]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Wool, R.P.; O’Connor, K.M. A theory crack healing in polymers. J. Appl. Phys. 1981, 52, 5953–5963. [Google Scholar] [CrossRef]
- Herbst, F.; Döhler, D.; Michael, P.; Binder, W.H. Self-Healing Polymers via Supramolecular Forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Swait, T.J.; Rauf, A.; Grainger, R.; Bailey, P.B.S.; Lafferty, A.D.; Fleet, E.J.; Hand, R.J.; Hayes, S.A. Smart composite materials for self-sensing and self-healing. Plast. Rubber Compos. 2012, 41, 215–224. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar]
- Karger-Kocsis, J. Self-healing properties of epoxy resins with poly(ε-caprolactone) healing agent. Polym. Bull. 2016, 73, 3081–3093. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Hailstone, R.; Lewis, C.L. Thermoplastic Blend Exhibiting Shape Memory-Assisted Self-Healing Functionality. ACS Appl. Mater. Interfaces 2020, 12, 46733–46742. [Google Scholar] [CrossRef]
- Bernatas, R.; Dagréou, S.; Despax-Ferreres, A.; Barasinski, A. Recycling of fiber reinforced composites with a focus on thermoplastic composites. Clean. Eng. Technol. 2021, 5, 100272. [Google Scholar] [CrossRef]
- Li, H.; Englund, K. Recycling of carbon fiber-reinforced thermoplastic composite wastes from the aerospace industry. J. Compos. Mater. 2016, 51, 1265–1273. [Google Scholar] [CrossRef]
- Stewart, R. Thermoplastic composites-recyclable and fast to process. Reinf. Plast. 2011, 55, 22–28. [Google Scholar] [CrossRef]
- Luo, X.; Ou, R.; Eberly, D.E.; Singhal, A.; Viratyaporn, W.; Mather, P.T. A Thermoplastic/Thermoset Blend Exhibiting Thermal Mending and Reversible Adhesion. ACS Appl. Mater. Interfaces 2009, 1, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Cohades, A.; Manfredi, E.; Plummer, C.J.G.; Michaud, V. Thermal mending in immiscible poly(ε-caprolactone)/epoxy blends. Eur. Polym. J. 2016, 81, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Fan, P.; Ren, J.; Cheng, Y.; Ren, J.; Zhao, J.; Song, R. Self-healing thermoplastic polyurethane (TPU)/polycaprolactone (PCL) /multi-wall carbon nanotubes (MWCNTs) blend as shape-memory composites. Compos. Sci. Technol. 2018, 168, 255–262. [Google Scholar] [CrossRef]
- Lai, S.-M.; Liu, J.-L.; Huang, Y.-H. Preparation of Self-healing Natural Rubber/Polycaprolactone (NR/PCL) Blends. J. Macromol. Sci. Part B 2020, 59, 587–607. [Google Scholar] [CrossRef]
- Nguyen-Tran, H.-D.; Hoang, V.-T.; Do, V.-T.; Chun, D.-M.; Yum, Y.-J. Effect of Multiwalled Carbon Nanotubes on the Mechanical Properties of Carbon Fiber-Reinforced Polyamide-6/Polypropylene Composites for Lightweight Automotive Parts. Materials 2018, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic Self-Healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef]
- Dorigato, A.; Mahmood, H.; Pegoretti, A. Optimization of the thermal mending process in epoxy/cyclic olefin copolymer blends. J. Appl. Polym. Sci. 2021, 138, 49937. [Google Scholar] [CrossRef]
- Millot, C.; Fillot, L.-A.; Lame, O.; Sotta, P.; Seguela, R. Assessment of polyamide-6 crystallinity by DSC. J. Therm. Anal. Calorim. 2015, 122, 307–314. [Google Scholar] [CrossRef]
- Gupta, B.; Geeta; Ray, A.R. Preparation of poly(ε-caprolactone)/poly(ε-caprolactone-co-lactide) (PCL/PLCL) blend filament by melt spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Wang, J.F.; Carson, J.K.; North, M.F.; Cleland, D.J. A knotted and interconnected skeleton structural model for predicting Young’s modulus of binary phase polymer blends. Polym. Eng. Sci. 2010, 50, 643–651. [Google Scholar] [CrossRef]
- Zovi, R.C.; Mahmood, H.; Dorigato, A.; Fredi, G.; Pegoretti, A. Cyclic Olefin Copolymer Interleaves for Thermally Mendable Carbon/Epoxy Laminates. Molecules 2020, 25, 5347. [Google Scholar] [CrossRef] [PubMed]
- Sadiku-Agboola, O.; Sadiku, E.R.; Adegbola, A.T.; Biotidara, O.F. Rheological Properties of Polymers: Structure and Morphology of Molten Polymer Blends. Mater. Sci. Appl. 2011, 2, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Khalili, R.; Jafari, S.H.; Saeb, M.R.; Khonakdar, H.A.; Wagenknecht, U.; Heinrich, G. Toward In Situ Compatibilization of Polyolefin Ternary Blends through Morphological Manipulations. Macromol. Mater. Eng. 2014, 299, 1197–1212. [Google Scholar] [CrossRef]
- Poslinski, A.J.; Ryan, M.E.; Gupta, R.K.; Seshadri, S.G.; Frechette, F.J. Rheological Behavior of Filled Polymeric Systems I. Yield Stress and Shear-Thinning Effects. J. Rheol. 1988, 32, 703–735. [Google Scholar] [CrossRef]
- Lee, H.M.; Park, O.O. Rheology and dynamics of immiscible polymer blends. J. Rheol. 1994, 38, 1405–1425. [Google Scholar] [CrossRef]
- Butnaru, I.; Fernández-Ronco, M.P.; Czech-Polak, J.; Heneczkowski, M.; Bruma, M.; Gaan, S. Effect of Meltable Triazine-DOPO Additive on Rheological, Mechanical, and Flammability Properties of PA6. Polymers 2015, 7, 1541–1563. [Google Scholar] [CrossRef] [Green Version]
- Salehiyan, R.; Ray, S.S.; Stadler, F.J.; Ojijo, V. Rheology–Microstructure Relationships in Melt-Processed Polylactide/Poly(vinylidene Fluoride) Blends. Materials 2018, 11, 2450. [Google Scholar] [CrossRef] [Green Version]
- Codou, A.; Anstey, A.; Misra, M.; Mohanty, A.K. Novel compatibilized nylon-based ternary blends with polypropylene and poly(lactic acid): Morphology evolution and rheological behaviour. RSC Adv. 2018, 8, 15709–15724. [Google Scholar] [CrossRef] [Green Version]
- Han, C.D.; Kim, J. Rheological technique for determining the order–disorder transition of block copolymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1741–1764. [Google Scholar] [CrossRef]
- Chopra, D.; Kontopoulou, M.; Vlassopoulos, D.; Hatzikiriakos, S.G. Effect of maleic anhydride content on the rheology and phase behavior of poly(styrene-co-maleic anhydride)/poly(methyl methacrylate) blends. Rheol. Acta 2002, 41, 10–24. [Google Scholar]
- Jafari, S.H.; Pötschke, P.; Stephan, M.; Warth, H.; Alberts, H. Multicomponent blends based on polyamide 6 and styrenic polymers: Morphology and melt rheology. Polymer 2002, 43, 6985–6992. [Google Scholar] [CrossRef]
- Lamnawar, K.; Vion-Loisel, F.; Maazouz, A. Rheological, morphological, and heat seal properties of linear low density polyethylene and cyclo olefine copolymer (LLDPE/COC) blends. J. Appl. Polym. Sci. 2010, 116, 2015–2022. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, Morphological and Mechanical Studies of Sustainably Sourced Polymer Blends Based on Poly(Lactic Acid) and Polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yin, B.; Zhou, Y.; Gong, L.; Yang, M.B.; Xie, B.; Chen, C. Characterization of PA6/EPDM-g-MA/HDPE ternary blends: The role of core-shell structure. Polymer 2012, 53, 3043–3051. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, M.; Song, Y.; Yan, X.; Zheng, Q. Dynamic rheology and dielectric relaxation of poly(vinylidene fluoride)/poly(methyl methacrylate) blends. Compos. Sci. Technol. 2015, 106, 39–46. [Google Scholar] [CrossRef]
- Kwag, H.; Rana, D.; Cho, K.; Rhee, J.; Woo, T.; Lee, B.H.; Choe, S. Binary blends of metallocene polyethylene with conventional polyolefins: Rheological and morphological properties. Polym. Eng. Sci. 2000, 40, 1672–1681. [Google Scholar] [CrossRef]
- Dell′Erba, R.; Groeninckx, G.; Maglio, G.; Malinconico, M.; Migliozzi, A. Immiscible polymer blends of semicrystalline biocompatible components: Thermal properties and phase morphology analysis of PLLA/PCL blends. Polymer 2001, 42, 7831–7840. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L.A.; Shi, Z.H. Development of polymer blend morphology during compounding in a twin-screw extruder. Part I: Droplet dispersion and coalescence—A review. Polym. Eng. Sci. 1992, 32, 1824–1833. [Google Scholar] [CrossRef]
- Rossato, J.H.H.; Lemos, H.G.; Mantovani, G.L. The influence of viscosity and composition of ABS on the ABS/SBS blend morphology and properties. J. Appl. Polym. Sci. 2019, 136, 47075. [Google Scholar] [CrossRef]
- Marischal, L.; Cayla, A.; Lemort, G.; Campagne, C.; Devaux, É. Selection of Immiscible Polymer Blends Filled with Carbon Nanotubes for Heating Applications. Polymers 2019, 11, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, J.T.; Wei, W.; Tsai, F.C.; Lai, Y.C. Drawing Properties of Modified Polyamide 6 Fibers. Adv. Mater. Res. 2011, 189–193, 3031–3034. [Google Scholar] [CrossRef]
- Cakir, S.; Jasinska-Walc, L.; Villani, M.; Hansen, M.R.; Koning, C.E. Morphology and local chain structure of polyamide 6 modified in the solid state with a semi-aromatic nylon salt. Mater. Today Commun. 2015, 2, e62–e69. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, K.W.; Pesapane, A.; Kim, H.Y.; Rabolt, J.F. Polarized FT-IR Study of Macroscopically Oriented Electrospun Nylon-6 Nanofibers. Macromolecules 2008, 41, 1494–1498. [Google Scholar] [CrossRef]
- Colthup, N.B. Infrared Spectroscopy. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 793–816. [Google Scholar]
- Benkaddour, A.; Jradi, K.; Robert, S.; Daneault, C. Grafting of Polycaprolactone on Oxidized Nanocelluloses by Click Chemistry. Nanomaterials 2013, 3, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Finkenstadt, V.L.; Gordon, S.H.; Biresaw, G.; Palmquist, D.E.; Rayas-Duarte, P. Thermal properties of PCL/gluten bioblends characterized by TGA, DSC, SEM, and infrared-PAS. J. Appl. Polym. Sci. 2008, 110, 3256–3266. [Google Scholar] [CrossRef]
- Parodi, E.; Govaert, L.E.; Peters, G.W.M. Glass transition temperature versus structure of polyamide 6: A flash-DSC study. Thermochim. Acta 2017, 657, 110–122. [Google Scholar] [CrossRef]
- Amestoy, H.; Diego, P.; Meaurio, E.; Muñoz, J.; Sarasua, J.-R. Crystallization Behavior and Mechanical Properties of Poly(ε-caprolactone) Reinforced with Barium Sulfate Submicron Particles. Materials 2021, 14, 2368. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A. Mechanical Properties of Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Wilkie, C.A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1203–1297. [Google Scholar]
- Kılıçoğlu, M.; Bat, E.; Gündüz, G.; Yıldırım, M.U.; Urgun, K.; Maviş, B. Fibers of thermoplastic polymer blends activate multiple interlayer toughening mechanisms. Compos. Part A Appl. Sci. Manuf. 2022, 158, 106982. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Paul, D.R. Notched impact behavior of polymer blends: Part 1: New model for particle size dependence. Polymer 2009, 50, 5539–5548. [Google Scholar] [CrossRef] [Green Version]
- Bucknall, C.B.; Paul, D.R. Notched impact behaviour of polymer blends: Part 2: Dependence of critical particle size on rubber particle volume fraction. Polymer 2013, 54, 320–329. [Google Scholar] [CrossRef]
- Argon, A.S.; Cohen, R.E. Toughenability of polymers. Polymer 2003, 44, 6013–6032. [Google Scholar] [CrossRef]
- Bucknall, C.B. Deformation mechanisms in rubber-toughened polymers. In Polymer Blends Volume 2: Performance; Paul, D.R., Bucknall, C.B., Eds.; Wiley-Interscience: New York, NY, USA, 2000; pp. 83–117. [Google Scholar]
- Chen, Z.; Zhou, Y.; Wu, Y.; Liu, S.; Huang, H.; Zhao, J. Fluorinated polyimide with polyhedral oligomeric silsesquioxane aggregates: Toward low dielectric constant and high toughness. Compos. Sci. Technol. 2019, 181, 107700. [Google Scholar] [CrossRef]
- Musteață, A.; Pelin, G.; Botan, M.; Popescu, A.; Deleanu, L. The Behavior of Polymeric Blends (PP + PA6) in Tensile Tests. Mater. Plast. 2020, 57, 153–166. [Google Scholar] [CrossRef]
- Dorigato, A.; Rigotti, D.; Pegoretti, A. Novel Poly(Caprolactone)/Epoxy Blends by Additive Manufacturing. Materials 2020, 13, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]











| First Heating Scan | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Tm PCL (a) [°C] | ΔHm PCL (b) [J/g] | χ PCL(c) [%] | Tm1PA6 (d) [°C] | Tm2 PA6 (e) [°C] | ΔHm PA6 (f) [J/g] | χ PA6 (g) [%] |
| PA6 | - | - | - | 219.8 | 226.3 | 79.8 | 34.7 |
| PA6_5PCL | 60.3 | 6.2 | 89.2 | 217.6 | 224.2 | 67.3 | 30.8 |
| PA6_10PCL | 62.4 | 10.9 | 78.1 | - | 225.2 | 78.0 | 37.7 |
| PA6_20PCL | 63.7 | 20.7 | 74.1 | 220.0 | 225.9 | 55.6 | 30.2 |
| PA6_30PCL | 64.6 | 26.3 | 62.7 | 220.3 | 226.8 | 54.3 | 33.8 |
| PCL | 66.8 | 74.9 | 53.7 | - | - | - | - |
| Cooling Scan | |||||||
| Sample | Tc PA6 (h) [°C] | ΔHc PA6 (i) [J/g] | χ PA6 [%] | Tc PCL(j) [°C] | ΔHc PCL (k) [J/g] | χ PCL [%] | Tg(l)PCL [°C] |
| PA6 | 190.7 | 89.7 | 39.0 | - | - | - | - |
| PA6_5PCL | 190.5 | 80.2 | 36.7 | 30.3 | 2.4 | 34.4 | - |
| PA6_10PCL | 190.3 | 75.0 | 36.2 | 30.7 | 5.7 | 40.9 | - |
| PA6_20PCL | 190.5 | 67.9 | 36.9 | 29.7 | 12.3 | 44.1 | - |
| PA6_30PCL | 190.6 | 61.9 | 38.4 | 28.5 | 17.7 | 42.3 | - |
| PCL | - | - | - | 23.3 | 62.0 | 44.4 | -61.0 |
| Second Heating Scan | |||||||
| Sample | Tm PCL [°C] | ΔHm PCL [J/g] | χ PCL [%] | Tm1PA6 [°C] | Tm2PA6 [°C] | ΔHm PA6 [J/g] | χ PA6 [%] |
| PA6 | - | - | 42.4 | - | 224.3 | 90.1 | 39.2 |
| PA6_5PCL | 53.5 | 3.0 | 40.7 | - | 224.2 | 79.9 | 36.6 |
| PA6_10PCL | 54.1 | 5.7 | 41.6 | 218.6 | 224.9 | 75.8 | 36.6 |
| PA6_20PCL | 54.3 | 11.6 | 39.3 | 217.9 | 224.3 | 71.2 | 38.7 |
| PA6_30PCL | 54.4 | 16.4 | 52.5 | 217.8 | 224.4 | 62.6 | 38.9 |
| PCL | 54.5 | 73.3 | - | - | - | - | - |
| Sample | T1%(a) [°C] | T5% (b) [°C] | TD PCL (c) [°C] | mD PCL(d) [%] | TD PA6 (e) [°C] | mD PA6 (f) [%] |
|---|---|---|---|---|---|---|
| PA6 | 326.5 | 398.3 | - | - | 461.5 | 32.9 |
| PA6_5PCL | 278.0 | 390.0 | 419.5 | 81.1 | 459.3 | 25.4 |
| PA6_10PCL | 251.3 | 391.5 | 432.3 | 62.3 | 468.7 | 18.2 |
| PA6_20PCL | 283.7 | 390.2 | 423.7 | 58.7 | 463.1 | 9.0 |
| PA6_30PCL | 227.8 | 384.3 | 416.8 | 52.9 | 457.3 | 5.7 |
| PCL | 249.3 | 389.2 | 420.2 | 48.2 | - | - |
| Quasi-Static | Impact | ||
|---|---|---|---|
| Sample | KIC [MPa m1/2] (a) | GIC [kJ/m2] (b) | KIC [MPa m1/2] (c) |
| PA6 | 2.79 ± 0.08 | 2.20 ± 0.14 | 9.44 ± 0.86 |
| PA6_5PCL | 3.34 ± 0.23 | 3.25 ± 0.35 | 9.40 ± 0.87 |
| PA6_10PCL | 3.08 ± 0.31 | 3.33 ± 0.45 | 8.77 ± 1.03 |
| PA6_20PCL | 2.86 ± 0.30 | 3.54 ± 0.77 | 7.86 ± 1.36 |
| PA6_30PCL | 2.24 ± 0.31 | 2.68 ± 0.71 | 7.32 ± 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perin, D.; Odorizzi, G.; Dorigato, A.; Pegoretti, A. Development of Polyamide 6 (PA6)/Polycaprolactone (PCL) Thermoplastic Self-Healing Polymer Blends for Multifunctional Structural Composites. Appl. Sci. 2022, 12, 12357. https://doi.org/10.3390/app122312357
Perin D, Odorizzi G, Dorigato A, Pegoretti A. Development of Polyamide 6 (PA6)/Polycaprolactone (PCL) Thermoplastic Self-Healing Polymer Blends for Multifunctional Structural Composites. Applied Sciences. 2022; 12(23):12357. https://doi.org/10.3390/app122312357
Chicago/Turabian StylePerin, Davide, Giada Odorizzi, Andrea Dorigato, and Alessandro Pegoretti. 2022. "Development of Polyamide 6 (PA6)/Polycaprolactone (PCL) Thermoplastic Self-Healing Polymer Blends for Multifunctional Structural Composites" Applied Sciences 12, no. 23: 12357. https://doi.org/10.3390/app122312357








