Highly Enriched Uranium-Free Medical Radioisotope Production Methods: An Integrative Review
Abstract
:1. Introduction
2. Materials and Methods
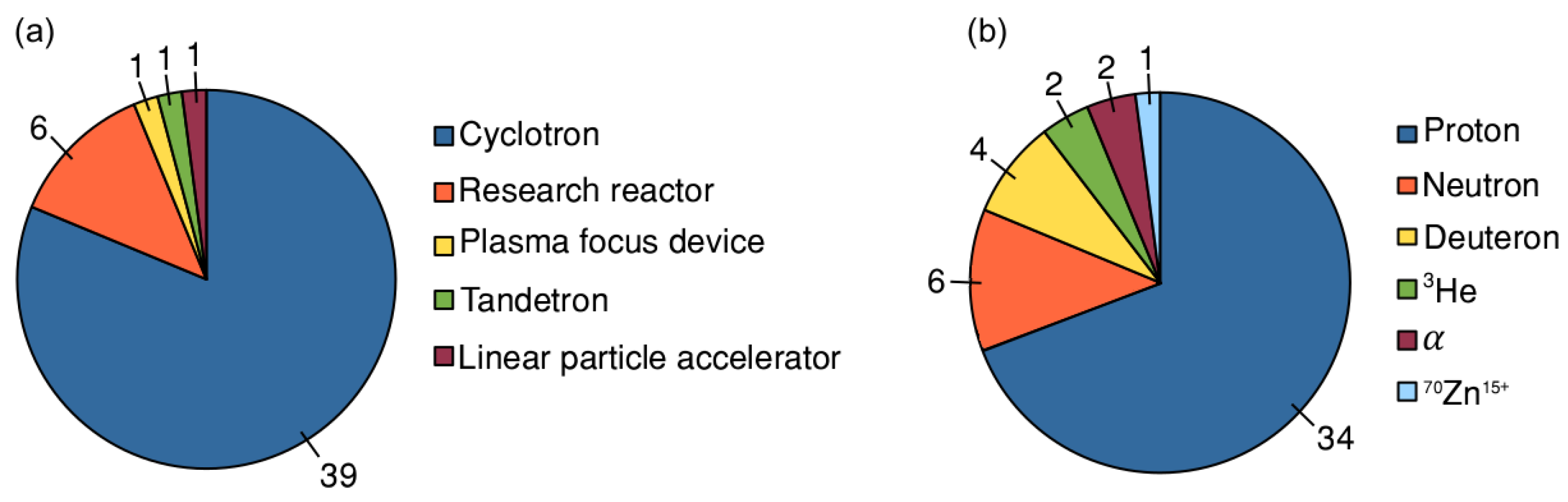
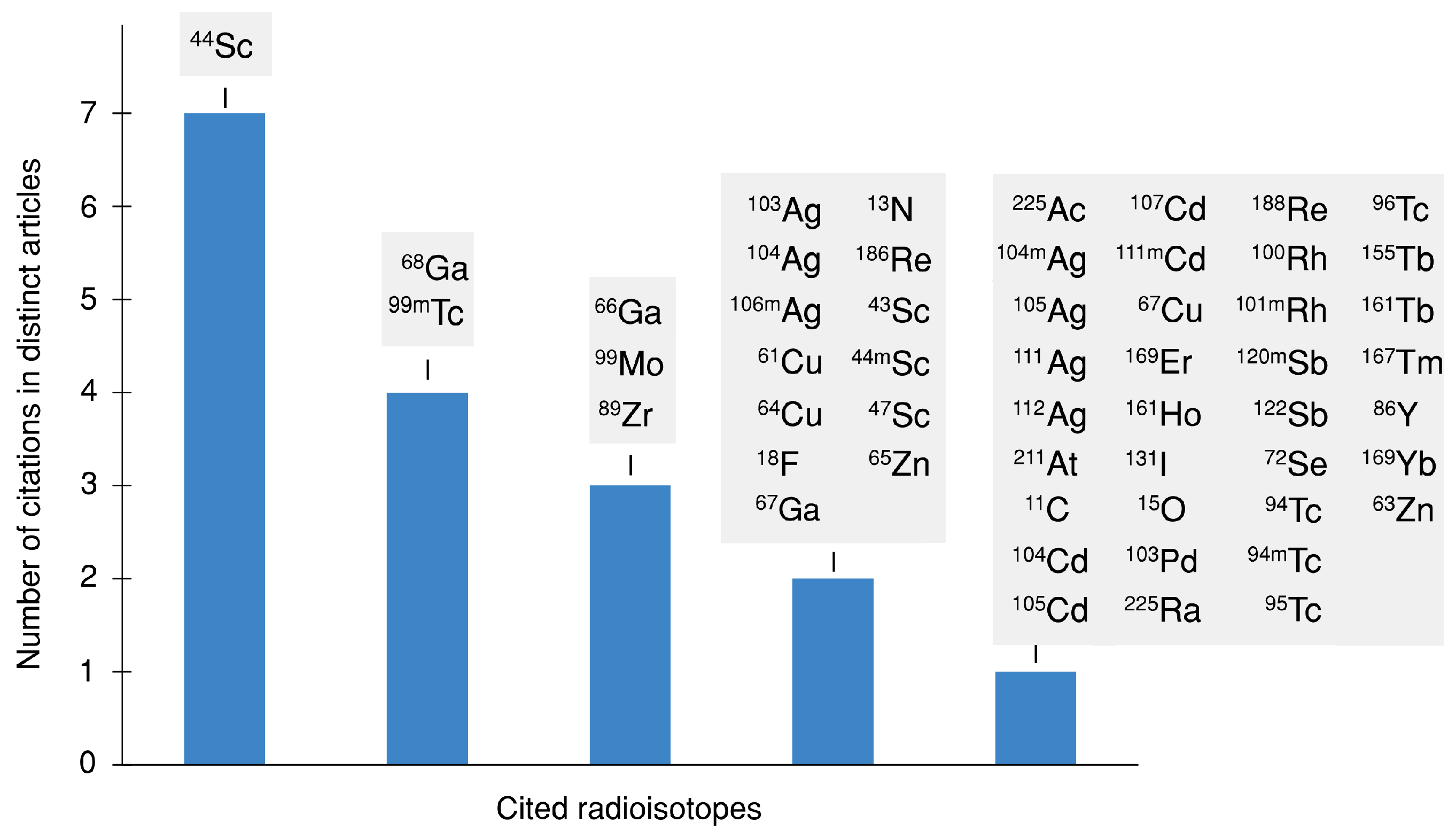
3. Results and Discussion
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPECT | single photon emission tomography |
| PET | positron emission tomography |
| HEU | highly enriched uranium |
| VHL | virtual health library |
| activity at the end of the bombardment | |
| TTY | thick target yield |
| SY | saturation yield |
| LINACS | linear particle accelerators |
| NPs | nanoparticles |
| LWFA | laser wakefield accelerator |
| TNSA | target normal sheath acceleration |
Appendix A
| n° | Intended Radioisotope | Half-Life [h] | Decay | Daughter Isotope | Technology | Accelerated Particle | Production Route | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu | 12.7 | (39%) | Zn | Cyclotron | Proton | Ni(p,n)Cu | Thisgaard et al. [35] |
| (61%) | ||||||||
| Sb | 138.2 | Sn | Sn(p,x)Sb | |||||
| Sb | 65.4 | Te | Sn(p,x)Sb | |||||
| 2 | F | 1.83 | O | Cyclotron | Proton | O(p,n)F | Roeda et al. [36] | |
| 3 | I | 192.5 | Xe | Research reactor | Neutron | Te(n,)TeI | Achoribo et al. [37] | |
| 4 | Tc | 0.867 | Mo | Cyclotron | Proton | Mo(p,n)Tc | Hoehr et al. [38] | |
| 5 | Mo | 66.0 | Tc | Cyclotron | Proton | Th(p,f)Mo | Abbas et al. [39] | |
| 6 | N | 0.166 | C | Plasma focus device | Deuteron | C(d,n)N | Shirani et al. [40] | |
| 7 | Ho | 2.48 | Ec | Dy | Cyclotron | Proton | Dy(p,xn)Ho | Tárkányi et al. [41] |
| 8 | Ga | 78.3 | Ec | Zn | Cyclotron | Proton | Zn(p,2n)Ga | Martins et al. [42] |
| 9 | Er | 225.4 | Tm | Research reactor | Neutron | Er(n,)Er | Chakravarty et al. [43] | |
| 10 | Tc | 6.01 | Tc | Cyclotron | Proton | Mo(p,2n)Tc | Bénard et al. [44] | |
| 11 | Sc | 4.04 | Ca | Cyclotron | Proton | Ca(p,n)Sc | Hoehr et al. [45] | |
| 12 | Zn | 0.641 | Cu | Cyclotron | Proton | Cu(p,n)Zn | DeGrado et al. [46] | |
| 13 | Ag | 1.10 | Pd | Cyclotron | He | Pd(He,pxn)Ag | Al-Abyad et al. [47] | |
| Ag | 1.16 | Pd | Pd(He,pxn)Ag | |||||
| Ag | 991.0 | Pd | Pd(He,pxn)Ag | |||||
| Ag | 198.7 | Cd | Pd(He,pxn)Ag | |||||
| Ag | 178.8 | Cd | Pd(He,pxn)Ag | |||||
| Ag | 3.13 | Cd | Pd(He,pxn)Ag | |||||
| Cd | 0.962 | Ag | Pd(He,xn)Cd | |||||
| Cd | 0.925 | Ag | Pd(He,xn)Cd | |||||
| Cd | 6.50 | Ag | Pd(He,xn)Cd | |||||
| Cd | 0.808 | Cd | Pd(He,xn)Cd | |||||
| 14 | At | 7.21 | Bi | Cyclotron | Proton | Bi(,2n)At | Martin et al. [48] | |
| 15 | Zr | 78.4 | Y | Cyclotron | Proton | Y(p,n)Zr | Siikanen et al. [49] | |
| 16 | Sc | 4.04 | Ca | Cyclotron | Proton | Ca(p,n)Sc | Valdovinos et al. [50] | |
| 17 | Tc | 6.01 | Tc | Cyclotron | Proton | Mo(p,2n)Tc | Schaffer et al. [51] | |
| 18 | Y | 14.7 | Sr | Cyclotron | Proton | Sr(p,n)Y | Oehlke et al. [52] | |
| Zr | 78.4 | Y | Y(p,n)Zr | |||||
| Ga | 1.13 | Zn | Zn(p,x)Ga | |||||
| 19 | Yb | 1024 | Ec | Tm | Research reactor | Neutron | YbO(n,)Yb | Saxena et al. [53] |
| 20 | C | 0.339 | B | Cyclotron | Proton | N(p,)C | Moon et al. [54] | |
| 21 | Sc | 4.04 | Ca | Cyclotron | Proton | Ca(p,n)Sc | van der Meulen et al. [55] | |
| 22 | Sc | 4.04 | Ca | Cyclotron | Deuteron | Ca(d,2n)Sc | Duchemin et al. [56] | |
| Sc | 58.6 | Sc | Ca(d,2n)Sc | |||||
| 23 | Cu | 3.33 | Ni | Cyclotron | Proton | Zn(p,)Cu | Asad et al. [57] | |
| Zn(p,)Cu | ||||||||
| 24 | Tb | 165.6 | Dy | Research reactor | Neutron | Gd(n,)GdTb | Aziz et al. [58] | |
| 25 | Re | 89.0 | Os | Cyclotron | Proton | W(p,n)Re | Kakavand et al. [59] | |
| 26 | Sc | 3.89 | Ca | Cyclotron | Ca(,p)Sc | Szkliniarz et al. [60] | ||
| Ca(,p)Sc | ||||||||
| Sc | 4.04 | Ca | Ca(,np+pn)Sc | |||||
| Sc | 58.6 | Sc | Ca(,np+pn)Sc | |||||
| 27 | Ga | 9.49 | Zn | Tandetron | Proton | Zn(p,x)Ga | Fraile et al. [61] | |
| Ga | 1.13 | Zn | Zn(p,x)Ga | |||||
| 28 | Ga | 9.49 | Zn | Cyclotron | Proton | Zn(p,n)Ga | Cho et al. [62] | |
| 29 | Cu | 3.33 | Ni | Cyclotron | Proton | Zn(p,)Cu | do Carmo et al. [63] | |
| Ga | 9.49 | Zn | Zn(p,x)Ga | |||||
| Ga | 78.2 | Ec | Zn | Zn(p,x)Ga | ||||
| Ga | 1.13 | Zn | Zn(p,n)Ga | |||||
| Zn | 5857 | Cu | Zn(p,pn)Zn | |||||
| 30 | Sc | 80.4 | Ti | Cyclotron | Proton | Ca(p,2n)Cu | Misiak et al. [64] | |
| 31 | Cu | 12.7 | (39%) | Zn | Cyclotron | Proton | Ni(p,n)Cu | Xie et al. [65] |
| (61%) | ||||||||
| 32 | Sc | 3.89 | Ca | Cyclotron | Deuteron | Ca(d,n)Sc | Sitarz et al. [66] | |
| Ca(p,n)Sc | ||||||||
| Sc | 4.04 | Ca | Ca(p,n)Sc | |||||
| Proton | Ca(p,n)Sc | |||||||
| Sc | 80.4 | Ti | Ca(p,2n)Sc | |||||
| Ti(p,2p)Sc | ||||||||
| 33 | Rh | 20.8 | Ru | Cyclotron | He | Rh(He,x)Rh | Ali et al. [67] | |
| Rh | 104.2 | Ec (94%) | Ru | Rh(He,+n)Rh | ||||
| (6%) | Rh | |||||||
| Ag | 1.10 | Pd | Rh(He,3n)Ag | |||||
| Ag | 0.558 | Pd | Rh(He,2n)Ag | |||||
| Ag | 1.15 | Rh(He,2n)Ag | ||||||
| Pd | 407.8 | Ec | Rh | Rh(He,n)Pd | ||||
| 34 | Zn | 5857 | Ec | Cu | Research reactor | Neutron | Zn(n,)Zn | Karimi et al. [68] |
| 35 | Ag | 19.2 | Pd | Cyclotron | Ag(,x)Ag | Ditrói et al. [69] | ||
| 36 | O | 0.034 | N | Cyclotron | Deuteron | N(d,n)O | Iguchi et al. [70] | |
| 37 | Se | 201.6 | Ec | As | Linear particle accelerator | Proton | As(p,4n)Se | DeGraffenreid et al. [71] |
| 38 | Re | 89.2 | Os | Research reactor | Neutron | Re(n,)Re | Pourhabib et al. [72] | |
| Re | 17.0 | Os | Re(n,)Re | |||||
| 39 | Cu | 61.8 | Zn | Cyclotron | Zn | H(Zn,+p)Cu | Souliotis et al. [73] | |
| 40 | Ac | 240.0 | Fr | Cyclotron | Proton | Th(p,x)Ac | Robertson et al. [74] | |
| Ra | 357.6 | Ac | Th(n,x)Ra | |||||
| 41 | Tc | 4.89 | Mo | Cyclotron | Proton | Mo(p,x)Tc | Ahmed et al. [75] | |
| Tc | 20.0 | Mo | Mo(p,x)Tc | |||||
| Tc | 1464 | Mo | Mo(p,x)Tc | |||||
| Tc | 102.7 | Mo | Mo(p,x)Tc | |||||
| Tc | 6.01 | Tc | Mo(p,x)Tc | |||||
| Mo | 66.0 | Tc | Mo(p,x)Mo | |||||
| 42 | Sc | 4.04 | Ca | Cyclotron | Proton | Ca(p,n)Sc | Alnahwi et al. [76] | |
| 43 | Tc | 6.01 | Tc | Cyclotron | Proton | Mo(p,x)Tc | Kambali et al. [77] | |
| Mo | 66.0 | Tc | Mo(p,x)Mo | |||||
| 44 | Sc | 4.04 | Ca | Cyclotron | Proton | Ca(p,n)Sc | van der Meulen et al. [78] | |
| 45 | N | 0.165 | C | Cyclotron | Proton | O(p,)N | Abel et al. [79] | |
| F | 1.82 | O | O(p,n)F | |||||
| 46 | Tm | 222.0 | Ec | Er | Cyclotron | Proton | Er(p,x)Tm | Heinke et al. [80] |
| 47 | Tb | 127.7 | Ec | Gd | Cyclotron | Proton | Gd(p,n)Tb | Favaretto et al. [81] |
| Gd(p,2n)Tb | ||||||||
| 48 | Zr | 78.4 | Y | Cyclotron | Proton | Y(p,n)Zt | Cisterino et al. [82] |
| n° | Production Route | Kinetic Energy [MeV] | Current [A] | Irradiation Time [h] | [kBq] | Reference |
|---|---|---|---|---|---|---|
| 1 | Ni(p,n)Cu | 16.1 | 121 | 1.26 | Thisgaard et al. [35] | |
| Sn(p,x)Sb | 150 | |||||
| Sn(p,x)Sb | ||||||
| 2 | O(p,n)F | 18 | 20 | 0.5 | Roeda et al. [36] | |
| 4 | Mo(p,n)Tc | 13 | 5 | 1 | Hoehr et al. [38] | |
| 5 | Th(p,f)Mo | 29.5 | 1 | 1 | Abbas et al. [39] | |
| 26.5 | ||||||
| 7 | Dy(p,xn)Ho | 36 | 61 | 1.18 | Tárkányi et al. [41] | |
| 8 | Zn(p,2n)Ga | 26 | 10 | 1 | Martins et al. [42] | |
| 10 | Mo(p,2n)Tc | 18 | 240 | 6.9 | Bénard et al. [44] | |
| 11 | Ca(p,n)Sc | 13 | 7.6 | 1 | Hoehr et al. [45] | |
| 12 | Cu(p,n)Zn | 14 | 20 | 1 | DeGrado et al. [46] | |
| 13 | Pd(He,pxn)Ag | 27 | 0.150 | 1 | Al-Abyad et al. [47] | |
| Pd(He,pxn)Ag | ||||||
| Pd(He,pxn)Ag | 30 | |||||
| Pd(He,pxn)Ag | 35.9 | |||||
| Pd(He,pxn)Ag | 26.9 | |||||
| Pd(He,pxn)Ag | 102 | |||||
| Pd(He,xn)Cd | ||||||
| Pd(He,xn)Cd | ||||||
| Pd(He,xn)Cd | 690 | |||||
| Pd(He,xn)Cd | 466 | |||||
| 14 | Bi(,2n)At | 27.8 | 0.327 | 4 | Martin et al. [48] | |
| 25.3 | 0.192 | |||||
| 15 | Y(p,n)Zr | 12.8 | 45 | 3 | Siikanen et al. [49] | |
| 16 | Ca(p,n)Sc | 15.6 | 25 | 1 | Valdovinos et al. [50] | |
| 17 | Mo(p,2n)Tc | 16.5 | 130 | 6 | Schaffer et al. [51] | |
| 18 | Sr(p,n)Y | 13 | 4.5 | 1 | Oehlke et al. [52] | |
| Y(p,n)Zr | 7 | |||||
| Zn(p,x)Ga | ||||||
| 20 | N(p,)C | 13 | 60 | 0.5 | Moon et al. [54] | |
| 21 | Ca(p,n)Sc | 11 | 50 | 1.5 | van der Meulen et al. [55] | |
| 22 | Ca(d,2n)Sc | 30 | 0.054 | 0.5 | Duchemin et al. [56] | |
| Ca(d,2n)Sc | ||||||
| 23 | Zn(p,)Cu | 11.7 | 20 | 0.5 | Asad et al. [57] | |
| Zn(p,)Cu | 40 | |||||
| 25 | W(p,n)Re | 15 | 20 | 5 | Kakavand et al. [59] | |
| 26 | Ca(,p)Sc | 20 | 0.050 | 1.5 | Szkliniarz et al. [60] | |
| Ca(,p)Sc | ||||||
| Ca(,np+pn)Sc | 29 | |||||
| Ca(,np+pn)Sc | 250 | |||||
| 28 | Zn(p,n)Ga | 14.2 | 5 | 0.083 | 508 | Cho et al. [62] |
| 8 | 195 | |||||
| 29 | Zn(p,)Cu | 16.9 | 30 | 0.75 | do Carmo et al. [63] | |
| Zn(p,x)Ga | ||||||
| Zn(p,x)Ga | ||||||
| Zn(p,n)Ga | ||||||
| Zn(p,pn)Zn | 225 | |||||
| 30 | Ca(p,2n)Sc | 20.5 | 0.03 | 5 | 21.1 | Misiak et al. [64] |
| 31 | Ni(p,n)Cu | 12.5 | 20 | 5-7 | Xie et al. [65] | |
| Ca(d,n)Sc | 6.8 | 4 | ||||
| 32 | Ca(p,n)Sc | 15.2 | 1 | 8 | Sitarz et al. [66] | |
| Ca(p,n)Sc | ||||||
| Ca(p,n)Sc | ||||||
| Ca(p,2n)Sc | 22.8 | |||||
| Ti(p,2p)Sc | 28 | |||||
| 33 | Rh(He,x)Rh | 27 | 0.1 | 1 | 118 | Ali et al. [67] |
| Rh(He,+n)Rh | 30 | |||||
| Rh(He,3n)Ag | ||||||
| Rh(He,2n)Ag | ||||||
| Rh(He,2n)Ag | ||||||
| Rh(He,n)Pd | 270 | |||||
| 35 | Ag(,x)Ag | 37.6 | 2 | 1 | 238 | Ditrói et al. [69] |
| 36 | N(d,n)O | 3.5 | 40 | 0.017 | Iguchi et al. [70] | |
| 39 | H(Zn,+p)Cu | 15 | 0.008 | 6.5 | 1.6 | Souliotis et al. [73] |
| 40 | Th(p,x)Ac | 480 | 72 | 36.5 | Robertson et al. [74] | |
| Th(p,x)Ra | ||||||
| 41 | Mo(p,x)Tc | 26 | 0.065 | 5 | Ahmed et al. [75] | |
| Mo(p,x)Tc | ||||||
| Mo(p,x)Tc | 86.9 | |||||
| Mo(p,x)Tc | ||||||
| Mo(p,x)Tc | ||||||
| Mo(p,x)Mo | ||||||
| 42 | Zn(p,n)Ga | 13 | 35 | 1.5 | Alnahwi et al. [76] | |
| 43 | Mo(n,x)Tc | 11 | 20 | 0.083 | 39 | Kambali et al. [77] |
| Mo(n,x)Mo | 33 | |||||
| 44 | Ca(p,n)Sc | 18 | 18.4 | 5.75 | van der Meulen et al. [78] | |
| 45 | O(p,)N | 16 | 5.6–34 | 3.3 | Abel et al. [79] | |
| O(p,n)F | ||||||
| 46 | Er(p,x)Tm | 22.8 | 50 | 8 | Heinke et al. [80] | |
| 47 | Gd(p,n)Tb | 10 | 50 | 8 | Favaretto et al. [81] | |
| Gd(p,2n)Tb | 23 | |||||
| 48 | Y(p,x)Zr | 12 | 50 | 5 | Cisterino et al. [82] |
| n° | Production Route | Target Mass [mg] | Neutron Flux [n/cms] | Irradiation Time [h] | [kBq] | Reference |
|---|---|---|---|---|---|---|
| 3 | Te(n,)TeI | 5000 | 4 | 400 | Achoribo et al. [37] | |
| 9 | Er(n,)Er | 10 | 504 | Chakravarty et al. [43] | ||
| 19 | YbO(n,)Yb | 5 | 168 | Saxena et al. [53] | ||
| 24 | Gd(n,)GdTb | 100 | 108 | Aziz et al. [58] | ||
| 34 | Zn(n,)Zn | 1000 | 0.5 | Karimi et al. [68] | ||
| 38 | Re(n,)Re | 1 | 96 | Pourhabib et al. [72] | ||
| Re(n,)Re |
| n° | Technology | Production Route | Kinetic Energy [MeV] | Current [A] | Irradiation Time [h] | [kBq] | Reference |
|---|---|---|---|---|---|---|---|
| 6 | Plasma focus device | C(d,n)N | 0.33 | not applicable | 0.097 | 0.014 | Shirani et al. [40] |
| 27 | Tandetron | Zn(p,n)Ga | 9 | 0.106 | 8.5 | Fraile et al. [61] | |
| Zn(p,n)Ga | 99 | ||||||
| 37 | Linear particle accelerator | As(p,4n)Se | 49.5 | 163.5 | 68.9 | DeGraffenreid et al. [71] |
References
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef] [Green Version]
- Crestoni, M.E. Radiopharmaceuticals for diagnosis and therapy. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Radioisotopes in Medicine. Available online: https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx (accessed on 23 August 2022).
- Qaim, S.M. Therapeutic radionuclides and nuclear data. Radiochim. Acta 2001, 89, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Mallard, J.R.; Myers, M.J. Clinical Applications of a Gamma Camera. Phys. Med. Biol. 1963, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Mcafee, J.G. A New Complex of 99mTc for Skeletal Imaging. Radiology 1971, 99, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Bisi, G.; Sciagrà, R.; Santoro, G.M.; Cerisano, G.; Vella, A.; Zerauschek, F.; Fazzini, P.F. Myocardial scintigraphy with Tc-99m-teboroxime: Its feasibility and the evaluation of its diagnostic reliability. A comparison with thallium-201 and coronary angiography. G Ital. Cardiol. 1992, 22, 795–805. [Google Scholar]
- De Klerk, J.M.; van Rijk, P.P.; van Dongen, A.J.; Deenstra, M.; Bànki, J.H.; van het Schup, A.D. Can technetium 99 m bisdiethylphosphinoethanebis-t butylisocyanide (99mTc-DEPIC) be used for routine radionuclide ventriculography? Eur. J. Nucl. Med. 1991, 18, 317–320. [Google Scholar] [CrossRef]
- Ludwing, C.; Chicherio, C.; Terraneo, L.; Magistretti, P.; de Ribaupierre, A.; Slosman, D. Functional imaging studies of cognition using 99mTc-HMPAO SPECT: Empirical validation using the n-back working memory paradigm. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Seok, J.W.; Choi, Y.S.; Chong, S.; Kwon, G.Y.; Chung, Y.J.; Kum, B.G.; Park, S.J. Sentinel lymph node identification with radiopharmaceuticals in patients with breast cancer: A comparison of 99mTc-tin colloid and 99mTc-phytate efficiency. Breast Cancer Res. Treat. 2010, 122, 453–457. [Google Scholar] [CrossRef]
- Willkomm, P.; Bender, H.; Bangard, M.; Decker, P.; Grünwald, F.; Biersack, H.J. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J. Nucl. Med. 2000, 41, 1657–1663. [Google Scholar]
- Popescu, H.I.; Lessem, J.; Erjavec, M.; Füger, G.F. In vivo labelling of RBC with 99mTc for blood pool imaging using different stannous radiopharmaceuticals. J. Nucl. Med. 1984, 9, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Sunil, H.V.; Mittal, B.R.; Bhattacharya, A.; Singh, B.; Chawla, Y. Role of Tc99m sulfur colloid scintigraphy in differentiating non-cirrhotic portal fibrosis from cirrhosis liver. Indian J. Nucl. Med. 2010, 25, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Diamond, R.H.; Rothstein, R.D.; Alavi, A. The role of cimetidine-enhanced technetium-99m-pertechnetate imaging for visualizing Meckel’s diverticulum. J. Nucl. Med. 1991, 32, 1422–1424. [Google Scholar] [PubMed]
- Kanoun, S.; Rossi, C.; Casasnovas, O. [18F]FDG-PET/CT in Hodgkin Lymphoma: Current Usefulness and Perspectives. Cancers 2018, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT imaging in oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, E.; Lutsenko, S.; Bandmann, O. Animal models of Wilson disease. J. Neurochem. 2018, 146, 356–373. [Google Scholar] [CrossRef] [Green Version]
- Hancock, C.N.; Stockwin, L.H.; Han, B.; Divelbuss, R.D.; Jun, J.H.; Malhotra, S.V.; Hollingshead, M.G.; Newton, D.L. A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Parry, J.J.; Andrews, R.; Rogers, B.E. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res. Treat. 2007, 101, 175–183. [Google Scholar] [CrossRef]
- Lewis, S.J.; Laforest, R.; Buettner, T.L.; Song, S.-K.; Fujibayashi, Y.; Connett, J.M.; Welch, M.J. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): An agent for radiotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Radioisotope Production for Nuclear Medicine. Available online: https://www.radioactivity.eu.com/site/pages/Radioisotope_Production.htm (accessed on 23 August 2022).
- Qaim, S.M. Comparison of reactor and cyclotron production of medically important radioisotopes, with special reference to 99Mo/99mTc, 64,67Cu and 103Pd. In Utilization Related Design Features of Research Reactors: A Compendium; International Atomic Energy Agency (IAEA): Vienna, Austria, 2007; pp. 135–143. [Google Scholar]
- International Atomic Energy Agency. The Applications of Research Reactors. Report of An Advisory Group Meeting, Vienna. 2001, p. 71. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:32048858 (accessed on 25 August 2022).
- Bibler, N.E.; Coleman, C.J.; Kinard, W.F. Relative Yields of U-235 Fission Products Measured in a High Level Radioactive Sludge at Savannah River Site. Westinghouse Savannah River Company. 1992. Available online: https://digital.library.unt.edu/ark:/67531/metadc1194158 (accessed on 25 August 2022).
- Storage and Disposal of Radioactive Waste. Available online: https://world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-waste/storage-and-disposal-of-radioactive-waste.aspx (accessed on 23 August 2022).
- Cho, W.-K. Radiation risk assessment for the transport of radioisotopes using KRI-BGM B(U) type container. In IRPA12: 12. Congress of the International Radiation Protection Association: Strengthening Radiation Protection Worldwide, Highlights, Global Perspective and Future Trends; International Atomic Energy Agency (IAEA): Vienna, Austria, 2010. [Google Scholar]
- United States Environmental Protection Agency. Transportation of Radioactive Material. Available online: https://www.epa.gov/radtown/transportation-radioactive-material (accessed on 7 November 2022).
- Adedapo, K.S.; Onimode, Y.A.; Ejeh, J.E.; Adepoju, A.O. Avoidable challenges of a nuclear medicine facility in a developing nation. Indian J. Nucl. Med. 2013, 28, 195–199. [Google Scholar] [CrossRef]
- Van Noorden, R. Radioisotopes: The medical testing crisis. Nature 2013, 504, 202–204. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee on Medical Isotope Production without Highly Enriched Uranium. Medical Isotope Production without Highly Enriched Uranium; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pesquisa.bvsalud.org/portal/advanced/?lang=en (accessed on 25 August 2022).
- Available online: https://www.sciencedirect.com/search (accessed on 25 August 2022).
- Available online: https://www.scopus.com/ (accessed on 25 August 2022).
- Thisgaard, H.; Jensen, M.; Elema, D.R. Medium to large scale radioisotope production for targeted radiotherapy using a small PET cyclotron. Appl. Radiat. Isot. 2011, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roeda, D.; Kuhnast, B.; Damont, A.; Dollé, F. Synthesis of fluorine-18-labeled TSPO ligands for imaging neuroinflammation with Positron Emission Tomography. J. Fluor. Chem. 2012, 134, 107–114. [Google Scholar] [CrossRef]
- Elom Achoribo, A.S.; Akaho, E.H.; Nyarko, B.J.; Osae Shiloh, K.D.; Odame Duodu, G.; Gibrilla, A. Feasibility study for production of I-131 radioisotope using MNSR research reactor. Appl. Radiat. Isot. 2012, 70, 76–80. [Google Scholar] [CrossRef]
- Hoehr, C.; Morley, T.; Buckley, K.; Trinczek, M.; Hanemaayer, V.; Schaffer, P.; Ruth, T.; Bénard, F. Radiometals from liquid targets: 94mTc production using a standard water target on a 13 MeV cyclotron. Appl. Radiat. Isot. 2012, 70, 2308–2312. [Google Scholar] [CrossRef]
- Abbas, K.; Holzwarth, U.; Simonelli, F.; Kozempel, J.; Cydzik, I.; Bulgheroni, A.; Cotogno, G.; Apostolidis, C.; Bruchertseifer, F.; Morgenstern, A. Feasibility of 99Mo production by proton-induced fission of 232Th. Nucl. Instrum. Methods Phys. Res. B Nucl. Instrum. Meth. B 2012, 278, 20–25. [Google Scholar] [CrossRef]
- Shirani, B.; Abbasi, F.; Nikbakht, M. Production of 13N by 12C(d,n)13N reaction in a medium energy plasma focus. Appl. Radiat. Isot. 2013, 74, 86–90. [Google Scholar] [CrossRef]
- Tárkányi, F.; Ditroi, F.; Hermanne, A.; Takács, S.; Ignatyuk, A. Investigation of production routes for the 161Ho Auger-electron emitting radiolanthanide, a candidate for therapy. J. Radioanal. Nucl. Chem. 2013, 298, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.d.A.; Osso Junior, J.A. Thermal diffusion of 67Ga from irradiated Zn targets. Appl. Radiat. Isot. 2013, 82, 279–282. Available online: http://repositorio.ipen.br/handle/123456789/4005 (accessed on 25 August 2022). [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Chirayil, V.; Dash, A. Reactor production and electrochemical purification of 169Er: A potential step forward for its utilization in in vivo therapeutic applications. Nucl. Med. Biol. 2014, 41, 163–170. [Google Scholar] [CrossRef]
- Bénard, F.; Buckley, K.R.; Ruth, T.J.; Zeisler, S.K.; Klug, J.; Hanemaayer, V.; Vuckovic, M.; Hou, X.; Celler, A.; Appiah, J.P.; et al. Implementation of Multi-Curie Production of 99mTc by Conventional Medical Cyclotrons. J. Nucl. Med. 2014, 55, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hoehr, C.; Oehlke, E.; Benard, F.; Lee, C.J.; Hou, X.; Badesso, B.; Ferguson, S.; Miao, Q.; Yang, H.; Buckley, K.; et al. 44gSc production using a water target on a 13 MeV cyclotron. Nucl. Med. Biol. 2014, 41, 401–406. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, T.R.; Pandey, M.K.; Byrne, J.F.; Engelbrecht, H.P.; Jiang, H.; Packard, A.B.; Thomas, K.A.; Jacobson, M.S.; Curran, G.L.; Lowe, V.J. Preparation and preliminary evaluation of 63Zn-zinc citrate as a novel PET imaging biomarker for zinc. J. Nucl. Med. 2014, 55, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Abyad, M.; Tárkányi, F.; Ditrói, F.; Takács, S. Excitation function of 3He-particle induced nuclear reactions on natural palladium. Appl. Radiat. Isot. 2014, 94, 191–199. [Google Scholar] [CrossRef]
- Martin, T.M.; Bhakta, V.; Al-Harbi, A.; Hackemack, M.; Tabacaru, G.; Tribble, R.; Shankar, S.; Akabani, G. Preliminary production of 211At at the Texas A&M University Cyclotron Institute. Health Phys. 2014, 107, 1–9. [Google Scholar] [CrossRef]
- Siikanen, J.; Tran, T.A.; Olsson, T.G.; Strand, S.-E.; Sandell, A. A solid target system with remote handling of irradiated targets for PET cyclotrons. Appl. Radiat. Isot. 2014, 94, 294–301. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Hernandez, R.; Barnhart, T.E.; Graves, S.; Cai, W.; Nickles, R.J. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl. Radiat. Isot. 2015, 95, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, P.; Benard, F.; Bernstein, A.; Buckley, K.; Celler, A.; Cockburn, N.; Corsaut, J.; Dodd, M.; Economou, C.; Eriksson, T.; et al. Direct Production of 99mTc via 100Mo(p,2n) on Small Medical Cyclotrons. Physics Procedia 2015, 66, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Oehlke, E.; Hoehr, C.; Hou, X.; Hanemaayer, V.; Zeisler, S.; Adam, M.J.; Ruth, T.J.; Celler, A.; Buckley, K.; Benard, F.; et al. Production of Y-86 and other radiometals for research purposes using a solution target system. Nucl. Med. Biol. 2015, 42, 842–849. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, Y.; Jagadeesan, K.C.; Nuwad, J.; Bamankar, Y.R.; Dash, A. Studies on the development of 169Yb-brachytherapy seeds: New generation brachytherapy sources for the management of cancer. Appl. Radiat. Isot. 2015, 101, 75–82. [Google Scholar] [CrossRef]
- Moon, B.; Lee, H.; Lee, W.; Hur, M.G.; Yang, S.; Lee, B.; Kim, S. Development of additive [11C]CO2 target system in the KOTRON-13 cyclotron and its application for [11C]radiopharmaceutical production. Nucl. Instrum. Methods Phys. Res. B Nucl. Instrum. Meth. B 2015, 356, 1–7. [Google Scholar] [CrossRef]
- Van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, C.; Guertin, A.; Haddad, F.; Michel, N.; Métivier, V. Production of scandium-44m and scandium-44g with deuterons on calcium-44: Cross section measurements and production yield calculations. Phys. Med. Biol. 2015, 60, 6847–6864. [Google Scholar] [CrossRef]
- Asad, A.H.; Smith, S.V.; Morandeau, L.M.; Chan, S.; Jeffery, C.M.; Price, R.I. Production of 61Cu by the natZn(p,α) reaction: Improved separation and specific activity determination by titration with three chelators. J. Radioanal. Nucl. Chem. 2015, 307, 899–906. [Google Scholar] [CrossRef]
- Aziz, A.; Artha, W. Radiochemical Separation of 161Tb from Gd/Tb Matrix Using Ln Resin Column. Indones. J. Chem. 2016, 16. [Google Scholar] [CrossRef]
- Kakavand, T.; Mirzaii, M.; Khaleghi, M.; Eslami, M. Optimization of sedimentation of tungsten on copper substrate for production of 168gRe via 168W(p,n) nuclear reaction: Feasibility of using high current, long irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 366, 224–226. [Google Scholar] [CrossRef]
- Szkliniarz, K.; Sitarz, M.; Walczak, R.; Jastrzebski, J.; Bilewicz, A.; Choinski, J.; Jakubowski, A.; Majkowska, A.; Stolarz, A.; Trzcinska, A.; et al. Production of medical Sc radioisotopes with an alpha particle beam. Appl. Radiat. Isot. 2016, 118, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Fraile, L.; Lopez Herraiz, J.; Udías, J.; Cal-Gonzalez, J.; García Corzo, P.M.; España, S.; Herranz, E.; Pérez-Liva, M.; Picado, E.; Vicente, E.; et al. Experimental validation of gallium production and isotope-dependent positron range correction in PET. Nucl. Instrum. Methods Phys. Res. Sect. B Nucl. Instrum. Methods A 2016, 814, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Wang, M.; Gonzalez-Lepera, C.; Mawlawi, O.; Cho, S.H. Development of bimetallic (Zn@Au) nanoparticles as potential PET-imageable radiosensitizers. Med. Phys. 2016, 43. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo, S.J.C.; Alves, V.H.P.; Alves, F.; Abrunhosa, A.J. Fast and cost-effective cyclotron production of 61Cu using a natZn liquid target: An opportunity for radiopharmaceutical production and R&D. Dalton Trans. 2017, 46, 14556–14560. [Google Scholar] [CrossRef]
- Misiak, R.; Walczak, R.; Wąs, B.; Bartyzel, M.; Mietelski, J.W.; Bilewicz, A. 47Sc production development by cyclotron irradiation of 48Ca. J. Radioanal. Nucl. Chem. 2017, 313, 429–434. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, H.; Wang, F.; Meng, X.; Ren, Q.; Xia, C.; Yang, Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules 2017, 22, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarz, M.; Szkliniarz, K.; Jastrzębski, J.; Choiński, J.; Guertin, A.; Haddad, F.; Jakubowski, A.; Kapinos, K.; Kisieliński, M.; Majkowska, A.; et al. Production of Sc medical radioisotopes with proton and deuteron beams. Appl. Radiat. Isot. 2018, 142, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Al-Abyad, M.; Kandil, S.; Solieman, A.H.M.; Ditrói, F. Excitation functions of 3He-particle-induced nuclear reactions on 103Rh: Experimental and theoretical investigations. Eur. Phys. J. Plus 2018, 133, 9. [Google Scholar] [CrossRef] [Green Version]
- Karimi, Z.; Sadeghi, M.; Rostampour, M. Assessment and estimation of 65Zn production yield via neutron induced reaction on natZnO and natZnONPs. Appl. Radiat. Isot. 2018, 141, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ditrói, F.; Takács, S.; Haba, H.; Komori, Y.; Aikawa, M.; Saito, M.; Murata, T. Investigation of alpha particle induced reactions on natural silver in the 40–50 MeV energy range. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 436, 119–129. [Google Scholar] [CrossRef]
- Iguchi, S.; Moriguchi, T.; Yamazaki, M.; Hori, Y.; Koshino, K.; Toyoda, K.; Teuho, J.; Shimochi, S.; Terakawa, Y.; Fukuda, T.; et al. System evaluation of automated production and inhalation of 15O-labeled gaseous radiopharmaceuticals for the rapid 15O-oxygen PET examinations. EJNMMI Phys. 2018, 5, 37. [Google Scholar] [CrossRef]
- Degraffenreid, A.; Medvedev, D.; Phelps, T.; Gott, M.; Smith, S.; Jurisson, S.; Cutler, C. Cross-section measurements and production of 72Se with medium to high energy protons using arsenic containing targets. Radiochim. Acta. 2019, 107, 279–287. [Google Scholar] [CrossRef]
- Pourhabib, Z.; Ranjbar, H.; Bahrami Samani, A.; Shokri, A.A. Experimental and theoretical study of rhenium radioisotopes production for manufacturing of new compositional radiopharmaceuticals. Appl. Radiat. Isot. 2019, 145, 176–179. [Google Scholar] [CrossRef]
- Souliotis, G.A.; Rodrigues, M.R.D.; Wang, K.; Iacob, V.E.; Nica, N.; Roeder, B.; Tabacaru, G.; Yu, M.; Zanotti-Fregonara, P.; Bonasera, A. A novel approach to medical radioisotope production using inverse kinematics: A successful production test of the theranostic radionuclide 67Cu. Appl. Radiat. Isot. 2019, 149, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.K.H.; Lobbezoo, A.; Moskven, L.; Schaffer, P.; Hoehr, C. Design of a Thorium Metal Target for 225Ac Production at TRIUMF. Instruments 2019, 3, 18. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Wrońska, A.; Magiera, A.; Bartyzel, M.; Mietelski, J.W.; Misiak, R.; Wąs, B. Reexamination of Proton-induced Reactions on natMo at 19–26 MeV and Study of Target Yield of Resultant Radionuclides. Acta Phys. Pol. B 2019, 50, 1583. [Google Scholar] [CrossRef]
- Alnahwi, A.H.; Samia Ait-Mohand, S.T.; Beaudoin, J.-F.; Guérin, B. Automated radiosynthesis of 68Ga for large-scale routine production using 68Zn pressed target. Appl. Radiat. Isot. 2020, 156, 109014. [Google Scholar] [CrossRef] [PubMed]
- Kambali, I.; Rajiman, P.; Marlina, K.; Huda, N.; Listiawadi, F.D.; Astarina, H.; Ismuha, R.R.; Heranudin, S. Hari Spectral analysis of proton-irradiated natural MoO3 relevant for technetium-99 m radionuclide production. J. Math. Fundam. Sci. 2020, 52, 222–231. [Google Scholar] [CrossRef]
- Van der Meulen, N.P.; Hasler, R.; Talip, Z.; Grundler, P.V.; Favaretto, C.; Umbricht, C.A.; Müller, C.; Dellepiane, G.; Carzaniga, T.S.; Braccini, S. Developments toward the Implementation of 44Sc Production at a Medical Cyclotron. Molecules 2020, 25, 4706. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.P.; Domnanich, K.; Kalman, C.; Walker, W.; Engle, J.W.; Barnhart, T.E.; Severin, G. Durability test of a flowing-water target for isotope harvesting. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2020, 478, 34–45. [Google Scholar] [CrossRef]
- Heinke, R.; Chevallay, E.; Chrysalidis, K.; Cocolios, T.E.; Duchemin, C.; Fedosseev, V.N.; Hurier, S.; Lambert, L.; Leenders, B.; Marsh, B.A.; et al. Efficient Production of High Specific Activity Thulium-167 at Paul Scherrer Institute and CERN-MEDICIS. Front. Med. 2021, 8, 712374. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, C.; Talip, Z.; Borgna, F.; Grundler, P.V.; Dellepiane, G.; Sommerhalder, A.; Zhang, H.; Schibli, R.; Braccini, S.; Müller, C.; et al. Cyclotron production and radiochemical purification of terbium-155 for SPECT imaging. EJNMMI Radiopharm. Chem. 2021, 6, 37. [Google Scholar] [CrossRef]
- Cisternino, S.; Cazzola, E.; Skliarova, H.; Amico, J.; Malachini, M.; Gorgoni, G.; Anselmi-Tamburini, U.; Esposito, J. Target manufacturing by Spark Plasma Sintering for efficient 89Zr production. Nucl. Med. Biol. 2021, 104, 38–46. [Google Scholar] [CrossRef]
- Krasnov, N.N. Thick target yield. The International Appl. Radiat. Isot. 1974, 25, 223–227. [Google Scholar] [CrossRef]
- Brady, F.; Clark, J.C.; Luthra, S. Building on a 50-year legacy of the MRC Cyclotron Unit: The Hammersmith radiochemistry pioneering journey. J. Label. Compd. Radiopharm. 2007, 50, 903–926. [Google Scholar] [CrossRef]
- Peeva, A. Cyclotrons—What Are They and Where Can You Find Them. Available online: https://www.iaea.org/newscenter/news/cyclotrons-what-are-they-and-where-can-you-find-them (accessed on 25 August 2022).
- García-Toraño, E.; Pryrés, V.; Roteta, M.; Sánchez-Cabezudo, A.I.; Romero, E.; Ortega, A.M. Standardisation and precise determination of the half-life of 44Sc. Appl. Radiat. Isot. 2016, 109, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the (44)Ti/(44)Sc generator system. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Buchholz, H.G.; Michels, S.; Hoffmann, M.A.; Piel, M.; Waldmann, C.M.; Rösch, F.; Reuss, S.; Schreckenberger, M. Image quality analysis of 44Sc on two preclinical PET scanners: A comparison to 68Ga. EJNMMI Phys. 2020, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolsky, K.L.; Joshi, V.; Mausner, L.F.; Srivastava, S.C. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl. Radiat. Isot. 1998, 49, 1541–1549. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meuler, N.P.; Türler, A.; Schibli, R. Promising Prospects for 44Sc-/47Sc-Based Theragnostics: Application of 47Sc for Radionuclide Tumor Therapy in Mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef] [Green Version]
- Koumarianou, E.; Loktionova, N.S.; Fellner, M.; Roesch, F.; Thews, O.; Paelak, D.; Archumandritis, S.C.; Mikolajczak, R. 44Sc-DOTA-BN[2-14]NH2 in comparison to 68Ga-DOTA-BN[2-14]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl. Radiat. Isot. 2012, 70, 2669–2676. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Medvedev, D.G.; Maassen, J.M.; Naranjo, C.M.; Unc, G.A.; Meyer, C.A.L.; Mastren, T.; Brugh, M.; Mausner, L.; et al. Proton-induced production and radiochemical isolation of 44Ti from scandium metal targets for 44Ti/44Sc generator development. Nucl. Med. Biol. 2017, 50, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Oroujeni, M.; Xu, T.; Gagnon, K.; Rinne, S.S.; Weis, J.; Garousi, J.; Andersson, K.G.; Löfblom, J.; Orlova, A.; Tolmachev, V. The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule. Pharmaceutics 2021, 13, 292. [Google Scholar] [CrossRef]
- Goethals, P.; Coene, M.; Slegers, G.; Agon, P.; Deman, J.; Schelstraete, K. Cyclotron production of carrier-free 66Ga as a positron emitting label of albumin colloids for clinical use. Eur. J. Nucl. Med. 1988, 14, 152–154. [Google Scholar] [CrossRef]
- Engle, J.W.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Myklejord, D.V.; Barnhart, T.E.; Theuer, C.P.; Nickles, R.J.; Cai, W. Positron emission tomography imaging of tumor angiogenesis with a 66Ga-labeled monoclonal antibody. Mol. Pharm. 2012, 9, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Ugur, Ö.; Kothari, P.J.; Finn, R.D.; Zanzonico, P.; Ruan, S.; Guenther, I.; Maecke, H.R.; Larson, S.M. Ga-66 labeled somatostatin analogue DOTA-DPhe1-Tyr3-octreotide as a potential agent for positron emission tomography imaging and receptor mediated internal radiotherapy of somatostatin receptor positive tumors. Nucl. Med. Biol. 2002, 29, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Gagnon, K.; Abouzayed, A.; Tolnachev, V.; Orlova, A. 66Ga-PET-Imaging of GRPR-expression in prostate cancer: Production and characterization of [66Ga]Ga-NOTA-PEG2-RM26. Sci. Rep. 2021, 11, 3631. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhu, M.; Kopka, K.; Haberkorn, U. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, M.M.; Gu, X.; Ginader, T.; Breheny, P.; Sunderland, J.J. 68Ga-DOTATOC Imaging of neuroendocrine tumors: A systematic review and metaanalysis. J. Nucl. Med. 2017, 58, 1452–1458. [Google Scholar] [CrossRef] [Green Version]
- Ehrhardt, G.J.; Welcj, M.J. A new germanium-63/gallium-68 generator. J. Nucl. Med. 1978, 19, 925–929. [Google Scholar]
- Breeman, W.A.; Verbruggen, A.M. The 68Ge/68Ga generator has highpotential, but when can we use 68Ga-labeled tracers in clinicalroutine? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 978–981. [Google Scholar] [CrossRef] [Green Version]
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.K.; Park, B.N.; Ryu, E.K.; An, Y.S.; Lee, S.J. Current perspectives on 89Zr-PET imaging. Int. J. Mol. Sci. 2020, 21, 4309. [Google Scholar] [CrossRef]
- Hovhannisyan, G.H.; Bakhshiyan, T.M.; Balabekyan, A.R.; Kerobyan, I.A. Production of 47Sc in photonuclear reactions on natTi targets at the bremsstrahlung endpoint energy of 30 and 40 MeV. Appl. Radiat. Isot. 2022, 182, 110138. [Google Scholar] [CrossRef]
- Sabel’nikov, A.V.; Maslov, O.D.; Molokanova, L.G.; Gustova, M.V.; Dmitriev, S.N. Preparation of 99Mo and 99mTc by 100Mo(γ,n) photonuclear reaction on an electron accelerator, MT-25 microtron. Radiochemistry 2006, 48, 191–194. [Google Scholar] [CrossRef]
- Vieira, N.D.; Maldonado, E.P.; Bonatto, A.; Nunes, R.P.; Banerjee, S.; Genezini, F.A.; Moralles, M.; Zuffi, A.V.F.; Samad, R.E. Laser wakefield electron accelerator: Possible use for radioisotope production. In Proceedings of the 2021 SBFoton International Optics and Photonics Conference (SBFoton IOPC), Sao Carlos, Brazil, 31 May–2 June 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Tajima, T.; Dawson, J.M. Laser electron accelerator. Phys. Rev. Lett. 1979, 43, 267. [Google Scholar] [CrossRef] [Green Version]
- Esarey, E.; Schroeder, C.B.; Leemans, W.P. Physics of laser-driven plasma-based electron accelerator. Rev. Mod. Phys 2009, 81, 1229–1285. [Google Scholar] [CrossRef]
- Gonsalves, A.J.; Nakamura, K.; Daniels, J.; Benedetti, C.; Pieronek, C.; de Raadt, T.C.H.; Steinke, S.; Bin, J.H.; Bulanov, S.S.; van Tilborg, J.; et al. Petawatt Laser Guiding and Electron Beam Acceleration to 8 GeV in a Laser-Heated Capillary Discharge Waveguide. Phys. Rev. Lett. 2019, 122, 084801. [Google Scholar] [CrossRef] [Green Version]
- Rovige, L.; Huijts, J.; Andriyash, I.; Vernier, A.; Tomkus, V.; Girdauskas, V.; Raciukaitis, G.; Dudutis, J.; Stankevic, V.; Gecys, P.; et al. Demonstration of stable long-term operation of a kilohertz laser-plasma accelerator. Phys. Rev. Accel. Beams 2020, 23, 093401. [Google Scholar] [CrossRef]
- Tajima, T. Laser acceleration and its future. Proc. Jpn. Acad. Ser. B 2010, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z. Review: Production of nuclear medicine radioisotopes with ultra-intense lasers. AIP Adv. 2021, 11, 040701. [Google Scholar] [CrossRef]
- Lobok, M.G.; Brantov, A.V.; Bychenkov, V.Y. Laser-based photonuclear production of medical isotopes and nuclear waste transmutation. Plasma Phys. Control. Fusion 2022, 64, 054002. [Google Scholar] [CrossRef]
- Shalloo, R.J.; Dann, S.J.D.; Gruse, J.-N.; Underwood, C.I.D.; Antoine, A.F.; Arran, C.; Backhouse, M.; Baird, C.D.; Balcazar, M.D.; Bourgeois, N.; et al. Automation and control of laser wakefield accelerators using Bayesian optimization. Nat. Commun. 2020, 11, 6355. [Google Scholar] [CrossRef]
- Jalas, S.; Kirchen, M.; Messner, P.; Winkler, P.; Hübner, L.; Dirkwinkel, J.; Schnepp, M.; Lehe, R.; Maier, A.R. Bayesian Optimization of a Laser-Plasma Accelerator. Phys. Rev. Lett. 2021, 126, 104801. [Google Scholar] [CrossRef]
- Snavely, R.A.; Key, M.H.; Hatchett, S.P.; Cowan, T.E.; Roth, M.; Phillips, T.W.; Stoyer, M.A.; Henry, E.A.; Sangster, T.C.; Singh, M.S.; et al. Intense High-Energy Proton Beams from Petawatt-Laser Irradiation of Solids. Phys. Rev. Lett. 2000, 85, 2945. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, S.P.; Brown, C.G.; Cowan, T.E.; Henry, E.A.; Johnson, J.S.; Key, M.H.; Koch, J.A.; Langdon, A.B.; Lasinski, B.F.; Lee, R.W.; et al. Electron, photon, and ion beams from the relativistic interaction of Petawatt laser pulses with solid targets. Phys. Plasmas 2000, 7, 2076–2082. [Google Scholar] [CrossRef] [Green Version]
- Afshari, M.; Hornung, J.; Kleinschmidt, A.; Neumayer, P.; Bertini, D.; Bagnoud, V. Proton acceleration via the TNSA mechanism using a smoothed laser focus. AIP Adv. 2020, 10, 035023. [Google Scholar] [CrossRef] [Green Version]
- Perego, C.; Batani, D.; Zani, A.; Passoni, M. Target normal sheath acceleration analytical modeling, comparative study and developments. Rev. Sci. Instrum. 2012, 83, 02B502. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Dorkings, S.; Neely, D.; Musgrave, I. The production of patient dose level 99mTc medical radioisotope using laser-driven proton beams. In Proceedings of the Laser Acceleration of Electrons, Protons, and Ions II; and Medical Applications of Laser-Generated Beams of Particles II, and Harnessing Relativistic Plasma Waves III International Society for Optics and Photonics, Prague, Czech Republic, 15–18 April 2013; Volume 8779, p. 87791C. [Google Scholar]
- Romanov, D.V.; Bychenkov, V.Y.; Rozmus, W.; Capjack, C.E.; Fedosejevs, R. Self-organization of a plasma due to 3D evolution of the Weibel instability. Phys. Rev. Lett. 2004, 93, 215004. [Google Scholar] [CrossRef] [PubMed]
- Bychenkov, V.Y.; Brantov, A.V.; Mourou, G. Tc-99m production with ultrashort intense laser pulses. Laser Part. Beams 2014, 32, 605–611. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, B.S.; Rodrigues, E.R.F.; Fruscalso, J.A.P.; Nunes, R.P.; Bonatto, A.; Alva-Sánchez, M.S. Highly Enriched Uranium-Free Medical Radioisotope Production Methods: An Integrative Review. Appl. Sci. 2022, 12, 12569. https://doi.org/10.3390/app122412569
Nunes BS, Rodrigues ERF, Fruscalso JAP, Nunes RP, Bonatto A, Alva-Sánchez MS. Highly Enriched Uranium-Free Medical Radioisotope Production Methods: An Integrative Review. Applied Sciences. 2022; 12(24):12569. https://doi.org/10.3390/app122412569
Chicago/Turabian StyleNunes, Bruno Silveira, Enio Rodrigo Fernandes Rodrigues, Jonathan Alexander Prestes Fruscalso, Roger Pizzato Nunes, Alexandre Bonatto, and Mirko Salomón Alva-Sánchez. 2022. "Highly Enriched Uranium-Free Medical Radioisotope Production Methods: An Integrative Review" Applied Sciences 12, no. 24: 12569. https://doi.org/10.3390/app122412569






