Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Sample Preparation for Analysis
2.2.1. Preparation for Stable Isotope Analysis
- (i)
- the water was extracted from meat without isotopic fractionation by a procedure consisting of cryogenic distillation under vacuum [12]. The isotopic values of 2H and 18O were then determined from the extracted water;
- (ii)
- for 13C measurements, the first step of the preparation protocol consisted of drying the meat samples in an oven at 60 °C for 48 h. Each meat sample (5 mg) was then converted to CO2 by dry combustion in excess oxygen at 550 °C for 3 hours.
2.2.2. Sample Digestion Procedure for Elemental Profile Determinations
2.3. Sample Measurements
2.3.1. Stable Isotope Analysis
2.3.2. Elemental Profile Analysis
2.3.3. Statistical Data Processing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tostado, L. Meat consumption: Everyday food and luxury good. In Meat Atlas; European Union: Brussels, Belgium, 2021; Available online: https://eu.boell.org/en/2021/09/07/consumption-everyday-food-and-luxury-good (accessed on 14 November 2022).
- Jagtap, S.; Trollman, H.; Trollman, F.; Garcia-Garcia, G.; Parra-López, C.; Duong, L.; Martindale, W.; Munekata, P.E.S.; Lorenzo, J.M.; Hdaifeh, A.; et al. The Russia-Ukraine Conflict: Its Implications for the Global Food Supply Chains. Foods 2022, 11, 2098. [Google Scholar] [CrossRef]
- Hrbek, V.; Zdenkova, K.; Jilkova, D.; Cermakova, E.; Jiru, M.; Demnerova, K.; Pulkrabova, J.; Hajslova, J. Authentication of Meat and Meat Products Using Triacylglycerols Profiling and by DNA Analysis. Foods 2020, 9, 1269. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Salouros, H.; Prasad, S. Authenticity and geographic origin of global honeys determined using carbon isotope ratios and trace elements. Sci. Rep. 2018, 8, 14639. [Google Scholar] [CrossRef]
- Griboff, J.; Baroni, M.V.; Horacek, M.; Wunderlin, D.A.; Monferran, M.V. Multielemental isotopic fingerprint enables linking soil, water, forage and milk composition, assessing the geographical origin of Argentinean milk. Food Chem. 2019, 283, 549–558. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, T.; Tang, X.; Zhao, S.; Qie, M.; Chen, A.; Yang, S. Authentication of organic pork and identification of geographical origins of pork in four regions of China by combined analysis of stable isotopes and multi-elements. Meat Sci. 2020, 165, 108129. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, C.M.; Hong, J.H.; Jamila, N.; Naeem, K.; Jung, J.H.; Jung, Y.C.; Kyong, S.K. Origin discrimination of defatted pork via trace elements profiling, stable isotope ratios analysis, and multivariate statistical techniques. Meat Sci. 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Dehelean, A.; Cristea, G.; Feher, I.; Hategam, A.R.; Magdas, D.A. Differentiation of Transylvanian fruit distillates using supervised statistical tools based on isotopic and elemental fingerprint. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cristea, G.; Pirnau, A.; Feher, I.; Hategan, A.R.; Dehelean, A. Authentication of Transylvanian Spirits Based on Isotope and Elemental Signatures in Conjunction with Statistical Methods. Foods 2021, 10, 3000. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Cristea, G.; Voica, C.; Feher, I.; Puscas, R.; Magdas, D.A. Isotopic and elemental characterization of Romanian pork meat in corroboration with advanced chemometric methods: A first exploratory study. Meat Sci. 2022, 189, 108825. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for stable isotope ratio analysis 2013 (IUPAC). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Thiem, I.; Lupke, M.; Seifert, H. Factors influencing the 18O/16O-ratio in meat juices. Isot. Environ. Health Stud. 2004, 40, 191–197. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Perșoiu, A.; Bădăluță, C.-A.; Bogdevich, O.; Bănică, S.; Bîrsan, M.-V.; Boengiu, S.; Onaca, A.; Ionita, M. Defining a Precipitation Stable Isotope Framework in the Wider Carpathian Region. Water 2022, 14, 2547. [Google Scholar] [CrossRef]
- Horacek, M.; Eisinger, E.; Papesch, W. Reliability of stable isotope values from meat juice for the determination of the meat origin. Food Chem. 2010, 118, 910–914. [Google Scholar] [CrossRef]
- Rhodes, C.N.; Lofthouse, J.H.; Hird, S.; Rose, P.; Reece, P.; Christy, J.; Macarthur, R.; Brereton, P.A. The use of stable carbon isotopes to authenticate claims that poultry have been corn-fed. Food Chem. 2010, 118, 927–932. [Google Scholar] [CrossRef]
- Islam, M.A.; Young Jeong, J.Y.; Kim, E.J.; Khan, N.; Jamila, N.; Kim, K.S. Multielemental characterization of chicken breasts from conventional and sustainable farms by Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Anal. Lett. 2022, 1–14. [Google Scholar] [CrossRef]
- Pinto, A.M.T.P.; Boeira, A.C.S.; Lisboa, M.T.; Medina, A.L.; Ribeiro, A.S.; Vieira, M.A. Development of an Analytical Method for the Determination of Metals in Chicken Breast by Microwave Induced Plasma Optical Emission Spectrometry (MIP-OES). J. Braz. Chem. Soc. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Bilandzic, N.; Sedak, M.; Calopek, B.; Đokic, M.; Varenina, I.; Kolanovic, B.S.; Luburic, D.B.; Varga, I.; Hruskar, M. Dietary exposure of the adult Croatian population to meat, liver and meat products from the Croatian market: Health risk assessment. J. Food Compost. Anal. 2021, 95, 103672. [Google Scholar] [CrossRef]
- Batista, B.L.; Grotto, D.; Carneiro, M.F.H.; Barbosa, F., Jr. Evaluation of the concentration of nonessential and essential elements in chicken, pork, and beef samples produced in Brazil. J. Toxicol. Environ. Health-A 2012, 75, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Song, O.Y.; Islam, M.A.; Son, J.H.; Jeong, J.Y.; Kim, H.E.; Yeon, L.S.; Khan, N.; Jamila, N.; Kim, K.S. Elemental composition of pork meat from conventional and animal welfare farms by inductively coupled plasma-optical emission spectrometry (ICP-OES) and ICP-mass spectrometry (ICP-MS) and their authentication via multivariate chemometric analysis. Meat Sci. 2021, 172, 108344. [Google Scholar] [CrossRef] [PubMed]
- Tomovic, V.M.; Ljiljana, S.; Petrovic, M.S.; Tomovic, Z.S.; Kevrešan, N.; Dzinic, R. Determination of mineral contents of semimembranosus muscle and liver from pure and crossbred pigs in Vojvodina (northern Serbia). Food Chem. 2011, 124, 342–348. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Yang, S. Effect of organic and conventional rearing system on the mineral content of pork. Meat Sci. 2016, 118, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Heerden, S.M.V.; Smith, M.F. The nutrient composition of three cuts obtained from P-class South African pork carcasses. Food Chem. 2013, 140, 458–465. [Google Scholar] [CrossRef]
- Han, J.L.; Pan, X.D.; Chen, Q. Distribution and safety assessment of heavy metals in fresh meat from Zhejiang, China. Sci. Rep. 2022, 12, 3241. [Google Scholar] [CrossRef]
- Gerber, N.; Brogioli, R.; Hattendorf, B.; Scheeder, M.R.L.; Wenk, C.; Gunther, D. Variability of selected trace elements of different meat cuts determined by ICP-MS and DRC-ICPMS. Animal 2009, 3, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.M.; Gremaud, G.; Hardorn, R.; Kreuzer, M. Geographic origin of meat-elements of an analytical approach to its authentication. Eur. Food Res. Technol. 2005, 221, 493–505. [Google Scholar] [CrossRef]
- Jukna, V.; Valaitiene, V.; Meskinyte-Kausiliene, E.; Jankauskas, A. Comparative evaluation of large white pigs and their crossbreeds meat nutritional value and mineral content. Vet. Med. Zoot. 2013, 62, 44–49. [Google Scholar]
- Wang, X.; Zhang, Y.; Geng, Z.; Liu, Y.; Guo, L.; Xiao, G. Spatial analysis of heavy metals in meat products in China during 2015–2017. Food Control. 2019, 104, 174–180. [Google Scholar] [CrossRef]
- Mazivila, S., Jr.; Gontijo, L.C.; Bachion de Santana, F.; Mitsutake, H.; Santos, D.Q.; Neto, W.B. Fast Detection of Adulterants/Contaminants in Biodiesel/Diesel Blend (B5) Employing Mid-Infrared Spectroscopy and PLS-DA. Energy Fuels 2015, 29, 227–232. [Google Scholar] [CrossRef]
- Bassbasi, M.; De Luca, M.; Ioele, G.; Oussama, A.; Ragno, G. Prediction of the geographical origin of butters by partial least square discriminant analysis (PLS-DA) applied to infrared spectroscopy (FTIR) data. J. Food Compos. Anal. 2014, 33, 210–215. [Google Scholar] [CrossRef]
- Rainey, C.J.; Nyquist, L.A.; Christensen, R.E.; Strong, P.L.; Culver, B.D.; Coughlin, J.R. Daily boron intake from the American diet. J. Am. Diet. Assoc. 1999, 99, 335–340. [Google Scholar] [CrossRef]
- Nielsen, F.H. Boron. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 451–455. ISBN 9780123849533. [Google Scholar] [CrossRef]
- Eisler, R. Boron Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. 1990. Available online: https://www.pwrc.usgs.gov/eisler/CHR_20_Boron.pdf (accessed on 14 November 2022).
- Cristea, G.; Feher, I.; Voica, C.; Radu, S.; Magdas, D.A. Isotopic and elemental profiling alongside with chemometric methods for vegetable differentiation. Isot. Environ. Health Stud. 2020, 56, 69–82. [Google Scholar] [CrossRef]
- Magdas, D.A.; Dehelean, A.; Feher, I.; Radu, S. Isotopic and multielemental fingerprinting of organically and conventionally grown potatoes. Isot. Environ. Health Stud. 2017, 53, 610–619. [Google Scholar] [CrossRef]
- Franke, B.M.; Hadorn, R.; Bosset, J.O.; Gremaud, G.; Kreuzer, M. Is authentication of the geographic origin of poultry meat and dried beef improved by combining multiple trace element and oxygen isotope analysis? Meat Sci. 2008, 80, 944–947. [Google Scholar] [CrossRef]

| Element | Chicken Meat | Pork Meat | ||||
|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | |
| ‰ | ||||||
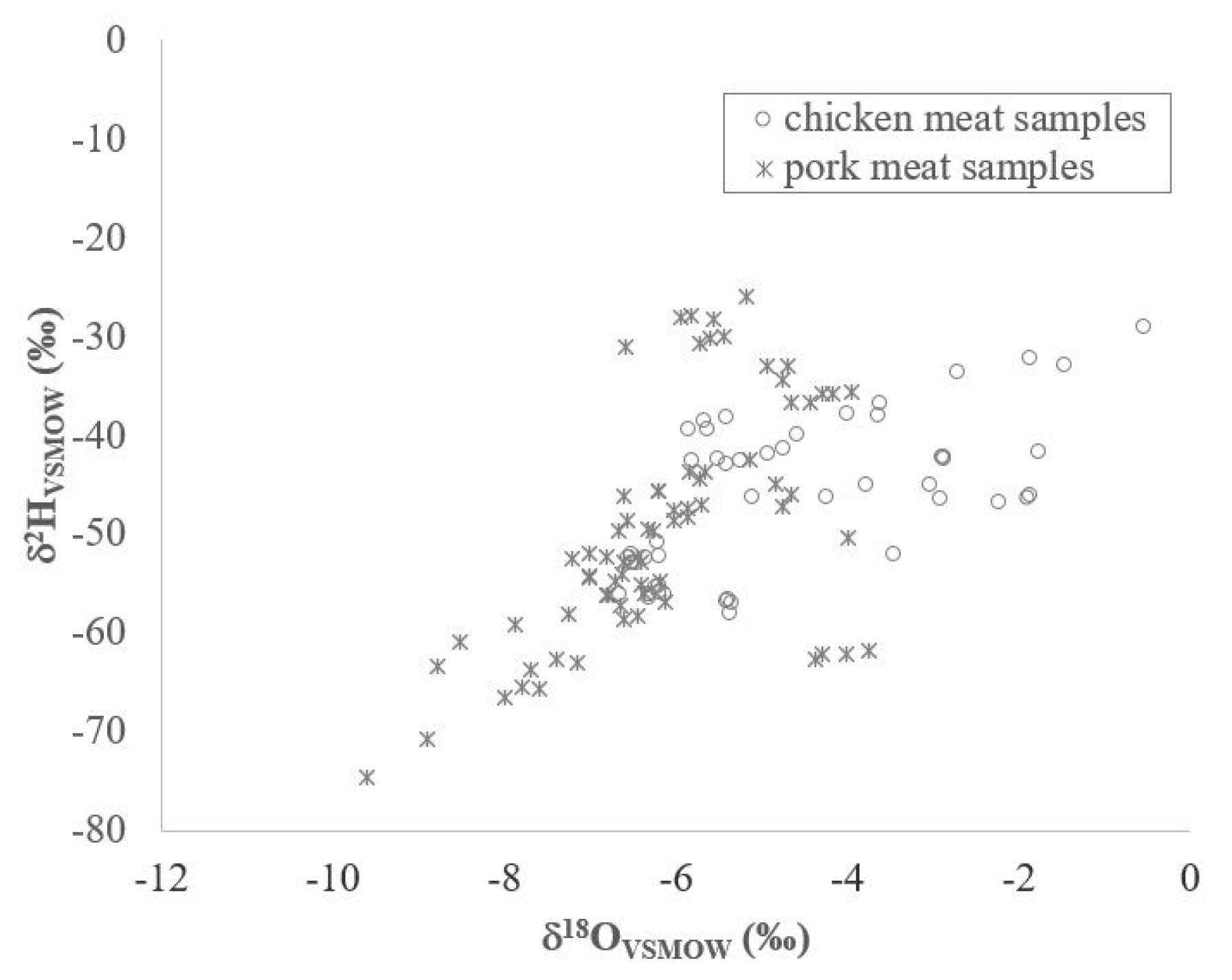
| δ13CVPDB | −22.3 | −12.8 | −19.3 | −24.1 | −14.2 | −19.0 |
| δ2HVSMOW | −58.1 | −29.1 | −46.3 | −74.7 | −25.9 | −49.5 |
| δ18OVSMOW | −6.6 | −0.5 | −4.6 | −9.6 | −3.7 | −6.1 |
| Concentration (mg/kg fresh meat) | ||||||
| K | 774.8 | 7818.7 | 4513.4 | 1093.1 | 4161.3 | 2724.1 |
| Na | 253.2 | 2621.2 | 1037.9 | 303.3 | 5681.1 | 837.7 |
| Mg | 261.1 | 1756.5 | 804.7 | 123.1 | 405.9 | 272.3 |
| Ca | 4.7 | 206.8 | 80.2 | 19.2 | 405.0 | 59.0 |
| Fe | 0.40 | 25.02 | 7.12 | 5.48 | 70.75 | 27.24 |
| Zn | 0.01 | 28.71 | 3.72 | 6.85 | 63.74 | 19.19 |
| Rb | 0.38 | 7.55 | 2.43 | 0.90 | 6.20 | 2.72 |
| Cu | 0.25 | 22.99 | 2.02 | 0.26 | 5.32 | 1.05 |
| B | 0.01 | 0.99 | 0.56 | LOQ | 0.76 | 0.19 |
| Ba | 0.04 | 0.79 | 0.18 | 0.01 | 0.63 | 0.09 |
| Se | 0.04 | 0.41 | 0.10 | 0.01 | 0.44 | 0.26 |
| Cr | 0.004 | 8.64 | 1.70 | 0.37 | 5.48 | 1.79 |
| Ni | 0.003 | 1.45 | 0.36 | 0.13 | 3.41 | 0.48 |
| Mn | 0.001 | 0.68 | 0.17 | 0.09 | 2.06 | 0.23 |
| Mo | <LOQ | 0.14 | 0.05 | LOQ | 0.20 | 0.04 |
| Li | LOQ | 0.34 | 0.03 | LOQ | 0.37 | 0.02 |
| Sr | LOQ | 0.16 | 0.03 | 0.01 | 0.23 | 0.04 |
| V | LOQ | 0.06 | 0.02 | 0.001 | 0.08 | 0.02 |
| Co | LOQ | 0.03 | 0.01 | 0.001 | 0.14 | 0.02 |
| La | LOQ | 0.13 | 0.004 | LOQ | 0.10 | 0.003 |
| Nb | LOQ | 0.01 | 0.004 | LOQ | 0.01 | 0.002 |
| Pd | LOQ | 0.05 | 0.003 | LOQ | 0.21 | 0.01 |
| In | LOQ | 0.01 | 0.001 | LOQ | 0.01 | 0.0006 |
| Pb | LOQ | 4.12 | 0.60 | 0.02 | 1.05 | 0.27 |
| Sn | LOQ | 0.16 | 0.05 | LOQ | 0.17 | 0.04 |
| Sb | LOQ | 0.01 | 0.001 | LOQ | 0.001 | 0.0005 |
| As | LOQ | 0.52 | 0.02 | LOQ | 0.05 | 0.005 |
| Cd | LOQ | 0.05 | 0.002 | LOQ | 0.01 | 0.002 |
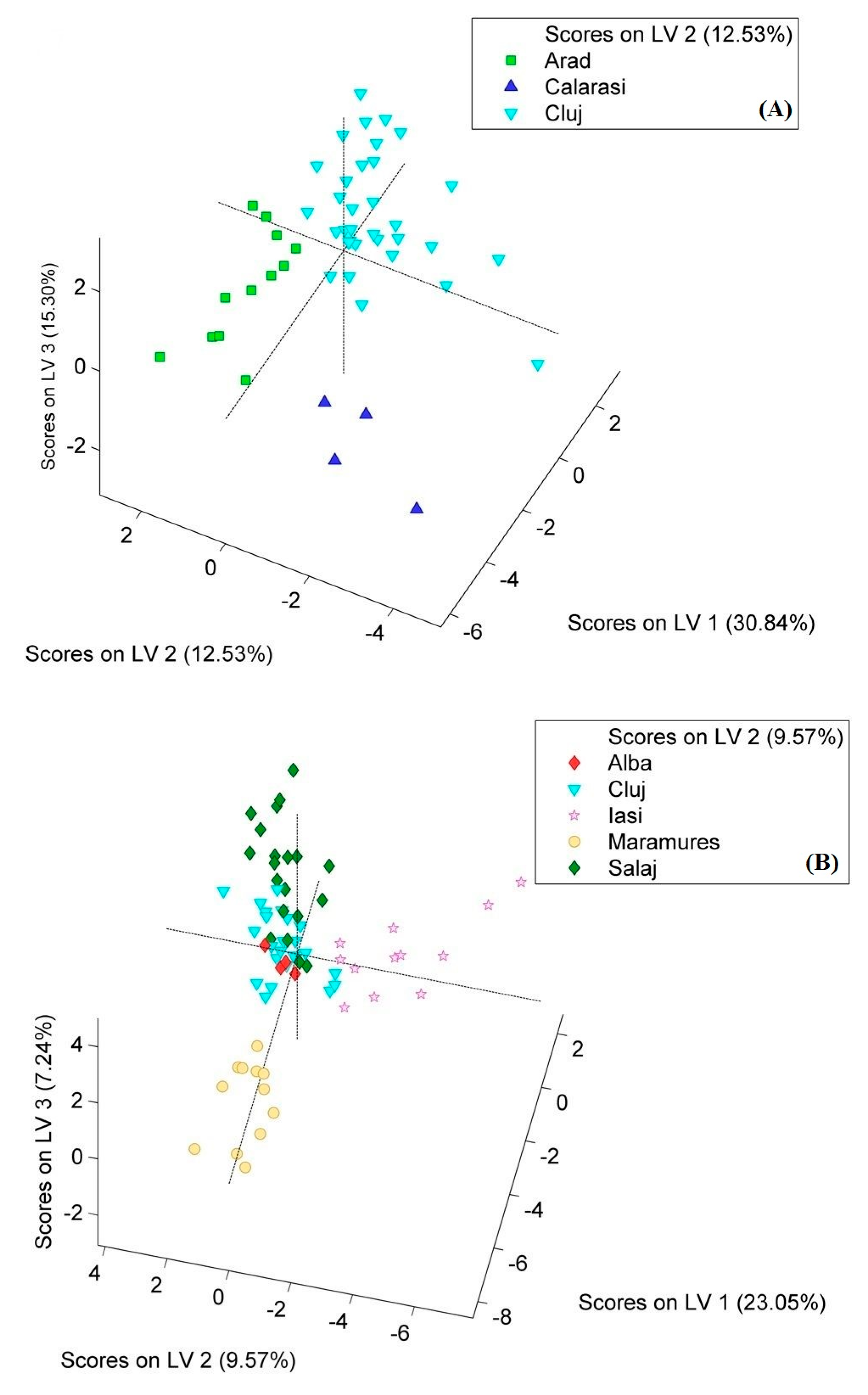
| Meat Models | Origin | RMSEC * | RMSECV ** | Sensitivity Cal | Specificity Cal | Sensitivity CV | Specificity CV |
|---|---|---|---|---|---|---|---|
| chicken | Arad | 0.26 | 0.35 | 1.00 | 0.94 | 0.91 | 0.91 |
| Calarasi | 0.19 | 0.29 | 1.00 | 0.97 | 1.00 | 0.95 | |
| Cluj | 0.25 | 0.30 | 0.97 | 1.00 | 0.97 | 1.00 | |
| pork | Alba | 0.22 | 0.23 | 1.00 | 0.64 | 0.75 | 0.65 |
| Cluj | 0.35 | 0.38 | 0.95 | 0.77 | 0.91 | 0.75 | |
| Iasi | 0.20 | 0.22 | 1.00 | 0.94 | 0.91 | 0.93 | |
| Maramures | 0.10 | 0.12 | 1.00 | 1.00 | 1.00 | 0.98 | |
| Salaj | 0.32 | 0.36 | 0.75 | 0.90 | 0.70 | 0.86 |
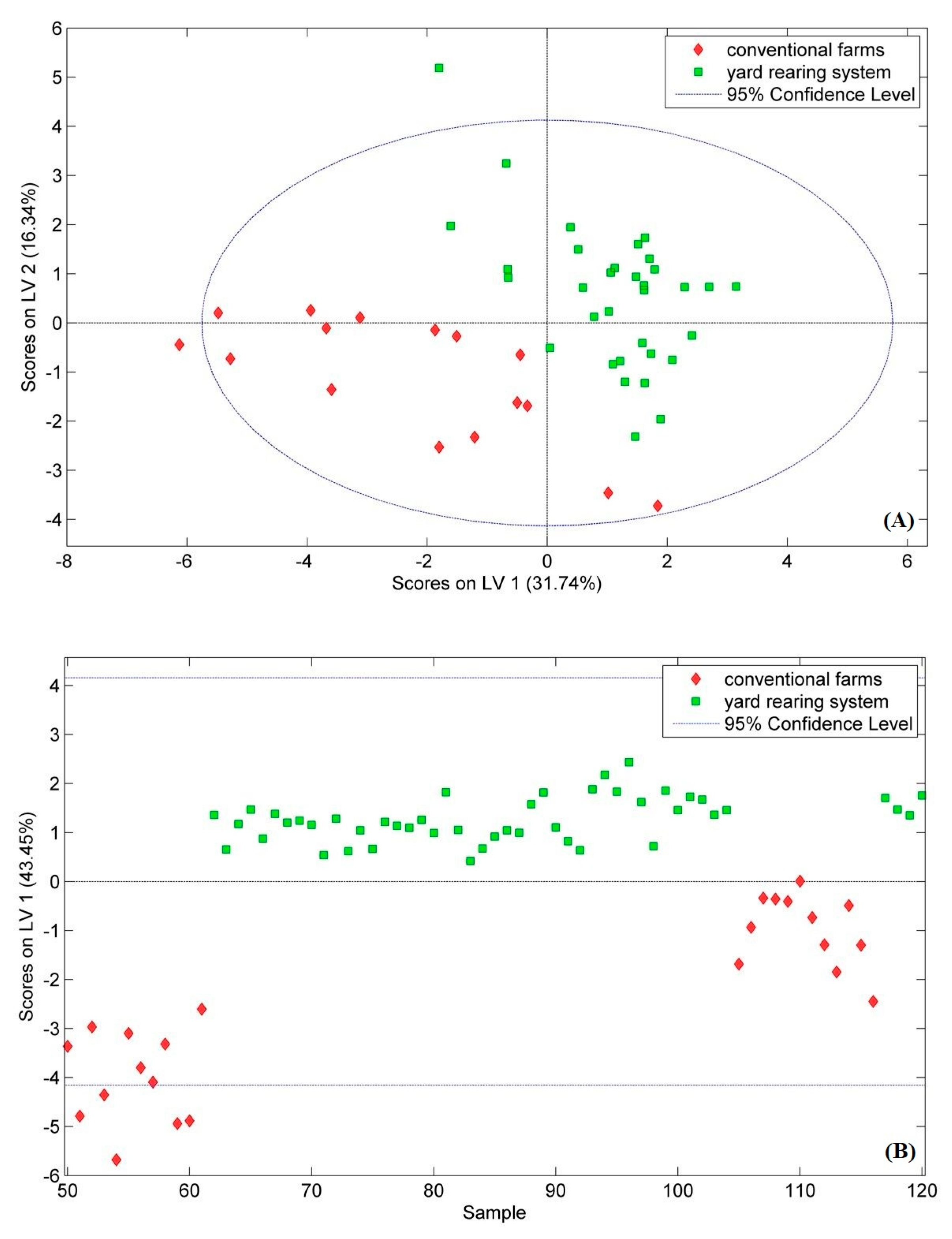
| Parameter | Animal Coming from Yard Rearing System | Animal Coming from Conventional System | ||
|---|---|---|---|---|
| Chicken | Pork | Chicken | Pork | |
| RMSEC * | 0.23 | 0.24 | 0.23 | 0.24 |
| RMSECV ** | 0.26 | 0.24 | 0.26 | 0.24 |
| Sensitivity (Cal) | 0.97 | 1.00 | 1.00 | 1.00 |
| Sensitivity (CV) | 0.97 | 1.00 | 1.00 | 1.00 |
| Specificity (Cal) | 1.00 | 1.00 | 0.97 | 1.00 |
| Sensitivity (CV) | 1.00 | 1.00 | 0.97 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehelean, A.; Cristea, G.; Puscas, R.; Hategan, A.R.; Magdas, D.A. Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints. Appl. Sci. 2022, 12, 12391. https://doi.org/10.3390/app122312391
Dehelean A, Cristea G, Puscas R, Hategan AR, Magdas DA. Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints. Applied Sciences. 2022; 12(23):12391. https://doi.org/10.3390/app122312391
Chicago/Turabian StyleDehelean, Adriana, Gabriela Cristea, Romulus Puscas, Ariana Raluca Hategan, and Dana Alina Magdas. 2022. "Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints" Applied Sciences 12, no. 23: 12391. https://doi.org/10.3390/app122312391
APA StyleDehelean, A., Cristea, G., Puscas, R., Hategan, A. R., & Magdas, D. A. (2022). Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints. Applied Sciences, 12(23), 12391. https://doi.org/10.3390/app122312391








