Influence of Activation Parameters on the Mechanical and Microstructure Properties of an Alkali-Activated BOF Steel Slag
Abstract
:1. Introduction
2. Materials and Methods
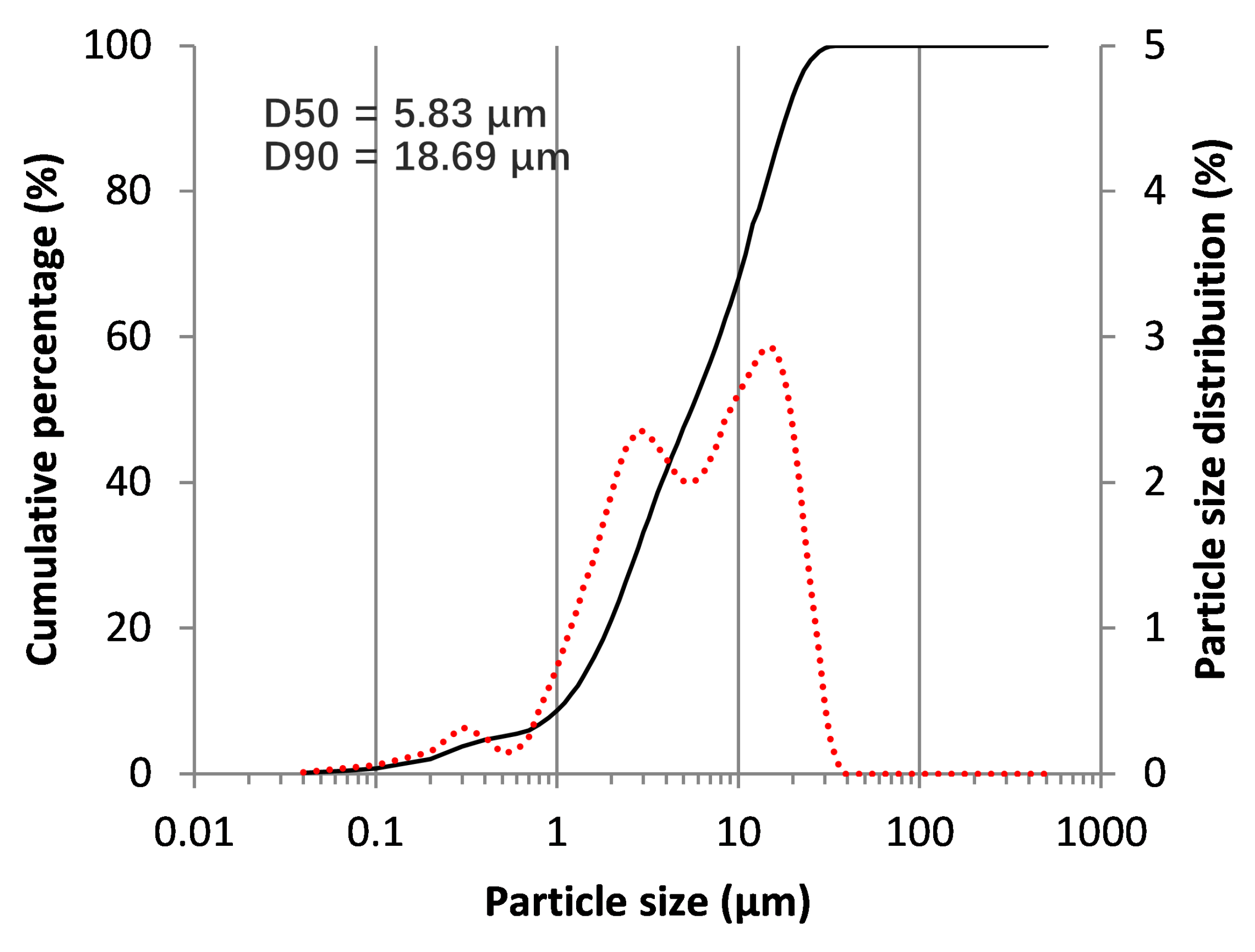
2.1. BOF Slag Characterization
2.2. Mix Proportions
2.3. Early Hydration
2.4. Hardened Properties
2.5. Structural and Microstructural Assessment
3. Results and Discussions
3.1. BOF Slag Pozzolanic/Reactivity Behavior
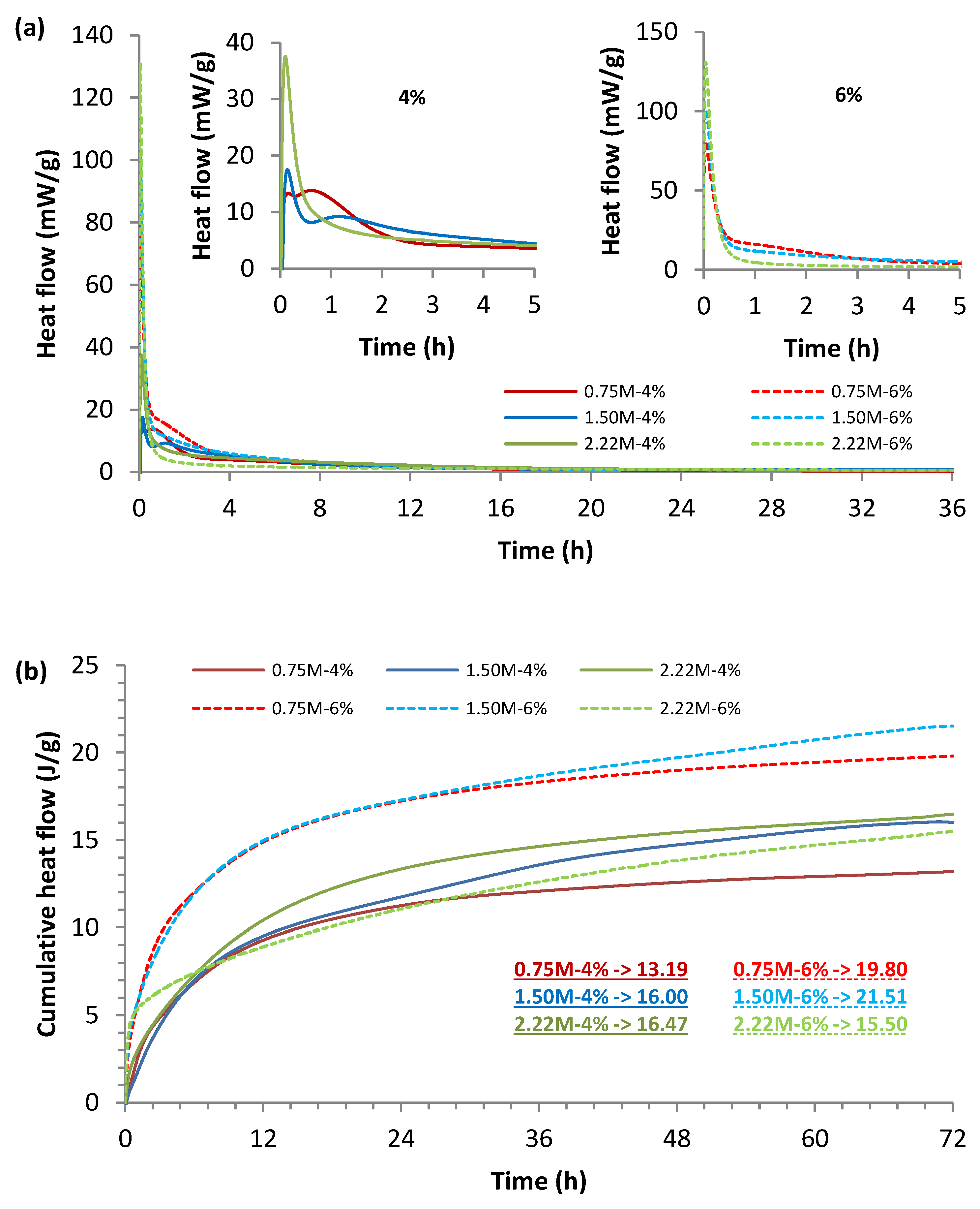
3.2. Early Hydration Results via Calorimetry
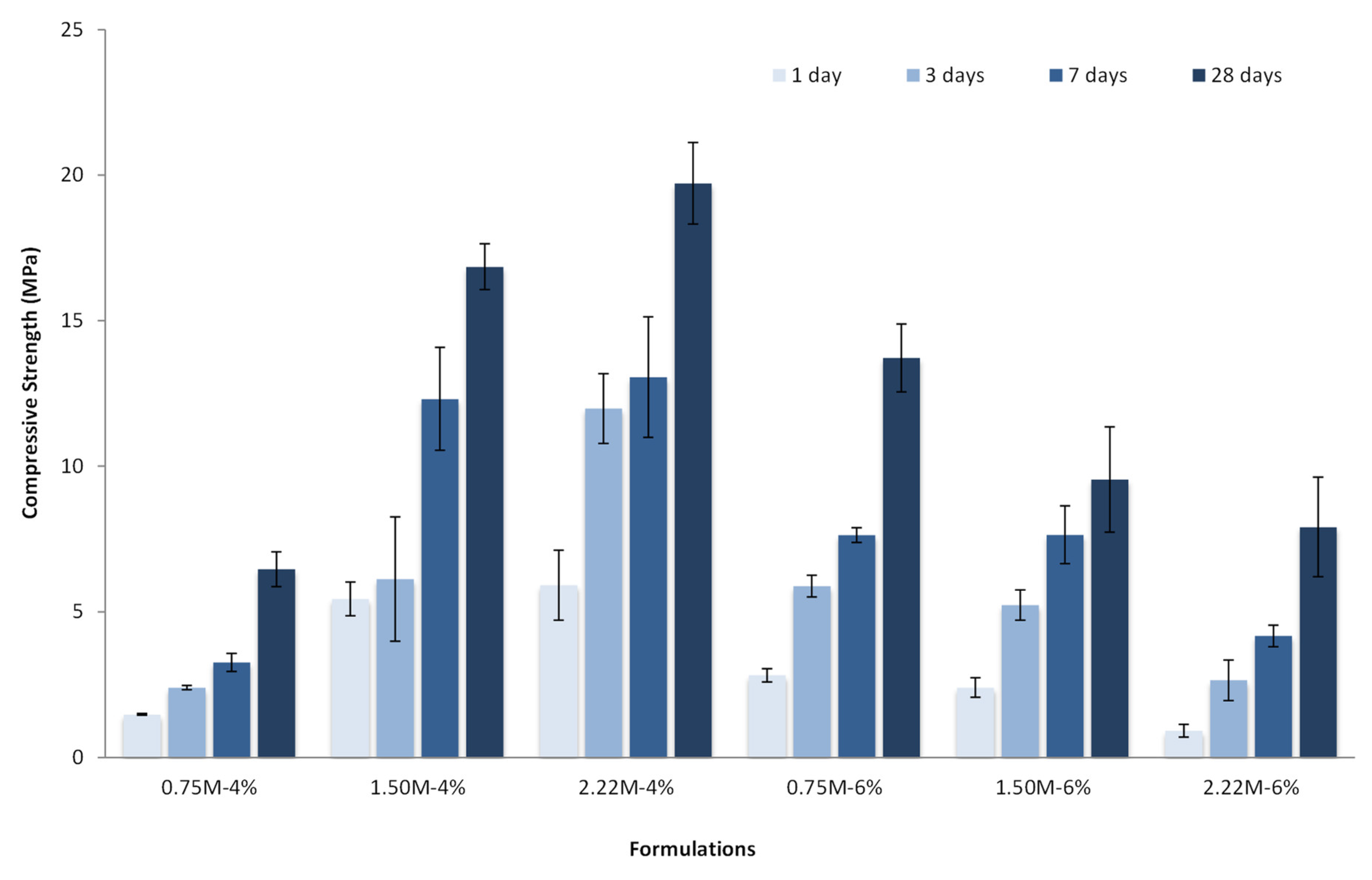
3.3. Hardened Properties
3.4. Structural and Microstructural Properties
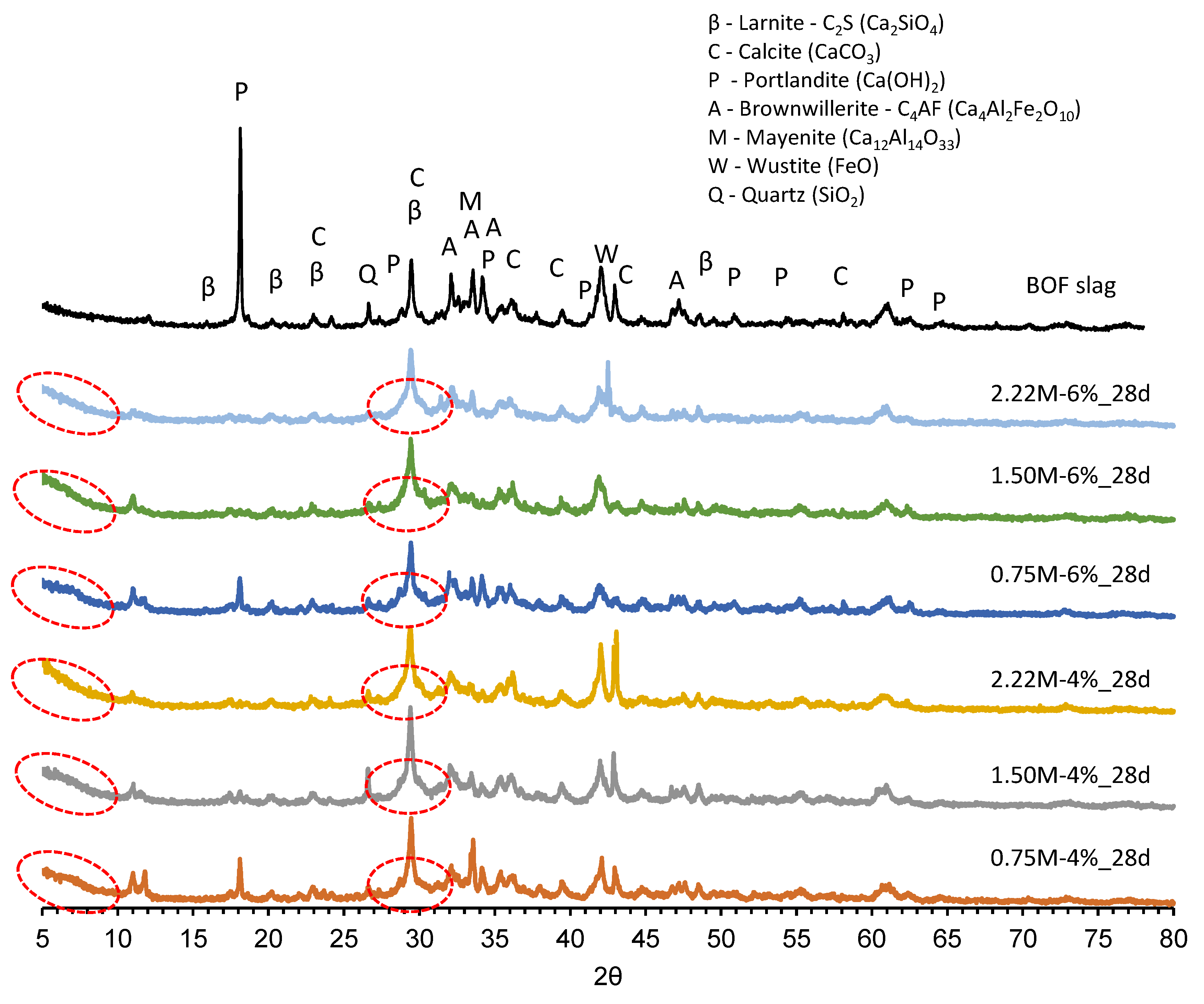
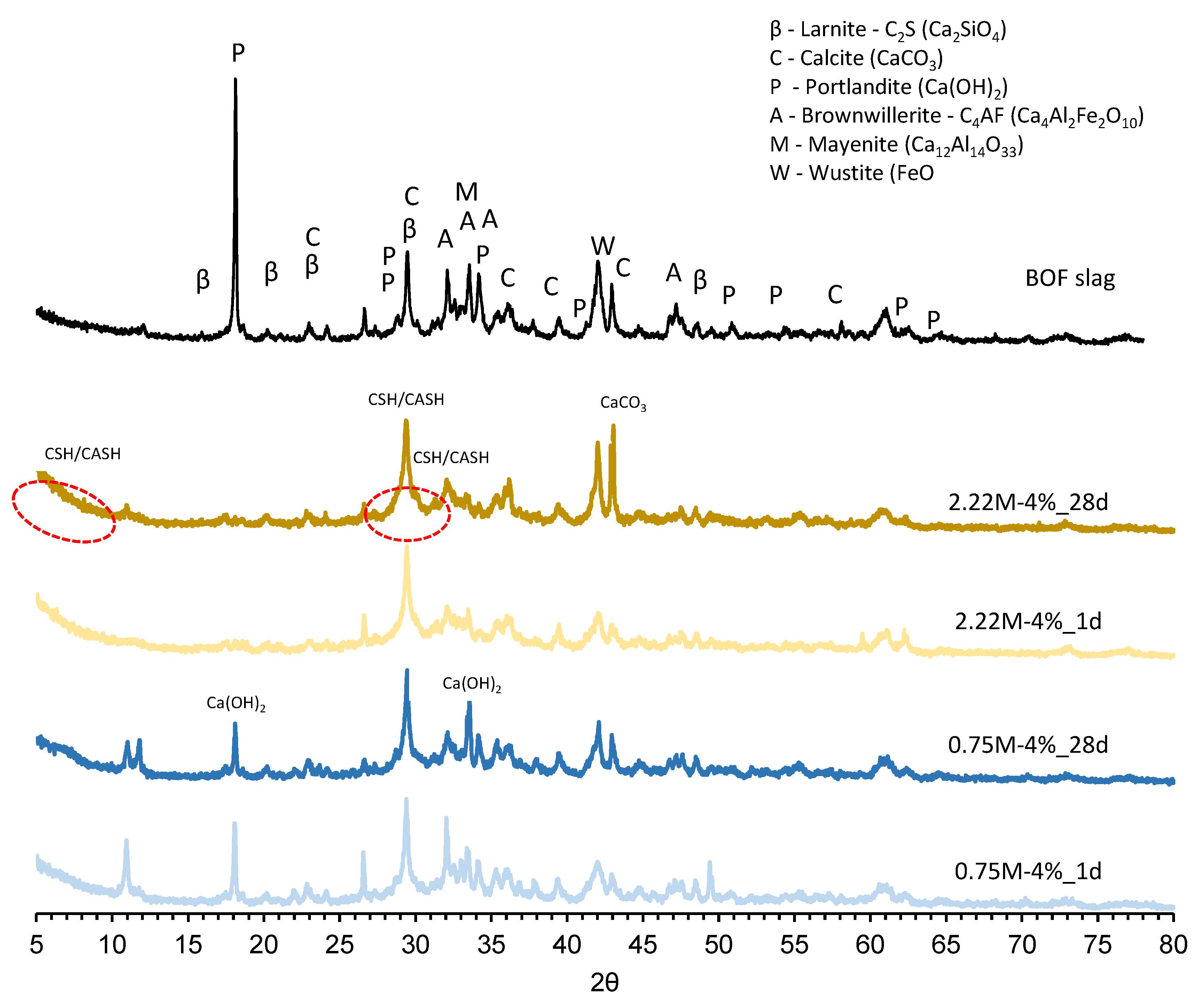
3.4.1. XRD Analysis
3.4.2. TG/DTG Analysis
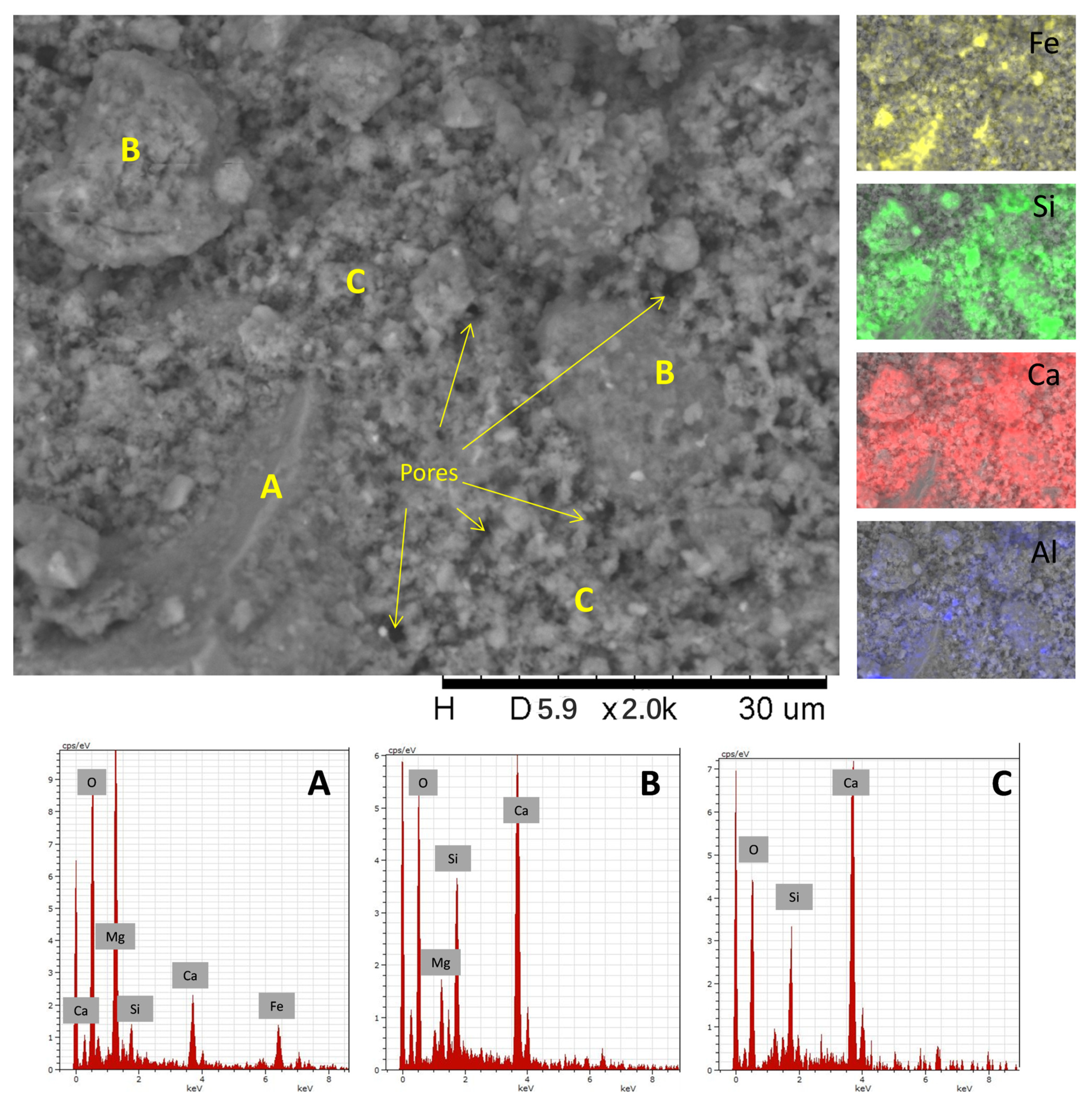
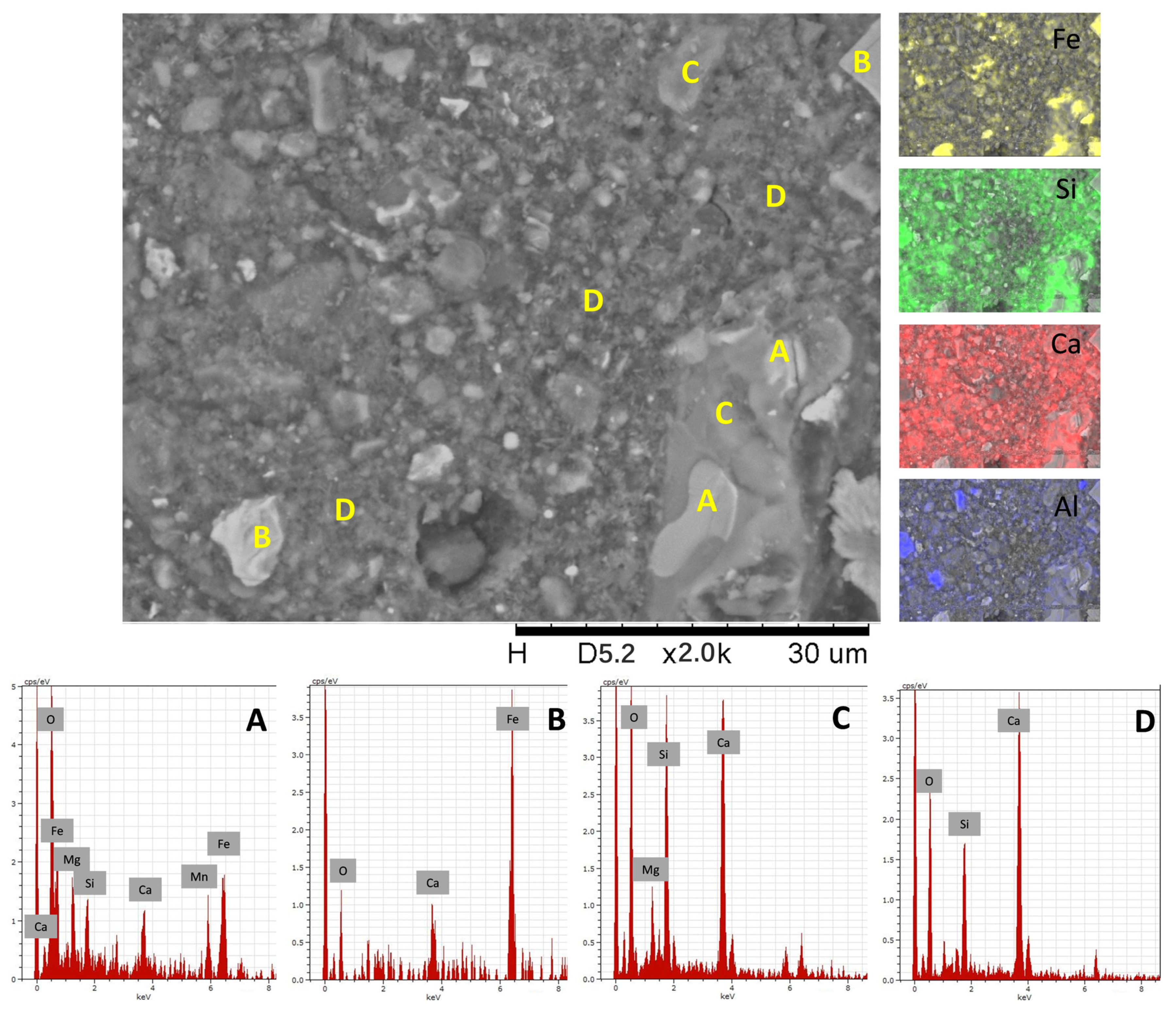
3.4.3. SEM-EDX Analysis
4. Concluding Remarks
- The BOF slag studied presented low pozzolanic behavior, low heat generation and may be considered a low-reactivity material. Nevertheless, the alkali activation process and hardening of pastes were confirmed in all studied cases.
- Crystalline phases of the BOF slag dissolved under the alkaline environment to produce hydrates in all studied formulations, as presented by the TGA and XRD results.
- The high initial heat release measured from the formulations with a high concentration of Na2O and a high silica modulus may have contributed to the rapid setting, thus compromising the casting process of those formulations.
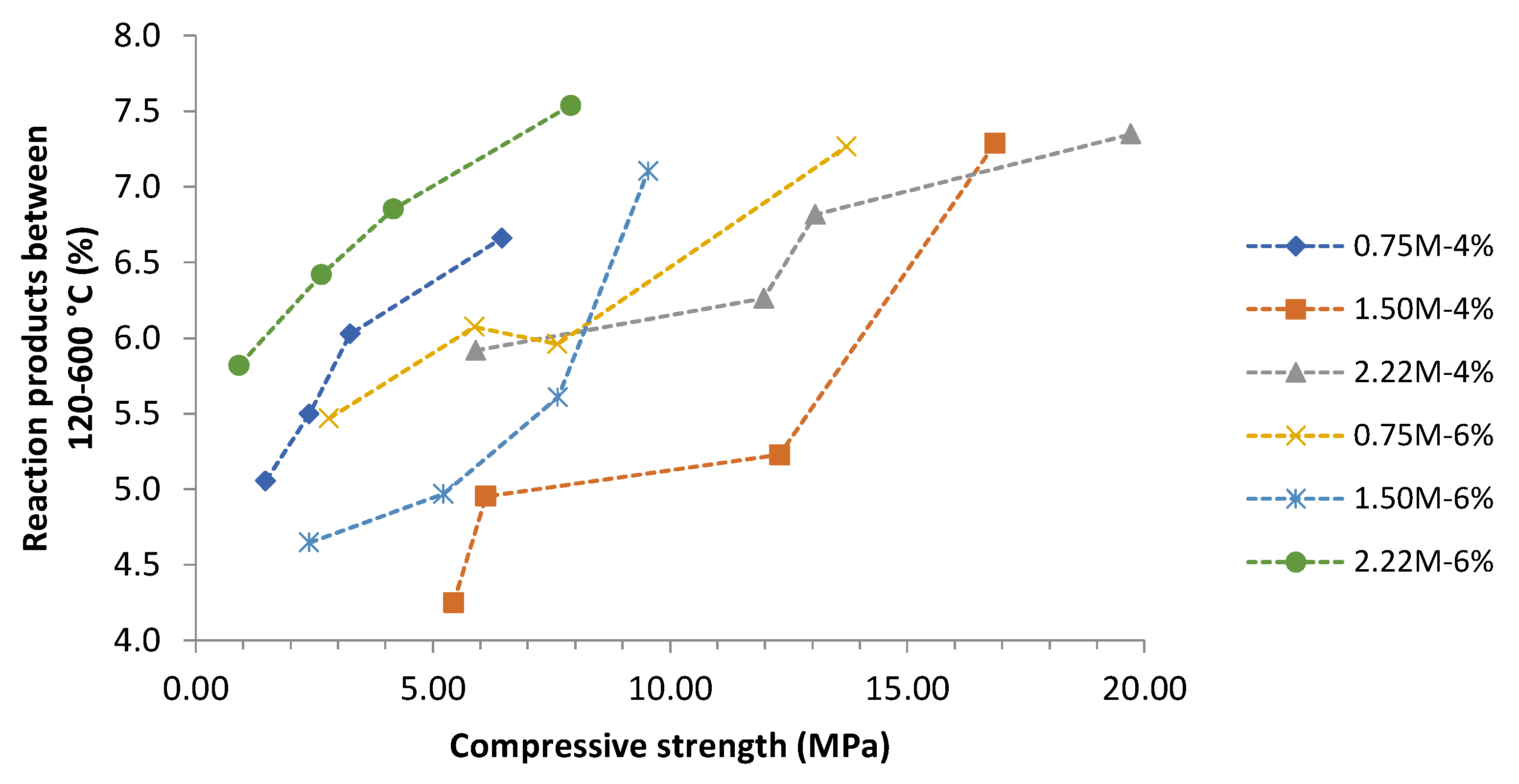
- All formulations presented the same behavior in terms of hydrate formation over the curing time. The TGA results showed the amount of hydrates by quantifying the mass loss, but no correlation with compressive strength development was determined. Other external factors are related to compressive strength, such as workability, molding process, water content and internal porosity.
- A low silica modulus combined with a low Na2O concentration led to the identification of residual Ca(OH)2 in the TGA and XRD results. This strongly suggested that the alkali reaction was weaker, and thus, less reaction products were formed, which jeopardized the strength development.
- A high silica modulus combined with a high Na2O concentration increased the alkalinity in the solution, thus increasing the intensity and speed of the heat release. Moreover, the excess of silicate and OH− limited the diffusion of ions, and thus, the reaction of Ca2+ with Si4+. As a result, the casting process and the strength development were compromised.
- Finally, better strength performance was achieved when an optimized combination of activator sources was considered. In this study, a low Na2O concentration (4%) with an intermediary silica modulus (1.50–2.22) presented the best mechanical results (compressive strength up to 20 MPa), a good amount of reaction products formed (C-S-H/C-A-S-H gel and CaCO3), and a low initial and cumulative amount of heat released. Although the compressive strength was lower than for the conventional ones, it is essential to highlight that BOF slag is an important industry waste that presents economic and environmental benefits and can be used in future engineering projects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, R.; Orloff, M.; Virsilas, T.; Pande, T. Confronting the Urban Housing Crisis in the Global South: Adequate, Secure, and Affordable Housing; World Resources Institute Working Paper; World Resources Institute: Washington, DC, USA, 2017. [Google Scholar]
- George, A.; Kaldany, R.-R.; Losavio, J. The World Is Facing a $15 Trillion Infrastructure Gap by 2040. Here’s How to Bridge It; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- IEA. Cement; IEA: Paris, France, 2020. [Google Scholar]
- Moumin, G.; Ryssel, M.; Zhao, L.; Markewitz, P.; Sattler, C.; Robinius, M.; Stolten, D. CO2 Emission Reduction in the Cement Industry by Using a Solar Calciner. Renew. Energy 2020, 145, 1578–1596. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to Improve Sustainability of Alkali Activated Materials: Review of Side-Stream Based Activators. J. Clean. Prod. 2020, 286, 125558. [Google Scholar] [CrossRef]
- Habert, G.; Ouellet-Plamondon, C. Recent Update on the Environmental Impact of Geopolymers. RILEM Tech. Lett. 2016, 1, 17–23. [Google Scholar] [CrossRef]
- Borges, P.H.R.; de Figueiredo Lourenço, T.M.; Foureaux, A.F.S.; Pacheco, L.S. Estudo Comparativo Da Análise de Ciclo de Vida de Concretos Geopoliméricos e de Concretos à Base de Cimento Portland Composto (CP II). Ambient. Construído 2014, 14, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutierrez, R.M.; Gordillo, M.; Provis, J.L. Mechanical and Thermal Characterisation of Geopolymers Based on Silicate-Activated Metakaolin/Slag Blends. J. Mater. Sci. 2011, 46, 5477. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review: Part 1. Historical Background, Terminology, Reaction Mechanisms and Hydration Products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Nunes, V.A.; Borges, P.H.R. Recent Advances in the Reuse of Steel Slags and Future Perspectives as Binder and Aggregate for Alkali-Activated Materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Fernández-Jiménez, A.; Krivenko, P.V.; Kavalerova, E.; Palacios, M.; Shi, C. Binder Chemistry–High-Calcium Alkali-Activated Materials. In Alkali Activated Materials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 59–91. ISBN 9400776713. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Binder Chemistry–Blended Systems and Intermediate Ca Content. In Alkali Activated Materials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 125–144. [Google Scholar]
- Shi, C.; Qu, B.; Provis, J.L. Recent Progress in Low-Carbon Binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. 16—Geopolymers and Other Alkali-Activated Materials. In Lea’s Chemistry of Cement and Concrete, 5th ed; Hewlett, P., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 779–805. ISBN 978-0-08-100773-0. [Google Scholar]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes. Woodhead Publishing: Amsterdam, The Netherlands, 2015; ISBN 1782422889. [Google Scholar]
- Cercel, J.; Adesina, A.; Das, S. Performance of Eco-Friendly Mortars Made with Alkali-Activated Slag and Glass Powder as a Binder. Constr. Build. Mater. 2021, 270, 121457. [Google Scholar] [CrossRef]
- WSA. World Steel Statistics. Available online: https://www.worldsteel.org/steel-by-topic/statistics/steel-data-viewer/MCSP_crude_steel_monthly/WORLD_ALL (accessed on 12 February 2022).
- Guo, J.; Bao, Y.; Wang, M. Steel Slag in China: Treatment, Recycling, and Management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- IAB. Relatório de Sustentabilidade 2018. Available online: https://acobrasil.org.br/site/publicacao/relatorio-de-sustentabilidade-2018/ (accessed on 10 February 2022).
- Fisher, L.V.; Barron, A.R. The Recycling and Reuse of Steelmaking Slags—A Review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Rosales, J.; Polanco, J.A.; Agrela, F. 7—Steel Slags. In Woodhead Publishing Series in Civil and Structural Engineering; de Brito, J., Agrela, F.B.T.-N.T., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 169–190. ISBN 978-0-08-102480-5. [Google Scholar]
- Wang, G.C. 5—Slag Processing. In The Utilization of Slag in Civil Infrastructure Construction; Wang, G.C., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 87–113. ISBN 978-0-08-100994-9. [Google Scholar]
- Wang, Y.; Suraneni, P. Experimental Methods to Determine the Feasibility of Steel Slags as Supplementary Cementitious Materials. Constr. Build. Mater. 2019, 204, 458–467. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z. Properties of 5-Year-Old Concrete Containing Steel Slag Powder. Powder Technol. 2018, 334, 27–35. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, J.; Jiang, X.; Xu, Y.; Jin, R.; Ma, J.; Zhuang, Y.; Diao, Z.; Zhang, S.; Si, Q.; et al. Investigating the Effects of Steel Slag Powder on the Properties of Self-Compacting Concrete with Recycled Aggregates. Constr. Build. Mater. 2019, 200, 570–577. [Google Scholar] [CrossRef]
- Mutuk, T.; Demirbaş, A.K.; Çevik, S.; Mesci Oktay, B. The Effects of Cast Industry Waste of Scale Instead of Cement on the Mechanical and Microstructural Properties. Int. J. Appl. Ceram. Technol. 2019, 17, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Shen, W.; Huang, J.; Yang, Y.; Zhang, D.; Huang, X.; Lv, Z.; Ji, X. Process to Utilize Crushed Steel Slag in Cement Industry Directly: Multi-Phased Clinker Sintering Technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Liu, J.; Yi, C.; Zhu, H.; Ma, H. Property Comparison of Alkali-Activated Carbon Steel Slag (CSS) and Stainless Steel Slag (SSS) and Role of Blast Furnace Slag (BFS) Chemical Composition. Materials 2019, 12, 3307. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient Use of Steel Slag in Alkali-Activated Fly Ash-Steel Slag-Ground Granulated Blast Furnace Slag Ternary Blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Liao, Y. Influence of Ground Granulated Blast Furnace Slag on the Early Hydration and Microstructure of Alkali-Activated Converter Steel Slag Binder. J. Therm. Anal. Calorim. 2022, 147, 243–252. [Google Scholar] [CrossRef]
- Wang, M.; Qian, B.; Jiang, J.; Liu, H.; Cai, Q.; Ma, B.; Hu, Y.; Wang, L. The Reaction between Ca2+ from Steel Slag and Granulated Blast-Furnace Slag System: A Unique Perspective. Chem. Pap. 2020, 74, 4401–4410. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, T.; Cao, L.; Kan, L.; Wu, M. Influence of Steel Slag on the Properties of Alkali-Activated Fly Ash and Blast-Furnace Slag Based Fiber Reinforced Composites. Cem. Concr. Compos. 2021, 116, 103875. [Google Scholar] [CrossRef]
- Bai, T.; Song, Z.-G.; Wu, Y.-G.; Hu, X.-D.; Bai, H. Influence of Steel Slag on the Mechanical Properties and Curing Time of Metakaolin Geopolymer. Ceram. Int. 2018, 44, 15706–15713. [Google Scholar] [CrossRef]
- Furlani, E.; Maschio, S.; Magnan, M.; Aneggi, E.; Andreatta, F.; Lekka, M.; Lanzutti, A.; Fedrizzi, L. Synthesis and Characterization of Geopolymers Containing Blends of Unprocessed Steel Slag and Metakaolin: The Role of Slag Particle Size. Ceram. Int. 2018, 44, 5226–5232. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of Steel Slag on Fresh, Hardened and Microstructural Properties of High-Calcium Fly Ash Based Geopolymers at Standard Curing Condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Wan, S.; Zheng, R.; Tong, J.; Hou, H.; Wang, T. Synthesis and Characterization of Geopolymer Composites Based on Gasification Coal Fly Ash and Steel Slag. Constr. Build. Mater. 2019, 211, 646–658. [Google Scholar] [CrossRef]
- Guo, X.; Yang, J. Intrinsic Properties and Micro-Crack Characteristics of Ultra-High Toughness Fly Ash/Steel Slag Based Geopolymer. Constr. Build. Mater. 2020, 230, 116965. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Properties and Durability of Alkali-Activated Ladle Slag. Mater. Struct. 2017, 50, 255. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, M.; Bankir, M.B.; Bolukbasi, O.S.; Sevim, U.K. Alkali Activation of Electric Arc Furnace Slag: Mechanical Properties and Micro Analyzes. J. Build. Eng. 2019, 21, 97–105. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z. Effect of Silicate Modulus of Water Glass on the Hydration of Alkali-Activated Converter Steel Slag. J. Therm. Anal. Calorim. 2019, 138, 47–56. [Google Scholar] [CrossRef]
- Morone, M.; Cizer, Ö.; Costa, G.; Baciocchi, R. Effects of Alkali Activation and CO2 Curing on the Hydraulic Reactivity and Carbon Storage Capacity of BOF Slag in View of Its Use in Concrete. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; LI, L.; Wang, J.; Shao, N.; Wang, D. Microstructure and Phase Evolution of Alkali-Activated Steel Slag during Early Age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Češnovar, M.; Traven, K.; Horvat, B.; Ducman, V. The Potential of Ladle Slag and Electric Arc Furnace Slag Use in Synthesizing Alkali Activated Materials; the Influence of Curing on Mechanical Properties. Materials 2019, 12, 1173. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration Properties and Microstructure Characteristics of Alkali–Activated Steel Slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Lancellotti, I.; Piccolo, F.; Traven, K.; Češnovar, M.; Ducman, V.; Leonelli, C. Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials 2021, 14, 639. [Google Scholar] [CrossRef]
- Wang, G.C. 13—Slag Use in Cement Manufacture and Cementitious Applications. In The Utilization of Slag in Civil Infrastructure Construction; Wang, G.C., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 305–337. ISBN 978-0-08-100994-9. [Google Scholar]
- Muhmood, L.; Vitta, S.; Venkateswaran, D. Cementitious and Pozzolanic Behavior of Electric Arc Furnace Steel Slags. Cem. Concr. Res. 2009, 39, 102–109. [Google Scholar] [CrossRef]
- Lu, T.-H.; Chen, Y.-L.; Shih, P.-H.; Chang, J.-E. Use of Basic Oxygen Furnace Slag Fines in the Production of Cementitious Mortars and the Effects on Mortar Expansion. Constr. Build. Mater. 2018, 167, 768–774. [Google Scholar] [CrossRef]
- Belhadj, E.; Diliberto, C.; Lecomte, A. Characterization and Activation of Basic Oxygen Furnace Slag. Cem. Concr. Compos. 2012, 34, 34–40. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 1687–8086. [Google Scholar] [CrossRef] [Green Version]
- N.B.R. 5752; Pozzolanic Materials–Determination of the Performance Index with Portland Cement at 28 Days. ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 2014.
- N.B.R. 11578; Portland Composite Cement-Specification. ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 1997.
- N.B.R. 7214; Areia Normal Para Ensaio de Cimento. ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 2015.
- Suraneni, P.; Weiss, J. Examining the Pozzolanicity of Supplementary Cementitious Materials Using Isothermal Calorimetry and Thermogravimetric Analysis. Cem. Concr. Compos. 2017, 83, 273–278. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Rec. 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Suraneni, P.; Hajibabaee, A.; Ramanathan, S.; Wang, Y.; Weiss, J. New Insights from Reactivity Testing of Supplementary Cementitious Materials. Cem. Concr. Compos. 2019, 103, 331–338. [Google Scholar] [CrossRef]
- N.B.R. 7215; Cimento Portland: Determinação Da Resistência à Compressão. ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 2019.
- N.B.R. 9778; Argamassa e Concreto Endurecidos–Determinação Da Absorção de Água, Índice de Vazios e Massa Específica. ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 2009.
- N.B.R. 12653Materiais Pozolânicos—Requisitos; ABNT (Technical Standards Brazilian Association): Rio de Janeiro, Brazil, 2014.
- Fernández-Jiménez, A.; Puertas, F.; Arteaga, A. Determination of Kinetic Equations of Alkaline Activation of Blast Furnace Slag by Means of Calorimetric Data. J. Therm. Anal. Calorim. 1998, 52, 945–955. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and Activator Effect on Early-Age Reaction Kinetics of Alkali-Activated Slag Binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Vollpracht, A. Isothermal Calorimetry and In-Situ XRD Study of the NaOH Activated Fly Ash, Metakaolin and Slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Kunhi Mohamed, A. Advances in Understanding Cement Hydration Mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- San Nicolas, R.; Bernal, S.A.; Mejía de Gutiérrez, R.; van Deventer, J.S.J.; Provis, J.L. Distinctive Microstructural Features of Aged Sodium Silicate-Activated Slag Concretes. Cem. Concr. Res. 2014, 65, 41–51. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P.L. Factors Affecting the Strength of Alkali-Activated Slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of Alkali Dosage and Silicate Modulus on Carbonation of Alkali-Activated Slag Mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; van Deventer, J.S.J. The Interrelationship between Surface Chemistry and Rheology in Alkali Activated Slag Paste. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Fonseca, L.F.; Nunes, V.A.; Panzera, T.H.; Martuscelli, C.C. Andreasen Particle Packing Method on the Development of Geopolymer Concrete for Civil Engineering. J. Mater. Civ. Eng. 2014, 26, 692–697. [Google Scholar] [CrossRef]
- You, N.; Li, B.; Cao, R.; Shi, J.; Chen, C.; Zhang, Y. The Influence of Steel Slag and Ferronickel Slag on the Properties of Alkali-Activated Slag Mortar. Constr. Build. Mater. 2019, 227, 116614. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, W.; Jiao, J.; Lu, D.; Li, D. Effect of Li+ on the Composition and Morphology of CASH. Constr. Build. Mater. 2021, 301, 123982. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H Gels with Various Ca/Si and Al/Si Ratio: Microstructural and Structural Characteristics with DTA/TG, XRD, FTIR and TEM Analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of Phase Evolution in Alkali-Activated Blast Furnace Slag by the Incorporation of Fly Ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The Use of Thermal Analysis in Assessing the Effect of Temperature on a Cement Paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of Alkali Activated Slag–Fly Ash Blends with Limestone Addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The Evolution of Strength and Crystalline Phases for Alkali-Activated Ground Blast Furnace Slag and Fly Ash-Based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]








| Chemical Composition of Oxides (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | FeO | SiO2 | MgO | Al2O3 | Cr2O3 | MnO | P2O5 | SO3 | |
| BOF slag | 36.66 | 24.19 | 12.10 | 10.60 | 3.84 | 0.10 | 4.53 | 1.11 | 0.71 |
| Formulation | Modulus | Na2O/Binder (%) | Water/Binder | Steel Slag (g) | Water (g) | Na2SiO3 (g) | NaOH (g) |
|---|---|---|---|---|---|---|---|
| 0.75M-4% | 0.75 | 4 | 0.35 | 540 | 159.03 | 48.78 | 18.68 |
| 1.50M-4% | 1.50 | 0.35 | 540 | 135.34 | 97.57 | 9.19 | |
| 2.22M-4% | 2.22 | 0.38 | 540 | 128.59 | 144.40 | - | |
| 0.75M-6% | 0.75 | 6 | 0.35 | 540 | 144.04 | 73.18 | 28.02 |
| 1.50M-6% | 1.50 | 0.40 | 540 | 135.50 | 146.35 | 13.79 | |
| 2.22M-6% | 2.22 | 0.40 | 540 | 101.39 | 216.60 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, V.A.; Suraneni, P.; Bezerra, A.C.S.; Thomas, C.; Borges, P.H.R. Influence of Activation Parameters on the Mechanical and Microstructure Properties of an Alkali-Activated BOF Steel Slag. Appl. Sci. 2022, 12, 12437. https://doi.org/10.3390/app122312437
Nunes VA, Suraneni P, Bezerra ACS, Thomas C, Borges PHR. Influence of Activation Parameters on the Mechanical and Microstructure Properties of an Alkali-Activated BOF Steel Slag. Applied Sciences. 2022; 12(23):12437. https://doi.org/10.3390/app122312437
Chicago/Turabian StyleNunes, Vitor A., Prannoy Suraneni, Augusto C. S. Bezerra, Carlos Thomas, and Paulo H. R. Borges. 2022. "Influence of Activation Parameters on the Mechanical and Microstructure Properties of an Alkali-Activated BOF Steel Slag" Applied Sciences 12, no. 23: 12437. https://doi.org/10.3390/app122312437








